Abstract
We investigated the degree of physiological damage to bacterial cells caused by optical trapping using a 1,064-nm laser. The physiological condition of the cells was determined by their ability to maintain a pH gradient across the cell wall; healthy cells are able to maintain a pH gradient over the cell wall, whereas compromised cells are less efficient, thus giving rise to a diminished pH gradient. The pH gradient was measured by fluorescence ratio imaging microscopy by incorporating a pH-sensitive fluorescent probe, green fluorescent protein or 5(6)-carboxyfluorescein diacetate succinimidyl ester, inside the bacterial cells. We used the gram-negative species Escherichia coli and three gram-positive species, Listeria monocytogenes, Listeria innocua, and Bacillus subtilis. All cells exhibited some degree of physiological damage, but optically trapped E. coli and L. innocua cells and a subpopulation of L. monocytogenes cells, all grown with shaking, showed only a small decrease in pH gradient across the cell wall when trapped by 6 mW of laser power for 60 min. However, another subpopulation of Listeria monocytogenes cells exhibited signs of physiological damage even while trapped at 6 mW, as did B. subtilis cells. Increasing the laser power to 18 mW caused the pH gradient of both Listeria and E. coli cells to decrease within minutes. Moreover, both species of Listeria exhibited more-pronounced physiological damage when grown without shaking than was seen in cells grown with shaking, and the degree of damage is therefore also dependent on the growth conditions.
Optical tweezers consist of a highly focused laser beam and have successfully been used for manipulation and force measurements on objects on the micro- and nanometer scale (27). Although the term optical tweezers is widely accepted, the system is also often referred to as an optical trap. In some cases the optical tweezers trap beads attached to the biological system of interest, e.g., in the study of molecular motors (24) or bacterial outer membrane proteins (19). In other cases the object under investigation, e.g., a whole living cell, is directly trapped. Within bacterial assays, this has been done in, e.g., studies of bacterial adhesion (9, 10, 26), the rotation of bacterial flagella (1), the propulsion of Listeria monocytogenes (4, 12), and the viability of cells (3). One concern with direct laser trapping of biological specimens is the potential optical damage, which has been ignored in many investigations.
Earlier investigations aiming at quantifying damage caused by optical tweezers have been performed on mammalian cells (11, 14, 15, 22), on E. coli cells (3, 18), and on the organism Caenorhabditis elegans (13). The use of fluorescence assays to assess the physiological status of optically trapped cells has been limited to the investigation of partially immotile sperm cells that died within 7 min of exposure to laser light, where the laser was used both as trap and as excitation source (15). Another experiment investigated the cloning efficiency of Chinese hamster ovary cells subsequent to exposure to different wavelengths and laser powers (14). When trapped at 1,064 nm with a laser power of 88 mW in the specimen plane, a fast decrease in cloning efficiency was observed, and after 20 min, the cloning efficiency was 0%. These observations suggest that long-term optical trapping has a severe effect on the physiology and viability of the mammalian cells being investigated. With C. elegans, the expression of a heat shock protein was measured to observe the stress response during exposure to optical tweezers (13). An increased stress response was observed at wavelengths between 700 nm and 850 nm, with 810 nm as the least-damaging wavelength, when the laser power or exposure time was increased.
To our knowledge, the only prokaryote for which damage during optical trapping has been investigated is Escherichia coli. The work reported in reference 3 shows that the doubling time of briefly optically trapped bacteria at room temperature remained constant (50 min) even for laser powers of up to 200 mW at the sample when a laser with a wavelength of 836 nm was used. The work reported in reference 18 shows that damage measured by the ability of the E. coli bacteria to rotate its flagella is strongly dependent on the trapping laser wavelength, exposure power, and duration, as well as oxygen availability within the sample.
Different mechanisms for the observed optical damage have been proposed. A local temperature increase in the trap has been suggested, but experiments and calculations have shown that in aqueous samples, the heating is typically less than 1°C (15, 21). Other possible mechanisms include multiphoton absorption in biological material (11) and the formation of singlet oxygen (18).
The purpose of the present study was to assess the optical damage to bacterial cells trapped for an extended period and to investigate the influence of laser power, bacterial species, and growth conditions. We have combined optical tweezers with fluorescence ratio imaging microscopy (FRIM) (2, 25) to obtain a real-time measure of intracellular pH (pHi) in the bacterial cells while they are optically trapped. FRIM utilizes pH-sensitive fluorescent probes, and the pHi is calculated from a standard curve based on the ratio between emissions at two different excitation wavelengths. FRIM and pHi have previously been utilized to predict the viability of individual cells (25), also while the cells were optically trapped (15). In this study, two different probes were used to measure the pHi: 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE), which easily stains many types of bacterial cells (2), and a pH-sensitive green fluorescent protein (GFP) molecule which is transformed into cells on a plasmid (16). Under most conditions, healthy cells will maintain a pH gradient over the cell membrane, with a more-alkaline pHi, whereas damaged cells often exhibit a diminished or absent pH gradient. Hence, we used the pHi as a measure of the optical damage induced. Cells were continuously trapped for up to 60 min at different laser powers. Different bacterial species were investigated using different fluorophores and grown under conditions of different oxygen availability (with and without shaking).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Listeria innocua (ATCC 33090) and Bacillus subtilis (ATCC 6633) strains originated from the American Type Culture Collection. The E. coli strain (Top 10; Invitrogen), transformed with a plasmid (pGFPratiometric) containing pH-sensitive GFP, was described in a previous work (20), and L. monocytogenes EGD was transformed with the same plasmid.
All subcultures were performed by adding 0.1 ml of a culture to 10 ml of fresh medium. All species were grown overnight (18 to 20 h) in brain heart infusion broth (Sigma). E. coli cells were grown with shaking in the presence of chloramphenicol (5 mg/liter), followed by an overnight incubation with similar conditions but without chloramphenicol.
L. innocua cells were grown at 37°C with or without shaking. The temperature was chosen to prevent the L. innocua cells from forming flagella, as active movement can prevent trapping at low laser powers.
L. monocytogenes cells were grown at 32°C, either with shaking in the presence of chloramphenicol (5 mg/liter) or with neither shaking nor chloramphenicol. The temperature was chosen to ensure that the GFP folded properly, while preventing the formation of flagella.
B. subtilis cells were grown at 37°C with shaking.
Solutions.
All buffers used in the experiment were citric acid-phosphate buffers adjusted to pH values from 5.5 to 8.0 by mixing 100 mM K2HPO4 and 50 mM citric acid in appropriate volumes. A 1 M stock solution of glucose was added to the buffer solutions to a final concentration of 1 mM prior to the experiments to provide an energy source for the cells.
The dye used for staining L. innocua and L. monocytogenes cells was CFDA-SE (Molecular Probes) dissolved in dimethyl sulfoxide to a concentration of 4.48 mM and stored in the freezer. For staining, this stock solution was added to a final concentration of 44.8 μM.
Staining of cells with CFDA-SE.
The staining procedures for L. innocua and L. monocytogenes cells were modified from the procedure described in reference 2. All cells were harvested by centrifugation at 10,000 × g for 2 min, resuspended in buffer at pH 7.0, and incubated for 30 min at 37°C in the presence of 44.8 μM CFDA-SE and 1 mM glucose. After incubation, cells were harvested and resuspended in buffer at pH 6.0 containing 1 mM glucose.
Preparation of GFP-transformed cells.
E. coli and L. monocytogenes cells were centrifuged at 10,000 × g for 2 min and resuspended in buffer at pH 6.0 containing 1 mM glucose.
Fluorescence microscopy.
All measurements were carried out in a room with a constant temperature of 22°C. The fluorescence microscope setup has been previously described (6) and consists of a monochromator (Monochromator B; TILL Photonics, Germany) with a 75-W xenon lamp that is coupled to the fluorescence port on the back of an inverted microscope (Axiovert 135; Zeiss, Germany) by an optical fiber. For assays with CFDA-SE, the excitation wavelengths were 490 nm and 435 nm, the dichroic mirrors were 510 nm, and the emissions were collected through a band-pass filter of 515 to 565 nm. For assays with GFP, the excitation wavelengths were 470 nm and 410 nm, the dichroic mirrors were 500 nm, and the emissions were collected through a band-pass filter of 510 to 560 nm. The emitted light was collected by using a charge-coupled-device (CCD) camera (Coolsnap fx; Roper Scientific, Inc.) with exposure times of 3 s. Transmission neutral-density (ND) filters were placed between the optical fiber and the fluorescence port to decrease the excitation intensity and, hence, prevent bleaching of the fluorophores. A 25% ND filter was used when illuminating GFP-transformed E. coli cells, and a 6% ND filter was used for CFDA-SE-stained cells. No filter was used with the GFP-transformed L. monocytogenes cells due to weak fluorescence.
Calibration of fluorescence.
The pHi was calibrated from the ratio of pH-equilibrated cells. A ratio of 490 nm/435 nm was used for CFDA-SE-stained cells (2), and a ratio of 470 nm/410 nm for GFP-transformed cells (16). L. innocua cells were pH equilibrated by incubating the stained cells with 67% ethanol for 30 min, harvesting them at 15,000 × g for 5 min, and resuspending them in buffers with pH values from 5.5 to 8.0. The cells were immobilized on a clean cover glass, and the 490/435 ratio for 20 to 30 cells at each pH value was calculated. The calibration curve for L. innocua cells was also used for CFDA-SE-stained L. monocytogenes cells.
The E. coli cells were treated with 10 μM carbonyl cyanide-m-chlorophenylhydrazone (CCCP) (Sigma-Aldrich) and calibrated in buffers with pH values from 5.5 to 8.0. The cells were immobilized on a cover glass pretreated with poly-l-lysine (Sigma-Aldrich) (20), and the 470/410 ratio for 20 to 30 cells at each pH value was calculated. The calibration curve from GFP-transformed E. coli cells was also used to calculate the pHi for the GFP-transformed L. monocytogenes.
Optical tweezer setup.
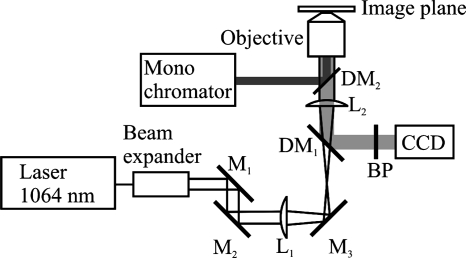
A neodymium-doped yttrium aluminum garnet laser with a wavelength of 1,064 nm (Compass 1064-4000 M; Coherent, Inc., Santa Clara, CA) was used as the trapping laser. The possibility of using the optical tweezers together with fluorescence was obtained by guiding the laser light through a hole in the optical table into the bottom of the microscope (Fig. 1). The laser head and necessary optics were arranged on an inverted optical table beneath the microscope. The bottom mirror of the microscope was replaced with a dichroic mirror that allows for the passage of the infrared laser light. All visible light is reflected from this mirror to retain the normal function of the microscope. The emission band-pass filter, which is usually placed in a filter cube together with the fluorescence dichroic mirror, was removed from the laser beam path and placed in front of the CCD camera. The laser light was focused through a 63× water immersion objective (Zeiss C-Apochromat; numerical aperture, 1.2) to form the optical trap in the specimen plane.
FIG. 1.
Drawing of the microscope equipped with optical tweezers and fluorescence. A neodymium-doped yttrium aluminum garnet laser with a wavelength of 1,064 nm was used as the trapping laser. The laser beam is guided through the different optical parts before entering the microscope, where the tube lens is used as the second lens in the tweezers telescope and the laser beam is focused by the water objective (Zeiss C-Apochromat; numerical aperture, 1.2) to form the trap. The fluorescence light is guided from the monochromator via an optical fiber through the fluorescence port on the back of the microscope and reflected into the sample by the fluorescence dichroic mirror (DM2). The fluorescence light is detected by the CCD camera. L1, lens 1; L2, lens 2; DM1, dichroic mirror 1; DM2, dichroic mirror 2; BP, bandpass filter; M1, mirror 1; M2, mirror 2; M3, mirror 3.
In this report, laser powers refer to the calculated laser power in the specimen plane. This was calculated by measuring the power at the entrance of the objective and corrected for the transmission efficiency of the objective at 1,064 nm (62% as specified by the manufacturer).
Measuring pHi in optically trapped cells.
To prevent interactions with the surface, the cells were individually trapped 10 μm from the surface with a laser power of 6 mW or 18 mW. Six milliwatts was the lowest laser power at which L. innocua cells could be trapped reliably. The first measurement of the pHi was initiated 10 s after trapping. While a cell was trapped, the pHi was measured to detect changes in the gradient across the cell wall. In experiments with L. innocua cells, the pHi was measured at 1-min intervals for 30 min. In experiments with GFP-transformed L. monocytogenes cells, photobleaching was pronounced, and to diminish exposure, the pHi was only measured every 20 min during the 60 min of the experiment. In all other experiments, the pHi was measured at 3-min intervals for 60 min.
Definition of τ1/2.
As a measure of physiological damage, we have defined a gradient decay time, τ1/2, which is the time range, t, from the time laser trapping is initiated, t = 0, until the pH gradient across the cell wall (pHi to extracellular pH [pHex]) is halved, calculated by the following equation: pH(t = τ1/2) = [pHi(t = 0) − pHex]/2. Due to experimental limitations, pHi(t = 0) is the value measured 10 s after trapping was initiated.
RESULTS
Calibration.
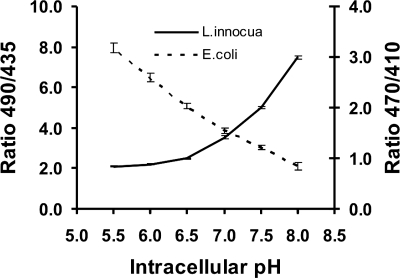
The calibration curve for GFP-transformed E. coli cells is depicted in Fig. 2. Due to a very weak fluorescence signal in equilibrated, GFP-transformed L. monocytogenes cells, the calibration curve for E. coli cells was also used to calculate the pHi in GFP-transformed L. monocytogenes cells.
FIG. 2.
Calibration of excitation ratios as a function of pHi of E. coli and L. innocua cells in buffers from pH 5.5 to pH 8.0. The GFP-transformed E. coli cells were pH equilibrated by using CCCP and an excitation ratio of 470 nm/410 nm. The CFDA-SE-stained L. innocua cells were pH equilibrated with ethanol and an excitation ratio of 490 nm/435 nm. Error bars show SEMs.
The calibration curve for CFDA-SE-stained L. innocua cells is also shown in Fig. 2, and was used for all CFDA-SE-stained Listeria sp. cells.
Optical damage to E. coli.
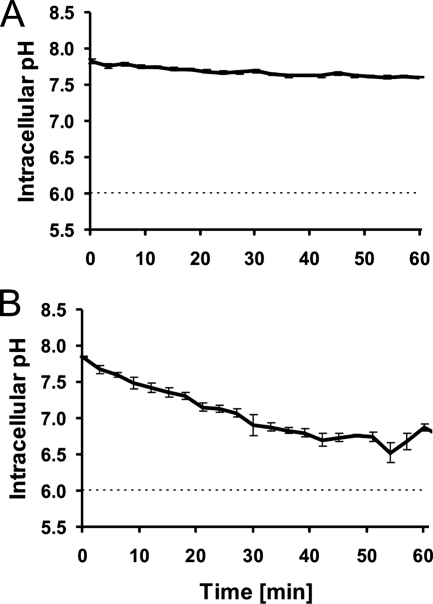
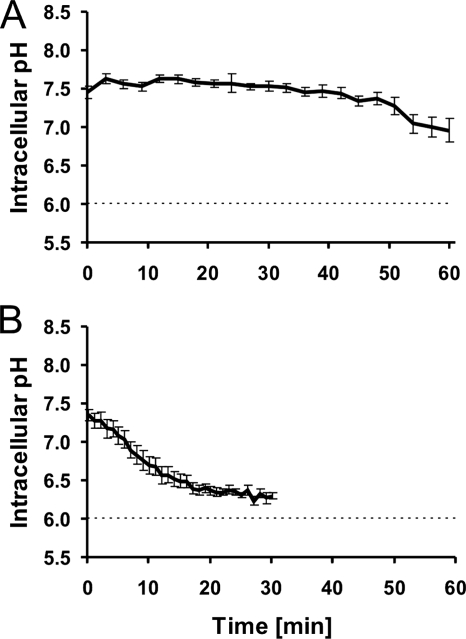
To determine the pHi of nontrapped E. coli cells, the cells were immobilized on a surface coated with poly-l-lysine and incubated for 60 min in buffer at pH 6.0. After this treatment, the pHi was measured as a reference point. The pHi of nontrapped E. coli bacteria in a pHex of 6.0 was 7.7 ± 0.3 (mean ± standard error of the mean [SEM]) (Table 1). Figure 3A shows the pHi as a function of time in E. coli bacteria during trapping with a laser power of 6 mW. During the 60 min of trapping, the bacteria showed only a small decrease in pHi. E. coli cells were also trapped with a laser power of 18 mW (Fig. 3B), and at this laser power, the cells exhibited a more-pronounced decrease in the pH gradient, with a τ1/2 of 30 min (Table 2).
TABLE 1.
pHi of nontrapped cellsa
| Species | Shaking during growth | pHi at end of exptb | No. of cells measured |
|---|---|---|---|
| E. coli | + | 7.7 ± 0.3 | 27 |
| L. innocua | + | 7.7 ± 0.2 | 30 |
| L. innocua | − | 7.3 ± 0.2 | 44 |
| L. monocytogenes | + | 7.5 ± 0.1 | 28 |
| L. monocytogenes | − | 7.4 ± 0.1 | 20 |
The pHi was measured after 60 min of incubation in a buffer with a pH of 6.0. +, with shaking; −, without shaking.
Values were determined after 60 min, except that the values for L. innocua cells without shaking were determined after 30 min.
FIG. 3.
pHi of GFP-transformed E. coli cells as a function of time within an optical trap. Dashed lines indicate pHex. (A) Average and SEM of the results for 20 individual cells trapped with a laser power of 6 mW. (B) Average and SEM of the results for five individual cells trapped with a laser power of 18 mW.
TABLE 2.
τ1/2 of trapped cells
| Species | Shaking during growtha | Fluorophore | Laser power (mW) | Initial pHi | τ1/2 (min)b |
|---|---|---|---|---|---|
| E. coli | + | GFP | 6 | 7.8 | »60 |
| E. coli | + | GFP | 18 | 7.8 | 30 |
| L. monocytogenes | + | GFP | 6 | 7.3 | »60 |
| L. monocytogenes | + | CFDA-SE | 6 | 7.7 | »60 |
| L. monocytogenes | − | CFDA-SE | 6 | 7.6 | 57 |
| L. innocua | + | CFDA-SE | 6 | 7.6 | »60 |
| L. innocua | − | CFDA-SE | 6 | 7.4 | 10 |
| B. subtilis | + | CFDA-SE | 6 | 7.2 | 21 |
+, with shaking; −, without shaking.
When the pH gradient is not halved within 60 min, τ1/2 is designated as »60 min.
Optical damage to Listeria monocytogenes.
To determine the pHi of nontrapped L. monocytogenes cells, the cells were immobilized on a clean glass surface and incubated for 60 min in buffer at pH 6.0. When L. monocytogenes cells were grown with shaking, the pHi was 7.5 ± 0.1, and when the same species was grown without shaking, the pHi was 7.4 ± 0.1 (Table 1).
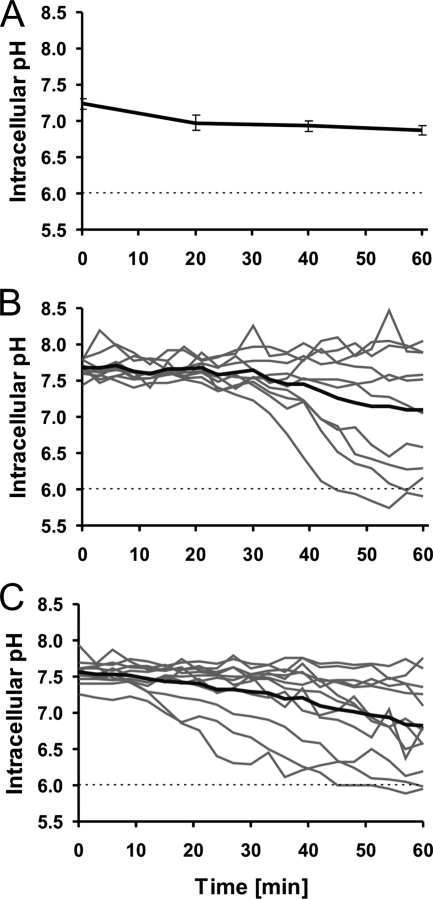
Figure 4 shows the evolution of pHi over time for L. monocytogenes cells during 60 min of optical trapping at 6 mW of laser power. When GFP-transformed cells were grown with shaking, the trapping at 6 mW had little effect on the pHi (Fig. 4A). When L. monocytogenes cells were grown with shaking but stained by CFDA-SE, the change in pHi during trapping was more heterogeneous. To illustrate this, the traces from 10 individual cells and the average of all traces are shown in Fig. 4B. The average pHi of all cells decreases during the trapping period, but the pH gradient is not halved within the observation time. The cells presented in Fig. 4B are also GFP transformed, but as the fluorescence from CFDA-SE is so much stronger than that from GFP, the fluorescence from GFP in Fig. 4B is negligible.
FIG. 4.
pHi of L. monocytogenes cells as a function of optical trapping time. Dashed lines indicate the pHex. (A) GFP-transformed L. monocytogenes cells grown with shaking and trapped with a laser power of 6 mW. The figure shows the average and SEM of the results for four cells. (B) L. monocytogenes cells grown with shaking, stained with CFDA-SE, and trapped with a laser power of 6 mW. Traces of the individual 10 cells investigated are shown in gray; the average of all cells is in black. (C) L. monocytogenes cells grown without shaking, stained with CFDA-SE, and trapped with 6 mW of laser power. Traces of the 12 individual cells investigated are shown in gray; the average of all cells is in black.
The behavior of the pHi over time for individual cells of L. monocytogenes grown without shaking and CFDA-SE stained is shown in Fig. 4C; the average pHi decreased even more than for the cells grown with shaking (Fig. 4B), and the τ1/2 for L. monocytogenes grown without shaking was 57 min (Table 2).
Optical damage to Listeria innocua cells.
The pHi of nontrapped L. innocua cells after 60 min of incubation was 7.7 ± 0.2 when cells were grown with shaking. When L. innocua cells were grown without shaking, the pHi was 7.4 ± 0.1 (Table 1), but this particular value was determined after only 30 min, as this was the maximum period of trapping of L. innocua cells grown without shaking.
Figure 5 depicts the pHi changes in L. innocua cells stained with CFDA-SE and trapped with a laser power of 6 mW. When L. innocua cells were grown with shaking (Fig. 5A), the population maintained a fairly constant pH gradient for a good fraction of the 60 min of treatment. When L. innocua cells were grown without shaking (Fig. 5B), no cells were able to maintain a pH gradient across the cell wall during trapping. The pHi decrease was pronounced from the onset of trapping, and all cells had reached the pHex after only 30 min, with a τ1/2 of only 10 min (Table 2).
FIG. 5.
pHi of L. innocua cells as a function of optical trapping time. The L. innocua cells were stained with CFDA-SE and trapped with a laser power of 6 mW. Dashed lines indicate the pHex. (A) L. innocua cells grown with shaking. The average and SEM of the results for 10 cells are shown. (B) L. innocua cells grown without shaking. The average and SEM of the results for 11 cells are shown.
All τ1/2 values are presented in Table 2. The results for B. subtilis cells grown with shaking indicate that this species is strongly influenced by trapping, as the τ1/2 is only 21 min.
DISCUSSION
As shown in Fig. 2, there is a difference in the calibration curves between the two types of fluorescent probes. The slope of the GFP curve (dashed line) is almost linear from pH 5.5 to pH 8.0, with SEMs of the same magnitude throughout the pH range. The slope of the CFDA-SE emissions (full line) is nonlinear and nearly flat below pH 6.5, and the SEM is dependent on the magnitude of the ratio. From these observations, GFP could be considered the most suitable choice; but from an experimental point of view, the dynamic range of the CFDA-SE emissions is larger, and the emission intensities from CFDA-SE are easily more than a magnitude stronger than the GFP emissions. Additionally, CFDA-SE easily stains gram-positive cells and some gram-negative cells.
Unfortunately, not all microbial cells stain well with CFDA-SE, as, e.g., E. coli cells require physiological alterations of the cell envelope (23), and for long-term, nonperturbing experiments, a probe such as GFP that is continuously synthesized by the organism is ideal. On the other hand, GFP transformation requires molecular manipulation that is not readily applied to all kinds of bacteria, and as noted above, the fluorescence signal from pH-sensitive GFP can be very weak.
The results of earlier studies of different microorganisms stained with CFDA-SE show that the calibration curves for different species can be interchanged (25), and consequently, we used the same CFDA-SE calibration curve for the two Listeria species because the species are very similar.
For the GFP-transformed L. monocytogenes cells, we were forced to use the calibration curve from the GFP-transformed E. coli cells because the original weak fluorescence signal from GFP-transformed L. monocytogenes cells was substantially reduced during pH equilibration and the resulting signal was too weak. This may lead to a slight imprecision in the calculated pHi of GFP-transformed L. monocytogenes cells, as earlier studies have shown a shift in ratios between organisms that are more distantly related (7).
For all types of cells investigated, the initial pHi in the trapping experiments and the pHi of nontrapped cells had good agreement, which leads us to conclude that the pHi of nontrapped cells is a good reference point.
As evident from the results in Fig. 3 and Table 2, optically trapped E. coli bacteria trapped at 6 mW of laser power showed only a small decrease in their pH gradient during 60 min of trapping. When the laser power was increased to 18 mW, the decrease in the pH gradient was faster and more pronounced, with a τ1/2 of 30 min. We can therefore conclude that the damage to E. coli is quite low at 6 mW but significant when the laser power is increased to 18 mW. In an earlier study, E. coli bacteria were briefly optically trapped, sorted, and subsequently subjected to a viability assay (3). It is unknown for exactly how long the bacteria were optically trapped, but in the subsequent viability assays, the doubling time was independent of the trapping laser power for powers up to 200 mW (measured at the output of the laser).
We investigated the effects of different fluorophores, GFP and CFDA-SE, in the same organism. When L. monocytogenes was grown with shaking and trapped with 6 mW of laser power, both the GFP-transformed (Fig. 4A) and CFDA-SE-stained cells (Fig. 4B) revealed populations that were almost unaffected by the trapping. However, the CFDA-SE-stained cells also revealed a number of cells that showed decreases in their pH gradients. Heterogeneity toward stress factors in populations is normal, and it has earlier been shown that, e.g., a moderate stress from bacteriocins on L. monocytogenes cells can lead to two subpopulations, one that maintains the pH gradient across the cell wall and one that cannot maintain this gradient (8). Therefore, we find it reasonable that subpopulations with different capabilities are observed and suggest that the subpopulation with a fast decrease in pHi consisted of the most-sensitive cells in the population. As the fluorescent signal in the GFP-transformed cells was quite weak, we speculate that the susceptible subpopulation observed with CFDA-SE staining was also present in the GFP-transformed population but that the fluorescent signal from these sensitive cells was so low that this second group was not detectable.
The healthy subpopulations with an almost-constant pH gradient have slightly different absolute pHi values for bacteria transformed with GFP and cells stained with CFDA-SE; this difference in pH values could be attributed to the imprecision of the calculated pHi in GFP-transformed L. monocytogenes cells described above, and small differences between the calculated pHi of GFP-transformed and CFDA-SE-stained cells of Lactococcus lactis subsp. lactis have also been observed previously (20). However, experiments with both fluorophores indicate that L. monocytogenes cells grown with shaking can be trapped for at least 20 min without inducing severe damage at a laser power of 6 mW (Fig. 4A and 4B).
L. monocytogenes cells were also grown without shaking, and under these conditions, a very heterogeneous response was observed (Fig. 4C). A subpopulation that decreased after 20 min was also observed in this population, but a number of cells showed an almost-instant decrease in pHi, which limits the time span in which all cells maintained a fairly constant pH gradient to a few minutes. The average τ1/2 for this experiment is 57 min, and L. monocytogenes cells grown without shaking are therefore less suitable for trapping experiments.
The difference between cells grown with and without shaking is more pronounced with L. innocua bacteria, and all the L. innocua cells grown without shaking were very susceptible to trapping, even with 6 mW of laser power (Fig. 5B). When L. innocua cells grown with shaking were trapped with 18 mW of laser power, a faster decrease was observed (results not shown), which is consistent with our observations of optically trapped E. coli (Fig. 3), as well as with earlier observations (10, 12) in which increasing laser power caused increasing damage.
All species investigated showed some degree of damage when trapped by a laser, although a laser power of 6 mW and cells grown with shaking in most cases caused a mild response. This indicates that optical trapping exerts a stress on the cell, although the nature of this stress is not clear. At the same time, cells grown with shaking must have experienced a higher degree of oxidative stress than cells grown without shaking. It is well known that stressing the cells with one type of stress may confer resistance to other types of stress or more-severe stress, such as the acid tolerance response (5). The previous exposure to oxidative stress could therefore be part of the explanation of why cells grown with shaking exhibit less damage than their counterparts grown without shaking upon optical trapping. Although B. subtilis can exhibit a fermentative metabolism under special conditions (17), it is usually considered an aerobic organism. Due to the respiratory metabolism, it is therefore unlikely that oxidative stress will trigger new defense mechanisms as would be the case for E. coli and Listeria bacteria, and this may reflect why this bacterium when grown with shaking exhibits the most-pronounced damage upon trapping, with a τ1/2 of 21 min (Table 2).
The focal volume of the laser trap is approximately 1 μm3, and in a small cell such as a Listeria cell (typical size, 0.5 μm by 1 μm), virtually all of the cell will be inside the focal volume, whereas only a smaller fraction of an E. coli (typical size, 1 μm by 3 μm) or B. subtilis (typical size, 2 μm by 6 μm) cell will be inside the focal volume. However, this parameter does not seem important, as the largest bacterial species, B. subtilis, exhibits the strongest damage, followed by the smallest, Listeria spp., and the intermediate-sized E. coli is the least affected when grown under identical conditions with shaking (Fig. 3A, 4A, 5A, and Table 2). As cells of the gram-negative E. coli were least damaged, one could reflect on whether the composition of the cell wall has any influence. Unfortunately, our data do not provide a clear answer to this question, but it seems unlikely, as e.g., cells of the gram-positive L. innocua were only marginally more damaged than E. coli cells when grown with shaking (Fig. 3A and 5A).
In summary, the common notion that infrared trapping is harmless to microbial cells is incorrect. Rather, the optically induced physiological damage is dependent on the trapping power and duration, the laser wavelength, and the growth conditions of the microorganism, which are important from a microbiological point of view. Hence, before using optical traps to investigate microbial properties, such as the propulsion of Listeria (12) or the rotation of the bacterial flagella (1), it is important to ensure that one is indeed in a regime in which the cells are not physiologically damaged.
Acknowledgments
This work received funding from the Danish Directorate for Food, Fisheries, and Agribusiness and from the EU through PathogenCombat under the FP6 program.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Block, S. M., D. F. Blair, and H. C. Berg. 1989. Compliance of bacterial flagella measured with optical tweezers. Nature 338:514-518. [DOI] [PubMed] [Google Scholar]
- 2.Breeuwer, P., J. Drocourt, F. M. Rombouts, and T. Abee. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol. 62:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ericsson, M., D. Hanstorp, P. Hagberg, J. Enger, and T. Nystrom. 2000. Sorting out bacterial viability with optical tweezers. J. Bacteriol. 182:5551-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerbal, F., V. Laurent, A. Ott, M. F. Carlier, P. Chaikin, and J. Prost. 2000. Measurement of the elasticity of the actin tail of Listeria monocytogenes. Eur. Biophys. 29:134-140. [DOI] [PubMed] [Google Scholar]
- 5.Greenacre, E. J., and T. F. Brocklehurst. 2006. The acetic acid tolerance response induces cross-protection to salt stress in Salmonella typhimurium. Int. J. Food Microbiol. 112:62-65. [DOI] [PubMed] [Google Scholar]
- 6.Guldfeldt, L. U., and N. Arneborg. 1998. Measurement of the effects of acetic acid and extracellular pH on intracellular pH of nonfermenting, individual Saccharomyces cerevisiae cells by fluorescence microscopy. Appl. Environ. Microbiol. 64:530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halm, M., T. Hornbaek, N. Arneborg, S. Sefa-Dedeh, and L. Jespersen. 2004. Lactic acid tolerance determined by measurement of intracellular pH of single cells of Candida krusei and Saccharomyces cerevisiae isolated from fermented maize dough. Int. J. Food Microbiol. 94:97-103. [DOI] [PubMed] [Google Scholar]
- 8.Hornbaek, T., P. B. Brockhoff, H. Siegumfeldt, and B. B. Budde. 2006. Two subpopulations of Listeria monocytogenes occur at subinhibitory concentrations of leucocin 4010 and nisin. Appl. Environ. Microbiol. 72:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, J. F., and D. Velegol. 2006. Laser trap studies of end-on E. coli adhesion to glass. Colloids Surf. B 50:66-71. [DOI] [PubMed] [Google Scholar]
- 10.Klein, J. D., A. R. Clapp, and R. B. Dickinson. 2003. Direct measurement of interaction forces between a single bacterium and a flat plate. J. Colloid Interface Sci. 261:379-385. [DOI] [PubMed] [Google Scholar]
- 11.Konig, K., H. Liang, M. W. Berns, and B. J. Tromberg. 1996. Cell damage in near-infrared multimode optical traps as a result of multiphoton absorption. Optics Lett. 21:1090-1092. [DOI] [PubMed] [Google Scholar]
- 12.Kuo, S. C., and J. L. McGrath. 2000. Steps and fluctuations of Listeria monocytogenes during actin-based motility. Nature 407:1026-1029. [DOI] [PubMed] [Google Scholar]
- 13.Leitz, G., E. Fallman, S. Tuck, and O. Axner. 2002. Stress response in Caenorhabditis elegans caused by optical tweezers: wavelength, power, and time dependence. Biophys. J. 82:2224-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, H., K. T. Vu, P. Krishnan, T. C. Trang, D. Shin, S. Kimel, and M. W. Berns. 1996. Wavelength dependence of cell cloning efficiency after optical trapping. Biophys. J. 70:1529-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, Y., G. J. Sonek, M. W. Berns, and B. J. Tromberg. 1996. Physiological monitoring of optically trapped cells: assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry. Biophys. J. 71:2158-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miesenbock, G., D. A. De Angelis, and J. E. Rothman. 1998. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192-195. [DOI] [PubMed] [Google Scholar]
- 17.Nakano, M. M., and P. Zuber. 1998. Anaerobic growth of a “strict aerobe” (Bacillus subtilis). Annu. Rev. Microbiol. 52:165-190. [DOI] [PubMed] [Google Scholar]
- 18.Neuman, K. C., E. H. Chadd, G. F. Liou, K. Bergman, and S. M. Block. 1999. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 77:2856-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oddershede, L., J. K. Dreyer, S. Grego, S. Brown, and K. Berg-Sorenson. 2002. The motion of a single molecule, the lambda-receptor, in the bacterial outer membrane. Biophys. J. 83:3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen, K. N., B. B. Budde, H. Siegumfeldt, K. B. Rechinger, M. Jakobsen, and H. Ingmer. 2002. Noninvasive measurement of bacterial intracellular pH on a single-cell level with green fluorescent protein and fluorescence ratio imaging microscopy. Appl. Environ. Microbiol. 68:4145-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterman, E. J. G., F. Gittes, and C. F. Schmidt. 2003. Laser-induced heating in optical traps. Biophys. J. 84:1308-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramser, K., J. Enger, M. Goksor, D. Hanstorp, K. Logg, and M. Kall. 2005. A microfluidic system enabling Raman measurements of the oxygenation cycle in single optically trapped red blood cells. Lab Chip 5:431-436. [DOI] [PubMed] [Google Scholar]
- 23.Riondet, C., R. Cachon, Y. Wache, G. Alcaraz, and C. Divies. 1997. Measurement of the intracellular pH in Escherichia coli with the internally conjugated fluorescent probe 5- (and 6-)carboxyfluorescein succinimidyl ester. Biotechnol. Tech. 11:735-738. [Google Scholar]
- 24.Schnitzer, M. J., K. Visscher, and S. M. Block. 2000. Force production by single kinesin motors. Nat. Cell Biol. 2:718-723. [DOI] [PubMed] [Google Scholar]
- 25.Siegumfeldt, H., K. B. Rechinger, and M. Jakobsen. 1999. Use of fluorescence ratio imaging for intracellular pH determination of individual bacterial cells in mixed cultures. Microbiology 145:1703-1709. [DOI] [PubMed] [Google Scholar]
- 26.Simpson, K. H., G. Bowden, M. Hook, and B. Anvari. 2003. Measurement of adhesive forces between individual Staphylococcus aureus MSCRAMMs and protein-coated surfaces by use of optical tweezers. J. Bacteriol. 185:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svoboda, K., and S. M. Block. 1994. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 23:247-285. [DOI] [PubMed] [Google Scholar]