Abstract
A series of molecular and geochemical studies were performed to study microbial, coal bed methane formation in the eastern Illinois Basin. Results suggest that organic matter is biodegraded to simple molecules, such as H2 and CO2, which fuel methanogenesis and the generation of large coal bed methane reserves. Small-subunit rRNA analysis of both the in situ microbial community and highly purified, methanogenic enrichments indicated that Methanocorpusculum is the dominant genus. Additionally, we characterized this methanogenic microorganism using scanning electron microscopy and distribution of intact polar cell membrane lipids. Phylogenetic studies of coal water samples helped us develop a model of methanogenic biodegradation of macromolecular coal and coal-derived oil by a complex microbial community. Based on enrichments, phylogenetic analyses, and calculated free energies at in situ subsurface conditions for relevant metabolisms (H2-utilizing methanogenesis, acetoclastic methanogenesis, and homoacetogenesis), H2-utilizing methanogenesis appears to be the dominant terminal process of biodegradation of coal organic matter at this location.
Isotopic signatures of methane accumulations in coals (56), shales (31), biodegraded oils (2, 34), and ocean floor sediments (35) demonstrate that much subsurface methane production results from microbial activity. Coal is extremely rich in complex organic matter (OM) and therefore could be considered a very attractive carbon source for microbial biodegradation. However, coal is a solid rock, often dominated by recalcitrant, partially aromatic, and largely lignin-derived macromolecules which tend to be relatively resistant to degradation. The rate-limiting step of coal biodegradation is the initial fragmentation of the macromolecular, polycyclic, lignin-derived aromatic network of coal. Lignin degradation can be achieved by extracellular enzymes used by fungi and some microbes (11, 14), and it has also been shown that up to 40% of the weight of some coals can be dissolved using extracted microbial enzymes (47). Furthermore, numerous microbiological studies have developed enrichments capable of anaerobic degradation of methylated and ethylated aromatic compounds (1, 5, 9, 20, 26, 57) or even polycyclic aromatic hydrocarbons (6, 7, 8, 33).
Methane generation from coal by microbial consortia has been documented previously. For example, microflora present in water leached from coal mines were shown to generate methane (56). Furthermore, a methane-generating consortium extracted from coal was observed to grow on low-volatile bituminous coal as a sole carbon source (50). A microbial community may also target the dissipated oil droplets that can be generated from coal by anaerobically degrading long-chain n-alkanes, the main constituents of nonbiodegraded oil (2, 57, 62, 64).
Several lines of geochemical evidence point to a microbial rather than a thermogenic origin of coal bed methane (CBM) along the eastern margin of the Illinois Basin, including the following: (i) very high values of C1/(C2+C3) ratios, typically >103; (ii) stable isotopic signatures of methane δ13CCH4 typically <−60‰ and δD typically about −200‰; and (iii) positive values of δ13CCO2 resulting in Δ13CCO2-CH4 values greater than 60‰, indicative of microbial H2-utilizing methanogenesis (53). The goals of the current study were to confirm the presence of methanogens in Illinois Basin coals and, using culture-dependent and culture-independent methods, to explore the complexity of the microbial communities required for complex OM biodegradation.
MATERIALS AND METHODS
Sampling site.
Coal water samples were collected from CBM-producing wells of a small production field in western Indiana, along the eastern margin of the Illinois Basin (Fig. 1). Our sampling target was the Seelyville Coal Member at a depth of 95 to 110 m. This coal contains significant reserves of biogenic methane, approximately 3 cm3/g, which corresponds to a total of 30 × 109 m3 in the Indiana part of the Illinois Basin (30, 53). The CBM wells coproduce significant quantities of water. Average in situ conditions at the depth studied are as follows: moisture content from 4 to 12% of total weight, pH from 7.5 to 8.8, Eh from −330 to −410 mV, temperature from 16.0 to 17.5°C, salinity from 1 to 12 g/liter, and oxygen content below the detection limit (1 mg/liter). The sampled coal is highly fractured, with a high permeability of ∼40 millidarcy and an average fracture density (including cleats) of 340 fractures/m (52). In addition to large-scale fractures and cleats (apertures from 4 to 250 μm), the main population (74%) are microfractures with apertures of <4 μm.
FIG. 1.
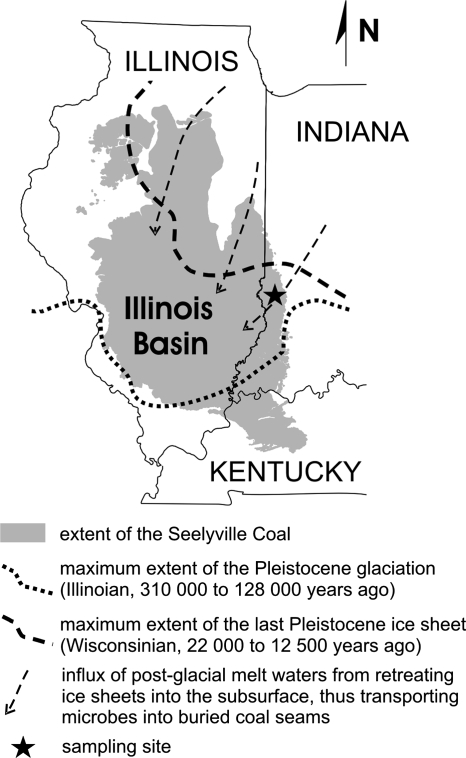
Map showing the extent of the Seelyville Coal formation in the Illinois Basin. The sampling site is located in the eastern marginal zone of the basin. The dotted and dashed lines represent the southernmost extents of the most recent Pleistocene glaciations. The arrows indicate the inferred direction of melt water influxes during inter- and postglacial periods.
Sample collection for enrichment experiments.
Prior to water sampling, 2-liter bottles were autoclaved and 1 ml of 1% resazurin, a redox indicator, was added. Forty milliliters of a reducing-agent mixture (1.25% cysteine-1.25% Na2S) was added inside an anaerobic chamber, and the bottles were subsequently purged with argon. Water for microbial enrichments was collected from three CBM-producing wells (INS-P8, INS-P11, and INS-P10) at 60- to 105-m depths during measurements of physicochemical properties of coal waters (53) done using a multifunctional probe equipped with a flowthrough chamber (YSI 600XL; Yellow Springs Instruments, Inc., Yellow Springs, OH). The chamber was purged for ∼10 min until the physicochemical parameters of the water (i.e., specific conductivity, pH, Eh) stabilized and oxygen levels were below detection limits. The outlet of the flowthrough chamber was then attached to one of two inlets of the 2-liter sample bottle equipped with double-port rubber stoppers. At the same time, sterile-filtered argon overpressure was applied to the other bottle inlet to prevent O2 intrusion. After several seconds, the sampling bottle outlet was opened to release overpressure created by inflowing water and argon. The bottle outlet was closed after sample collection, and the redox indicator showed successful anoxic sampling.
Enrichments.
Aliquots of sample water (2 liters) were passed through sterile 0.22-μm membrane filters (Whatman) in an anaerobic chamber. For enrichment of H2-utilizing methanogens, the filters were placed in 120-ml serum bottles containing 50 ml of prereduced anaerobic medium and crimp sealed with butyl rubber stoppers (Bellco Glass, Inc., Vineland, NJ). The basal medium contained the following (in g/liter): KCl (0.335), MgCl2·2H2O (2.75), MgSO4·7H2O (3.45), NH4Cl (0.25), CaCl2·2H2O (0.14), K2HPO4 (0.14), NaCl (11.0), and yeast extract (1.0) (Difco). In addition, the medium contained 10 mM HEPES buffer (pH 7.5), 0.001% resazurin, 1 ml of 0.2% Fe(NH4)2(SO4)2, 40 ml of 1.25% cysteine-1.25% Na2S, and 10 ml/liter each of trace metals and vitamin solutions. The trace mineral solution contained the following (in mg/liter): FeCl2·4H2O (1,500), ZnCl2 (70), MnCl2·4H2O (100), CuCl2 (2), CoCl2·6H2O (190), AlK(SO4)2 (10), NiCl2·6H2O (24), NaMoO4, 6 H3BO3 (36), and 10 ml/liter of 25% HCl. The vitamin solution contained the following (in mg/liter): biotin (2), folic acid (2), pyroxidine HCl (10), thiamine HCl (5), riboflavin (5), nicotinic acid (5), lipoic acid (5), lipobenzoic acid (5), and vitamin B12 (0.1). The headspace of enrichment bottles contained oxygen-free H2:CO2 (4:1, vol/vol) at 105 Pa (∼1 atm) overpressure. Headspace gases were replaced weekly, and 10% of the volume of the enrichments was transferred to fresh medium every 2 to 3 weeks. Beginning with the third transfer, the medium was amended with alternating pairs of antibiotics (either penicillin G and kanamycin or streptomycin and vancomycin) to inhibit growth of eubacteria. Concentrations of penicillin G, kanamycin, streptomycin, and vancomycin were 80, 80, 60, and 100 μg/ml, respectively. After eight transfers, we reduced the volume of enrichments to 5 ml and used Balch tubes instead of serum bottles. After a series of 15 transfers, we attempted to isolate pure cultures by using both roll tube (23) and bottle plate (17) methods but colonies produced on solid medium were very small and multiple attempts to transfer viable colonies back to liquid culture were unsuccessful.
Additional enrichments were prepared for acetoclastic methanogens and homoacetogens. For the former, the medium described above was modified by addition of 50 mM sodium acetate and use of N2 as the headspace gas. For the latter, we amended the original medium with the methanogen inhibitor 1 mM 2-bromoethanesulfonic acid and did not add antibiotics. The headspace was H2:CO2 (4:1, vol/vol) at 105 Pa overpressure.
Enrichments were tested for methane generation biweekly by use of gas chromatography with a flame ionization detector. Periodically, methanogenic enrichments were also tested for the presence of coenzyme F420 by use of epifluorescence microscopy (58). A highly purified enrichment showing plentiful methane production and coenzyme F420 fluorescence was selected for scanning electron microscopy (SEM) imaging. Selected enrichments were also evaluated for the rate of substrate consumption by use of gas chromatography/mass spectrometry to observe changes in the headspace CH4/CO2 ratios over the time of the enrichment's growth.
DNA extraction.
Twenty milliliters of enrichment culture (from the fifth transfer of the H2-utilizing and third transfer of the acetoclastic methanogens) was centrifuged (10 min, 7,000 rpm, 7°C) to collect cells for DNA extraction. For analysis of the in situ microbial community, cells contained in 8 liters of coal water were collected by filtration through two stacked, sterile, 1.0-μm glass fiber filters (Whatman). The filters were kept at −20°C until extraction. The DNA extraction procedure was the same for both the cell pellet and the filter. For the filter extraction, of the filter was used. Resuspension of the cells in 2-ml vials was achieved by adding a solution of buffer P1 (50 mM Tris-Cl, 10 mM EDTA, 100 μg/ml RNase A; Qiagen) and chloroform (1:1, vol/vol) and was followed by centrifugation (8 min, 12,000 rpm, 4°C). Cells were lysed by addition of 20 μl of 10% pyrophosphate and lysozyme (900 U/ml final concentration) and incubation for 40 min at 37°C. Afterwards, proteinase K (2 mg/ml) and 10 μl of 20% sodium dodecyl sulfate were added. The vials were stored at 50°C for 30 min. To wash and concentrate DNA, phenol-chloroform-isoamyl alcohol and 0.3 g of acid-washed silica beads were added. The mixture was vortexed at 6,000 rpm for 2 min and centrifuged for 3 min at 12,000 rpm, and the top aqueous phase was extracted once more with phenol-chloroform-isoamyl alcohol. DNA in the extracted aqueous phase was precipitated overnight at −20°C with 1:1 (vol/vol) isopropanol and 0.1 (vol) Na acetate. The DNA was subsequently purified using the Qiaex agarose gel extraction protocol (Qiaex II handbook; Qiagen).
16S rRNA analysis.
Purified DNA was amplified by PCR using the universal primers 1492r and 533f. The PCR began with initial melting at 94°C for 5 min, followed by 30 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 2 min and a final elongation at 72°C for 20 min. Although PCR amplification was additionally attempted using several other primer sets (archaeal 21f and 958r, mixed archaeal 21f and universal 1492r), only universal primers 533f and 1492r yielded PCR products.
PCR products were cloned into pCR4-TOPO plasmids and transferred into competent OneShot Mach1 Escherichia coli cells as specified by the manufacturer (TOPO TA cloning kit; Invitrogen). Following growth on LB agar supplemented with 50 μg/ml kanamycin, isolated colonies were picked for colony PCR. We selected 48 colonies containing plasmids with DNA from the highly purified, methanogenic enrichments and 65 colonies containing plasmids with DNA extracted from the coal water. DNA from picked colonies was amplified using M13 primers. The amplification parameters were as follows: initial heating to 80°C for 2 min and melting at 95°C for 7 min, followed by a series of annealing steps at different temperatures (two steps at 60°C, two at 58°C, two at 56°C, two at 54°C, two at 52°C, and 25 at 50°C). All annealing steps were preceded by a melting step at 95°C for 30 s and followed by an elongation step at 72°C for 1.5 min. The final elongation was at 72°C for 20 min, followed by cooling down to 4°C. Colony PCR products were purified by using a QIAquick PCR purification kit 250 (Qiagen) according to the manufacturer's protocols.
Sequencing and phylogenetic analysis.
Plasmids were sequenced at the Penn State University Biotechnology Center by using T3 and T7 primers. Partial sequences were assembled and bases manually checked using the CAP application of the BioEdit software (16). The chimera check was performed using the Bellephoron software (22). Sequences were then submitted to the NCBI BLAST internet library to compare levels of similarity to known phylotypes. All sequences were aligned using the ClustalW application in the BioEdit software (16). Additionally, Methanosarcina sp., as well as species from the Methanocorpusculaceae family and several bacterial species, were selected for phylogenetic analysis. A phylogenetic tree was constructed in the MEGA 3.1 software (29) using the neighbor-joining method (substitution method, p distance; bootstrap, 3,000 replicate trees).
IPLs.
Intact polar lipids (IPLs) of the microbial cell membranes were extracted using a modified Bligh-Dyer extraction protocol (60). The cell pellets obtained from 20 ml of the methanogenic enrichment were sonication extracted three times with 1:2:0.8 dichloromethane (DCM):methanol:phosphate buffer (8.7 g/ liter KH2PO4, pH 7.4) and three times with 1:2:0.8 DCM:methanol:trichloroacetic acid buffer (50 g/liter). Supernatants were combined to a separatory funnel where separation of organic and aqueous phases was achieved with addition of DCM and 5% NaCl in preextracted water. The organic phase was collected, and the aqueous phase was washed three times with DCM and then added to the organic fraction. The pooled organic phase was dried over Na2SO4, and the solvent lipid extract was dried under a stream of nitrogen gas, stored at −80°C, and shipped frozen to Bremen, Germany, for analysis.
The lipid components in the total lipid extract were separated according to head group polarity by use of high-performance liquid chromatography (HPLC) techniques described previously (3, 55). Briefly, lipid material was dissolved in DCM-methanol (1:1, vol/vol) and injected into a LiChrospher Diol-100 column (150 by 2.1 mm, 5 μm; Alltech GmbH, Germany) equipped with a guard column of the same packing material by use of a ThermoFinnigan Surveyor HPLC system (ThermoFinnigan, Bremen, Germany). HPLC-mass spectrometry experiments were performed using a ThermoFinnigan LCQ Deca XP Plus ion-trap mass spectrometer (ThermoFinnigan, Bremen, Germany) with an electrospray ionization interface in data-dependent ion-tree mode with automatic fragmentation up to MS3. Compound classes were identified based on characteristic molecular ions and daughter ion fragments identified previously (27, 28, 55).
Analysis of H2 and acetate concentrations.
The H2 concentration was analyzed using a Peak Performer 1 gas analyzer (Peak Laboratories, LLC, California) equipped with a reducing compound photometer. Gas samples were taken from serum bottles by use of a gas tight syringe and diluted to a concentration less than 10 ppm for injection. Measurements on replicate samples generally have a precision of about ±1 ppm. Measured gas-phase partial pressure values were converted to pore water concentration by use of solubility constants corrected for temperature and salinity (10).
Coal water samples for acetate concentration analysis were stored frozen until measurement. Acetate concentrations were obtained during isotopic analysis of acetate (values not reported due to very low acetate concentrations) according to a published protocol (18) by use of a ThermoFinnigan Surveyor HPLC coupled to a ThermoFinnigan Delta Plus XP isotope ratio mass spectrometer via the Finnigan LC IsoLink interface. Separation of acetate was achieved on a Nucleogel Sugar 810 H column (200 by 1.8 mm; Macherey-Nagel, Germany) equipped with a CC30/4 Nucleogel Sugar 810 H guard column (30 by 4 mm; Macherey-Nagel, Germany). Degassed aqueous phosphate buffer (5 mM) was used as a mobile phase with a flow rate of 300 μl/min. The oxidation reagent, converting acetate to CO2, was a solution of sodium peroxodisulfate in phosphoric acid (3 g Na2S2O8, 10 ml H3PO4, 300 ml MilliQ) pumped at a flow rate of 60 to 80 μl/min. Absolute values of acetate concentration were determined relative to external calibration by use of peak areas of acetate-derived CO2 signal recorded by isotope ratio mass spectrometry. Enrichment cultures were not tested for acetate concentrations.
Free energy calculations for coal bed conditions.
The equation for standard free energy, ΔG° = VΔP − SΔT (where V is volume and S is entropy), is a function of temperature (T) and pressure (P). Therefore, to calculate ΔG for the studied system, we calculated a “reference state” (ΔG°P,T) matching the in situ temperature and pressure conditions (10.5 atm, 17°C). This calculated reference state is not identical to the standard state of 105 Pa and 25°C. The free energies available for in situ microbial reactions, ΔG, can thus be correctly calculated based on ΔG°P,T for the in situ reference state and modifications due to in situ concentrations of substrates and products by using the formula ΔG = ΔG° − RT ln Q, where R is the universal gas constant 8.31 J·K−1·mol−1, T is the temperature in kelvins, and Q is the reaction quotient. ΔG°P,T values of three microbial reactions (Table 1) were calculated using SUPCRT92 (25), which uses published thermodynamic data (48). Calculations of Q involved activity corrections based on the average in situ salinity (3.11 g/liter) determined at pH 8 and an acetate concentration of 4.3 × 10−6 M. The ionic strength was 0.054 M, and the resulting activity coefficient γ of a single charged species (Davies equation) was 0.773. The molalities of aqueous-phase H2 (H2,aq) and CH4,aq were calculated using measured pH2 and pCH4 in coal gas and the appropriate Bunsen solubility coefficient, dependent on temperature and salinity (10). The activity coefficient of gaseous species was assumed to be 1; thus, their activities were equivalent to their molalities. The HCO3− activities (aHCO3−) were calculated based on water physicochemical properties measured in the wells and CO2 concentration data from fresh coal core desorption, representative of in situ values, by use of PHREEQC software (38).
TABLE 1.
Microbial process in Indiana coal beds and associated free energy changes
| Reaction no. | Microbial process | Chemical reaction | ΔG°P,T (kJ)a | ΔG (kJ)a |
|---|---|---|---|---|
| 1 | H2-utilizing methanogenesis | HCO3− + 4H2,aq + H+ → CH4 + 3H2O | −230.2 | −19.8 |
| 2 | Acetoclastic methanogenesis | CH3COO− + H2O → CH4,aq + HCO3− | −14.7 | −4.8 |
| 3 | Homoacetogenesis | 2HCO3− + 4H2,aq + H+ → CH3COO− + 4H2O | −215.6 | −15.1 |
ΔG°P,T is a calculated reference state at in situ temperature and pressure conditions (10.5 atm, 17°C) with all reactants and products at an activity of 1.0. ΔG values are derived from ΔG°P,T but modified for in situ conditions based on the following average measured concentrations at three wells (INS-P11, INS-P6, and INS-P8): H+, 10−8 M; H2,aq, 3.76 × 10−8 M; CH3COO−, 4.3 × 10−6 M; CH4,aq, 1.5 × 10−2 M; and HCO3−, 1.7 × 10−2 M. All free energy changes represent the values for the reactions as written.
The ΔG value was assigned to be −15 kJ (per mole of protons) as the minimum amount of energy that a microbial cell requires for each step of the three-step ATP synthase activation, which requires transfer of three protons to generate one ATP (19, 45). Some authors suggest that anaerobic metabolism can function even at lower levels of available energy near the thermodynamic limits of ΔG ≈ 0 kJ (24). In order to generate free energy plots presenting −15 or 0 kJ isolines, we used the formula given above: ΔG = ΔG° − RT ln Q. For example, −15 kJ isoclines for H2-utilizing methanogenesis (Table 1, reaction no. 1) were calculated as follows: −15 kJ = −230 kJ − RT ln [aCH4,aq/(aHCO3−·aH+·H2,aq4)].
Subsequently, this equation was recast for aHCO3− as a function of aH2,aq as follows: aHCO3− = {aCH4,aq/[e(15 − 230 kJ)/RT·aH+·H2,aq4]} (where e is the base of natural logarithm).
The same procedure was used for calculations for reaction no. 2 from Table 1. For calculation of the isolines ΔG = −15 kJ and ΔG = 0 kJ for reaction no. 3, the acetate concentration, similarly to the example above, was expressed as a function of HCO3− activity.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences determined in this study have been deposited in GenBank under accession no. EU168192 to EU168199 for the representative clones from the methanogenic enrichment (45 clones total sequenced) and accession no. EU168200 to EU168262 for all 65 clones from the filtered coal water sample.
RESULTS
Enrichments.
Biweekly headspace analysis of H2-utilizing, methanogenic enrichments from two wells showed the presence of methane and comparable methanogenesis rates. To more closely monitor rates of methanogenesis, we measured headspace CH4 every 6 h in selected enrichments for 4 days after transfer to fresh media. Typically, within 24 to 72 h after 10% enrichment culture transfers to fresh medium, we observed a significant drop in headspace pressure and an increase of the CH4 to CO2 ratios caused by consumption of headspace H2 and CO2. Under the microscope, about 80% of all cells from the fourth and subsequent transfers had F420 coenzyme fluorescence. Furthermore, the morphological homogeneity of cells observed under SEM confirmed that the culture was highly enriched and consisted primarily of one morphotype, i.e., spherical cells with a diameter of approximately 0.4 μm (Fig. 2). In enrichments for acetoclastic methanogens, initial low rates of methane generation ceased after the fourth transfer. In the homoacetogenic enrichments, we observed a significant decrease in the H2:CO2 headspace pressure during the first three transfers, although no CH4 was produced, likely due to the inhibitory presence of 2-bromoethanesulfonic acid. Since our work was focused on methane production rather than a detailed evaluation of C metabolism, we did not measure acetate production and discontinued efforts to enrich acetogens.
FIG. 2.
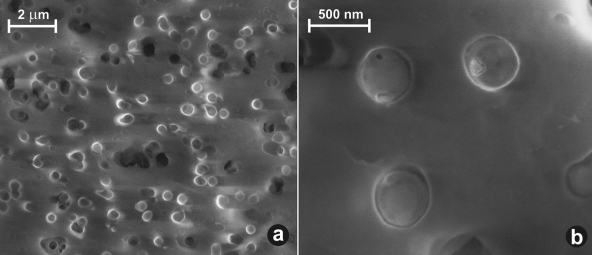
SEM pictures of the methanogenic enrichment on a 0.22-μm membrane filter. (a) Culture was predominantly one morphotype, consisting of spherical cells with diameters of ≤0.5 μm. (b) Higher magnification of three typical spherical cells, most likely Methanocorpusculum parvum.
Phylogeny of the microbial community from coal and methanogenic enrichments.
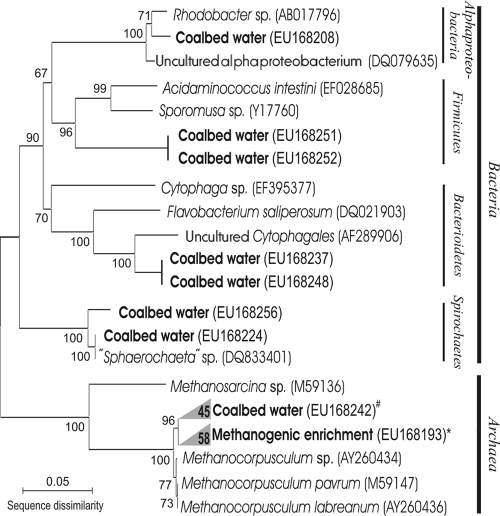
Of the 65 clones analyzed from the coal water sample, 13% were related to various bacteria of the following clades: Alphaproteobacteria, Firmicutes, Bacteroidetes, and Spirochaetes (Fig. 3). The rest of the clones were Archaea, represented exclusively by close relatives (98 to 100% similarity) of the genus Methanocorpusculum. The relative abundance of archaeal and bacterial clones in our clone library does not necessarily represent the distributions of species in the environmental populations, due to potential PCR bias.
FIG. 3.
Microbial diversity in water from coal gas-producing well INS-P11, Seelyville Coal, depth of 105 m. The tree was generated using MEGA 3.1 software (29) with neighbor joining, a p distance substitution method, and 3,000 bootstrap replicates. Archaeal coal bed water clones (#) and methanogenic enrichment clones (*) were almost identical and are represented as clades. The number of clones in each clade is shown. Designations in parentheses are NCBI accession numbers.
In the methanogenic enrichment, all of the archaeal clones (95% of total) were members of the genus Methanocorpusculum, with 99 to 100% similarity to Methanocorpusculum parvum and slightly lower similarity to Methanocorpusculum labreanum. SEM imaging confirmed the submicron size of the cells, which is a characteristic of the Methanocorpusculum genus (Fig. 2). The attempts at phylogenetic analysis of acetoclastic enrichment were unsuccessful due to low DNA yields.
Distribution of IPLs in the methanogenic enrichment.
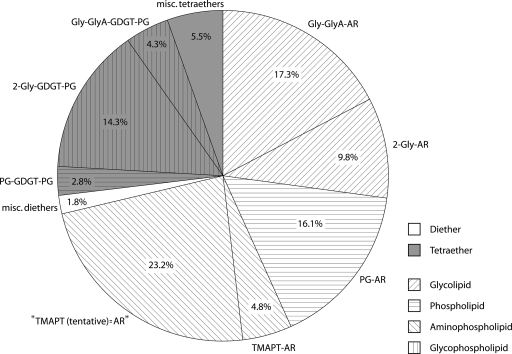
The IPL distribution in the methanogenic enrichment culture consists of 73% dialkyl glycerol diethers and 27% glycerol dialkyl glycerol tetraethers (GDGTs). The main compounds forming the cell membrane of our enriched methanogen were (i) diglycosyl-GDGT-phosphatidylglycerol (2-Gly-GDGT-PG) (two glycosyl head groups are attached on one side of the GDGT moiety and a phosphatidylglycerol head group on the other) (Fig. 4) and (ii) dialkyl glycerol diethers diglycosyl-archaeol (2-Gly-AR), phosphatidylglycerol-archaeol (PG-AR), and (N,N,N-trimethyl)-aminopentanetetrol-archeol (TMAPT-AR) (Fig. 4). The bacterial lipids were present only in traces (less than 1% of total IPLs), which confirms a high purity of the enrichment.
FIG. 4.
Distribution of archaeal IPLs in the methanogenic enrichment sample dominated by close relatives of Methanocorpusculum parvum. Core lipids were GDGT and AR. Polar head groups were mono- and diglycosyl, PG, TMAPT, Gly-GlyA (tentatively identified glycosyl-glycuronic acid), and TMAPT(tentative) (tentatively identified derivative of TMAPT with a similar fragmentation pattern and a 14-Da-higher mass). misc., miscellaneous.
DISCUSSION
Methanogens of the Illinois Basin.
The presence of viable methanogens in coal beds has previously been observed indirectly in incubation experiments from several basins, including low-volatile bituminous coal from Russia (50), Rhine River brown coal from Germany (4), and Ruhr Basin coals in Germany (56). Previous studies of CH4 and CO2 stable isotope ratios suggested that CH4 was being produced by H2-utilizing methanogens in the Illinois Basin coals (53). In the current study, enrichments from coal waters showed high rates of methane generation and F420 epifluorescence, confirming the presence of abundant methanogens. The submicrometer (0.4-μm) size of the predominant morphotype, typical for Methanocorpusculum sp., was revealed by SEM imaging of the enrichment culture (Fig. 2b). The phylogenetic study of water extracted from the coal bed and the enrichment cultures (Fig. 3) indicated dominance of one archaeal species, Methanocorpusculum parvum. Evidence of Methanocorpusculum parvum as the dominant methanogen is supported by the lipid composition of the cell membranes. The IPL distribution from the enrichment culture includes all the major phospho- and glycolipid components typical of Methanocorpusculum parvum (28), e.g., IPL speciation and the 2:1 ratio of diethers to tetraethers (Fig. 4). This combination of geochemical and biological evidence unambiguously implicates the presence and activity of close relatives of Methanocorpusculum parvum in the Illinois Basin coals.
Methanocorpusculum was first reported as Methanogenium aggregans in 1985 (37) and was subsequently isolated in 1987 (63). Recently the complete genome of Methanocorpusculum labreanum has been sequenced and submitted to NCBI (accession no. CP000559). Since the initial discovery, Methanocorpusculum parvum has been found in a variety of anoxic environments, including shales in the Michigan Basin (59), hydrothermally active sediments in the Guaymas Basin (12), river estuary sediments in the United Kingdom (40), a cold polluted pond in Russia (51), waste waters and landfills (21), animal waste storage pits (61), and as endosymbionts of ciliates (13). Anoxia, low salinity, and moderate temperatures are common characteristics of all described Methanocorpusculum niches. For example, in organic-matter-rich shales in the Michigan Basin there is a gradual shift toward more halophilic methanogens along a salinity gradient; Methanocorpusculaceae were found in large numbers only in formation waters and enrichments with lower than marine (∼19 g/liter) chlorinities of 0.7 to 8.1 g/liter, with smaller numbers in enrichments with chlorinities of 17.8 and 26.6 g/liter (59). Similarly, in the estuary of the Colne River, United Kingdom, Methanocorpusculum was observed only in freshwater and brackish sediments (<1 g/liter salinity) and not under estuarine or marine conditions (40).
Methanocorpusculum sp. can be considered a psychrotolerant mesophile, found at temperatures as low as 1°C, although it grows optimally from 25 to 35°C (51). High temperatures (∼90°C) that existed in the coalification stage about 310 million years ago most likely sterilized the Illinois Basin coals and removed the preburial microbial community associated with the peat bog stage. The erosional uplift of coal beds followed by post- and interglacial dilution of the original basinal brines, from typical original values of around 50 g/liter (54) to present-day values of approximately 5 g/liter, allowed shallow coals to be inoculated with microbial communities capable of methanogenesis (Fig. 1). The chemical conditions of coal water combined with in situ temperatures of ∼17°C created a habitable niche for Methanocorpusculum sp.
Biodegradation of coal OM and production of substrates for methanogenesis by complex microbial consortia.
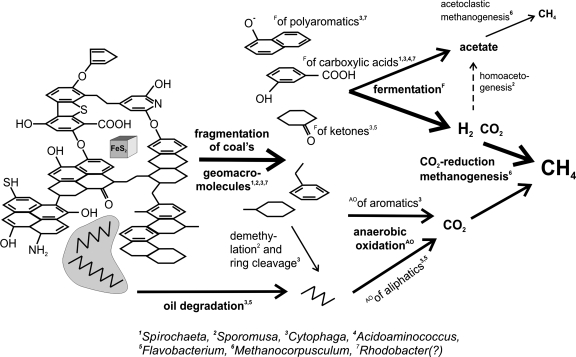
The aromatic structures of coal derived from cellulose and lignin are often interlinked by oxygen bridges and contain numerous oxygen-containing moieties (e.g., carboxyl, hydroxyl, or ketone functional groups) (Fig. 5). Although Indiana high-volatile bituminous B and C coals are considered moderately mature (vitrinite reflectance Ro of 0.6%), they still contain ∼8% weight of oxygen in the coal OM. These oxygen linkages and functional groups can be targeted by fermentation, providing important intermediates of OM degradation, such as succinate, propionate, acetate, CO2, and H2.
FIG. 5.
Proposed mechanisms of stepwise biodegradation of OM in coal, annotated with microbes related to those found in the clone library and potentially capable of performing the indicated processes: (i) defragmentation of coal geomacromolecular structure predominately by fermentation targeted at oxygen-linked moieties and oxygen-containing functional groups (this process detaches some of the oxygen-linked aromatic rings and generates some short organic acids); (ii) anaerobic oxidation of available aromatic and aliphatic moieties, derived from coal defragmentation or from dispersed oil; (iii) fermentation of products available from step i described above to H2, CO2, and acetate; and (iv) methanogenesis utilizing H2 and CO2 likely predominating over homoacetogenesis and acetoclastic methanogenesis. The dark area represents a droplet of oil. The molecular model of coal is adapted from reference 43.
While we recognize that the limited number of clones recovered from the coal waters and enrichments likely do not represent a comprehensive picture of the complexity of the in situ populations responsible for organic carbon metabolism, we can nonetheless use the clone libraries to develop simplified and hypothetical mechanisms for the degradation of OM in the coal beds. Fragmentation of the oxygen-interlinked geomacromolecular structure of coal could potentially be performed by the clones associated with Sphaerochaeta (Fig. 5). Related organisms from the Spirochaetes phylum are known as plant-polymer fermenters in bovine rumen fluids (39) and can also degrade higher plant-derived polymers, such as xylan, pectin, and arabinogalactan. Bacteroidetes, including some species of Cytophaga and Flavobacterium, are capable of anaerobic degradation of cellulose, proteins, and polysaccharides in methanogenic enrichments of freshwater sediments as well as polyaromatic hydrocarbons (15, 41). Additionally, some representatives of the Firmicutes found in our clone library, e.g., Sporomusa, can demethylate aromatic compounds, a key reaction preceding cleavage of aromatic rings (32). Acidoaminococcus sp., closely related to our recovered Sporomusa clones, can ferment simple amino acids as a sole energy source (42). This organism could then potentially participate in the recycling of microbial biomass in the coal ecosystem. The potential role of Rhodobacter (Alphaproteobacteria) (49) in the coal beds is not known.
Another possible carbon substrate for ultimate generation of methane precursors could be oil dispersed in the coal as micron-sized droplets. Well-known participants in oil biodegradation are microbes from the Cytophaga and Flavobacterium group (41) found to grow on n-hexane. Anaerobic oxidation of aliphatic compounds in the oil droplets or present as aliphatic side chains of the aromatic coal matrix may lead to production of CO2, which could ultimately serve as the carbon source for CH4 production by H2-utilizing methanogens. Although the proposed pathway (Fig. 5) for biodegradation of organic C in the coal beds is highly speculative, it is consistent with our results and can be tested in future studies.
Free energy considerations for coal bed methanogenesis.
In subsurface coal beds, H2 is typically found in concentrations so low (H2,aq of ∼10−8 mol/liter) that it can be considered the rate-limiting factor for H2-based methanogenesis. Microbial fermentations that produce H2 are therefore an important link in the sequential biodegradation of coal. Syntrophic relationships between fermenting microorganisms and H2-utilizing methanogens have been documented previously, and the low pH2 maintained by methanogenesis can create exergonic conditions for otherwise energetically unfavorable fermentations (44).
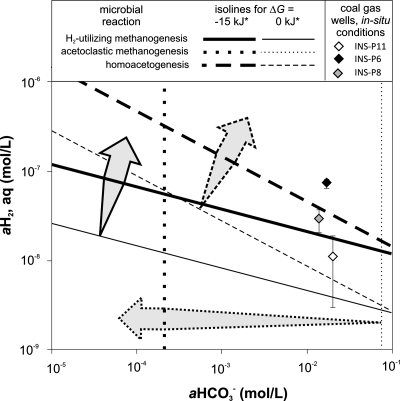
In order to evaluate the possible scenarios leading to terminal coal OM biodegradation, we examined the free energy balances of several possible microbial reactions. In addition to H2-based methanogenesis, we explored the potential role of homoacetogenesis in tandem with acetoclastic methanogenesis as the terminal OM degradation process. Since homoacetogenesis utilizes the same substrates as CO2/H2-utilizing methanogenesis, these two microbial reactions may compete, and the in situ conditions should select the more energetic system. As depicted in Fig. 6, our calculations show that H2-utilizing methanogenesis should be more energetically favorable at lower CO2 and H2 concentrations. Nonetheless, an important role for homoacetogenesis cannot be ruled out. Experimental observations that may support the latter possibility are (i) the two clones related to Sporomusa, a homoacetogen, in the clone library and (ii) the decrease in the H2:CO2 headspace pressure without methane in the initial homoacetogen-targeted enrichments. Since we discontinued enrichment of acetogens to better focus our efforts on methanogens, the role of acetogens or other possible H2-utilizing bacteria, e.g., sulfate reducers, is unknown. It should be noted, however, that known sulfate reducers were not observed in the clone library.
FIG. 6.
Free energy dependence on the substrate and product availability of microbial reactions calculated for typical measured in situ conditions (salinity of 3.11 g/liter, temperature of 17°C, pressure of 10.5 atm, H+ of 10−8 M, H2,aq of 3.8·10−8 M, CH3COO− of 4.3·10−6 M, CH4,aq of 1.5·10−2 M, and HCO3− of 1.7·10−2 M). The data points represent in situ conditions in three, coal gas-producing wells within 5 km of each other. The arrows indicate conditions under which the given reaction is more exergonic than the required minimum (15 or 0 kJ), based on the reactions shown in Table 1.
An alternative source of acetate for acetoclastic methanogenesis could be fermentative degradation of coal OM. Very low concentrations of acetate in the coal waters could imply active acetate consumption. Despite the low free energy yield (∼−5 kJ) (Table 1; Fig. 6), the potential for acetoclastic methanogenesis is supported by our initially successful acetoclastic enrichments. Another potential acetate sink could be syntrophic acetate oxidation to CO2 and H2, which has been documented in some methanogenic environments (36, 46), but our clone library contains no known acetate oxidizers and the process is highly endergonic (+15.1 kJ) under extant in situ conditions. All of the available stable isotopic (53), microbiological, phylogenetic, and thermodynamic data suggest that the importance and contribution of acetoclastic methanogenesis to bulk methane generated in the studied coal beds are minor. Overall, our studies conclude that past and ongoing biodegradation of coal and coal-derived oil followed predominantly by H2-utilizing methanogenesis by members of the Methanocorpusculum genus contribute to the significant CBM reserves along the eastern margins of the Illinois Basin.
Acknowledgments
We thank Katherine H. Freeman for use of her lab at Penn State University for IPL extractions. Thanks to Juergen Schieber for help with the SEM imaging. We greatly acknowledge Tom Hite for gas and water sampling opportunities. Grzegorz Lis, Jaofa Jiang, Zuoping Zheng, and Agnieszka Drobniak were very helpful field assistants.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Anderson, R. T., and D. R. Lovley. 2000. Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum-contaminated aquifer. Environ. Sci. Technol. 34:2261-2266. [Google Scholar]
- 2.Bekins, B. A., F. D. Hostettler, W. N. Herkelrath, G. N. Delin, E. Warren, and H. I. Essaid. 2005. Progression of methanogenic degradation of crude oil in the subsurface. Environ. Geosci. 12:139-152. [Google Scholar]
- 3.Biddle, J. F., J. S. Lipp, M. A. Lever, K. G. Lloyd, K. B. Sørensen, R. Anderson, H. F. Fredricks, M. Elvert, T. J. Kelly, D. P. Schrag, M. L. Sogin, J. E. Brenchley, A. Teske, C. H. House, and K.-U. Hinrichs. 2006. Heterotrophic archaea dominate sedimentary subsurface ecosystems off Peru. Proc. Natl. Acad. Sci. USA 103:3846-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catcheside, D. E. A., and J. P. Ralph. 1999. Biological processing of coal. Appl. Microbiol. Biotechnol. 52:16-24. [Google Scholar]
- 5.Chakraborty, R., S. M. O'Connor, E. Chan, and J. D. Coates. 2005. Anaerobic degradation of benzene, toluene, ethylbenzene, and xylene compounds by Dechloromonas strain RCB. Appl. Environ. Microbiol. 71:8649-8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, B. V., L. C. Shiung, and S. Y. Yuan. 2002. Anaerobic biodegradation of polycyclic aromatic hydrocarbon in soil. Chemosphere 48:717-724. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, N., D. J. Batstone, Z. He, I. Angelidaki, and J. E. Schmidt. 2004. Removal of polycyclic aromatic hydrocarbons (PAHs) from sewage sludge by anaerobic degradation. Water Sci. Technol. 50:237-244. [PubMed] [Google Scholar]
- 8.Coates, J. D., J. Woodward, J. Allen, P. Philp, and D. R. Lovley. 1997. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl. Environ. Microbiol. 63:3589-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates, J. D., R. Chakraborty, J. G. Lack, S. M. O'Connor, K. A. Cole, K. S. Bender, and L. A. Achenbach. 2001. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 411:1039-1043. [DOI] [PubMed] [Google Scholar]
- 10.Crozier, T. E., and S. Yamamoto. 1974. Solubility of hydrogen in water, seawater, and NaCl solutions. J. Chem. Eng. Data 19:242-244. [Google Scholar]
- 11.Deobald, L. A., and D. L. Crawford. 1987. Activities of cellulase and other extracellular enzymes during lignin solubilization by Streptomyces viridosporus. Appl. Environ. Microbiol. 26:158-163. [Google Scholar]
- 12.Dhillon, A., M. Lever, K. G. Lloyd, D. B. Albert, M. L. Sogin, and A. Teske. 2005. Methanogen diversity evidenced by molecular characterization of methyl coenzyme M reductase A (mcrA) genes in hydrothermal sediments of the Guaymas Basin. Appl. Environ. Microbiol. 71:4592-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Embley, T. M., and B. J. Finlay. 1993. Systematic and morphological diversity of endosymbiotic methanogens in anaerobic ciliates. Antonie van Leeuwenhoek 64:261-271. [DOI] [PubMed] [Google Scholar]
- 14.Fakoussa, R. M., and M. Hofrichter. 1999. Biotechnology and microbiology of coal degradation. Appl. Environ. Microbiol. 52:25-40. [DOI] [PubMed] [Google Scholar]
- 15.Haack, S. K., and J. A. Breznak. 1993. Cytophaga xylanolytica sp. nov., a xylan-degrading, anaerobic gliding bacterium. Arch. Microbiol. 159:6-15. [Google Scholar]
- 16.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 17.Hermann, M., K. M. Noll, and R. S. Wolfe. 1986. Improved agar bottle plate for isolation of methanogens or other anaerobes in a defined gas atmosphere. Appl. Environ. Microbiol. 51:1124-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuer, H., M. Elvert, S. Tille, M. Krummen, X. Prieto Mollar, L. R. Hmelo, and K.-U. Hinrichs. 2006. Online δ13C analysis of volatile fatty acids in sediment/porewater systems by liquid chromatography-isotope ratio mass spectrometry. Limnol. Oceanogr. Methods 4:346-357. [Google Scholar]
- 19.Hinrichs, K.-U., J. M. Hayes, W. Bach, A. J. Spivack, L. R. Hmelo, N. G. Holm, C. G. Johnson, and S. P. Sylva. 2006. Biological formation of ethane and propane in the deep marine subsurface. Proc. Natl. Acad. Sci. USA 103:14684-14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hostettler, F. D. 2004. Methanogenic biodegradation of n-alkanes and n-alkylated cyclohexanes and benzenes in the oil spill long-term study site at Bemidji, MN. GSA 2004 Denver Annual Meeting, abstr. no. 248-6. GSA Abstr. Program, Denver, CO, 7 to 10 November 2004.
- 21.Huang, L.-N., H. Zhou, Y.-Q. Chen, S. Luo, C.-Y. Lan, and L.-H. Qu. 2002. Diversity and structure of the archaeal community in the leachate of a full-scale recirculating landfill as examined by direct 16S rRNA gene sequence retrieval. FEMS Microbiol. Lett. 214:235-240. [DOI] [PubMed] [Google Scholar]
- 22.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon; a program to detect chimeric sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 23.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3B:117-132. [Google Scholar]
- 24.Jackson, B. E., and M. J. McInerney. 2002. Anaerobic microbial metabolism can proceed close to thermodynamic limits. Nature 415:454-456. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. W., E. H. Oelkers, and H. C. Helgeson. 1992. SUPCRT92: a software package for calculating the standard molal properties of minerals, gases, aqueous species and reactions among them from 1 to 5000 bars and 0 to 1000°C. Comput. Geosci. 18:899-947. [Google Scholar]
- 26.Jothimani, P., G. Kalaichelvan, A. Bhaskaran, D. A. Selvaseelan, and K. Ramasamy. 2003. Anaerobic biodegradation of aromatic compounds. Indian J. Exp. Biol. 41:1046-1067. [PubMed] [Google Scholar]
- 27.Koga, Y., M. Nishihara, H. Morii, and M. Akagawa-Matsushita. 1993. Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol. Rev. 57:164-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koga, Y., M. Morii, M. Akagawa-Matsushita, and M. Ohga. 1998. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens. Further analysis of lipid component parts. Biosci. Biotechnol. Biochem. 62:230-236. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 30.Mastalerz, M., A. Drobniak, J. Rupp, and N. Shaffer. 2004. Characterization of Indiana's coal resource: availability of the reserves, physical and chemical properties of the coal, and present potential uses. Indiana Geological Survey open-file study 04-02, July 2004. Indiana Geological Survey, Bloomington.
- 31.McIntosh, J. C., L. M. Walter, and A. M. Martini. 2002. Pleistocene recharge to midcontinent basins: effects on salinity structure and microbial gas generation. Geochim. Cosmochim. Acta 66:1681-1700. [Google Scholar]
- 32.Mechichi, T., M. Labat, B. K. C. Patel, T. H. S. Woo, P. Thomas, and J.-L. Garcia. 1999. Clostridium methoxybenzovorans sp. nov., a new aromatic o-demethylating homoacetogen from an olive mill wastewater treatment digester. Int. J. Syst. Bacteriol. 49:1201-1209. [DOI] [PubMed] [Google Scholar]
- 33.Meckenstock, R. U., C. Griebler, and M. Safinowski. 2004. Anaerobic degradation of polycyclic aromatic hydrocarbons. FEMS Microbiol. Ecol. 49:27-36. [DOI] [PubMed] [Google Scholar]
- 34.Milkov, A. V., and L. Dzou. 2007. Geochemical evidence of secondary microbial methane from very slight biodegradation of undersaturated oils in a deep hot reservoir. Geology 35:455-458. [Google Scholar]
- 35.Newberry, C. J., G. Webster, B. A. Cragg, R. J. Parkes, A. J. Weightman, and J. C. Fry. 2004. Diversity of prokaryotes and methanogenesis in deep subsurface sediments from the Nankai Trough, Ocean Drilling Program Leg 190. Environ. Microbiol. 6:274-287. [DOI] [PubMed] [Google Scholar]
- 36.Nüsslein, B., K.-J. Chin, W. Eckert, and R. Conrad. 2001. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3:460-470. [DOI] [PubMed] [Google Scholar]
- 37.Ollivier, B. M., R. A. Mah, J. L. Garcia, and R. Robinson. 1985. Isolation and characterization of Methanogenium aggregans sp. nov. Int. J. Syst. Bacteriol. 35:127-130. [Google Scholar]
- 38.Parkhurst, D. L., and C. A. J. Appelo. 1999. User's guide to PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inversegeochemical calculations. U.S. Geological Survey water resources investigations report 99-4259. U.S. Geological Survey, Denver, CO.
- 39.Paster, B. J., and E. Canale-Parola. 1982. Physiological diversity of rumen spirochetes. Appl. Environ. Microbiol. 43:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purdy, K. J., M. A. Munson, D. B. Nedwell, and T. M. Embley. 2002. Comparison of the molecular diversity of the methanogenic community at the brackish and marine ends of a UK estuary. FEMS Microbiol. Ecol. 39:17-21. [DOI] [PubMed] [Google Scholar]
- 41.Rahman, K. S. M., J. Thahira-Rahman, P. Lakshmanaperumalsamy, and I. M. Banat. 2002. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 85:257-261. [DOI] [PubMed] [Google Scholar]
- 42.Rogosa, M. 1969. Acidaminococcus gen. n., Acidaminococcus fermentans sp. n., anaerobic gram-negative diplococci using amino acids as the sole energy source for growth. J. Bacteriol. 98:756-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saroj, K. K., A. Gupta, and S. C. Srivastava. 2001. Bacterial gasification as an alternative to methane drainage and subsequent extraction of coal, p. 105-109. Proceedings of the 6th U.S. Mine Ventilation Symposium, 17 to 22 May 1997, Pittsburgh, PA. Society for Mining, Metallurgy, and Exploration, Inc., Littleton, CO.
- 44.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schink, B., and A. J. M. Stams. 2006. Syntrophy among prokaryotes, p. 309-335. In A. Balows, H. G. Trüper, M. Dworkin, and K.-H. Schleifer (ed.), The prokaryotes, 3rd ed. Springer, New York, NY.
- 46.Schnürer, A., G. Zellner, and B. H. Svensson. 1999. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol. Ecol. 29:249-261. [Google Scholar]
- 47.Scott, C. D., C. A. Woodward, and T. C. Scott. 1994. Use of chemically modified enzymes in organic solvents for conversion of coal to liquids. Catal. Today 19:381-394. [Google Scholar]
- 48.Shock, E. L., and H. C. Helgeson. 1990. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: standard partial molal properties of organic species. Geochim. Cosmochim. Acta 54:915-945. [Google Scholar]
- 49.Shoreit, A. M., and M. S. A. Shabeb. 1994. Utilization of aromatic compounds by phototrophic purple non sulfur bacteria. Earth Environ. Sci. 5:71-76. [Google Scholar]
- 50.Shumkov, S., S. Terekhova, and K. Laurinavichius. 1999. Effect of enclosing rocks and aeration on methanogenesis from coals. Appl. Environ. Microbiol. 52:99-103. [Google Scholar]
- 51.Simankova, M. V., O. R. Kotsyurbenko, T. Lueders, A. N. Nozhevnikova, B. Wagner, R. Conrad, and M. W. Friedrich. 2003. Isolation and characterization of new strains of methanogens from cold terrestrial habitats. Syst. Appl. Microbiol. 26:312-318. [DOI] [PubMed] [Google Scholar]
- 52.Solano-Acosta, W., M. Mastalerz, and A. Schimmelmann. 2007. Cleats and their relation to geologic lineaments and coalbed methane potential in Pennsylvanian coals in Indiana. Int. J. Coal Geol. 72:187-208.
- 53.Strąpoć, D., M. Mastalerz, C. Eble, and A. Schimmelmann. 2007. Characterization of the origin of coalbed gases in southeastern Illinois Basin by compound-specific carbon and hydrogen stable isotope ratios. Org. Geochem. 38:267-287. [Google Scholar]
- 54.Stueber, A. M., L. M. Walter, T. J. Huston, and P. Pushkar. 1993. Formation waters from Mississippian-Pennsylvanian reservoirs, Illinois Basin, USA: chemical and isotopic constraints on evolution and migration. Geochim. Cosmochim. Acta 57:763-784. [Google Scholar]
- 55.Sturt, H. F., R. E. Summons, K. J. Smith, M. Elvert, and K.-U. Hinrichs. 2004. Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Commun. Mass Spectrom. 18:617-628. [DOI] [PubMed] [Google Scholar]
- 56.Thielemann, T., B. Cramer, and A. Schippers. 2004. Coalbed methane in the Ruhr Basin, Germany: a renewable energy source? Org. Geochem. 35:1537-1549. [Google Scholar]
- 57.Townsend, G. T., R. C. Prince, and J. M. Suflita. 2004. Anaerobic biodegradation of alicyclic constituents of gasoline and natural gas condensate by bacteria from an anoxic aquifer. FEMS Microbiol. Ecol. 49:129-135. [DOI] [PubMed] [Google Scholar]
- 58.van Bruggen, J. J. A., C. K. Stumm, and G. D. Vogels. 1983. Symbiosis of methanogenic bacteria and sapropelic protozoa. Arch. Microbiol. 136:89-95. [Google Scholar]
- 59.Waldron, P. J., S. T. Petsch, A. M. Martini, and K. Nüsslein. 2007. Salinity constraints on subsurface archaeal diversity and methanogenesis in sedimentary rock rich in organic matter. Appl. Environ. Microbiol. 73:4171-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White, D. C., and D. B. Ringelberg. 1998. Signature lipid biomarker analysis, p. 255-272. In R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (ed.), Techniques in microbial ecology. Oxford University Press, New York, NY.
- 61.Whitehead, T. R., and M. A. Cotta. 1999. Phylogenetic diversity of methanogenic archaea in swine waste storage pits. FEMS Microbiol. Lett. 179:223-226. [DOI] [PubMed] [Google Scholar]
- 62.Widdel, F., and R. Rabus. 2001. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr. Opin. Biotechnol. 12:259-276. [DOI] [PubMed] [Google Scholar]
- 63.Zellner, G., C. Alten, E. Stackebrandt, E. Conway de Macario, and J. Winter. 1987. Isolation and characterization of Methanocorpusculum parvum, gen. nov., spec. nov., a new tungsten requiring, coccoid methanogen. Arch. Microbiol. 147:13-20. [Google Scholar]
- 64.Zengler, K., H. H. Richnow, R. Rosselló-Mora, W. Michaelis, and F. Widdel. 1999. Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 401:266-269. [DOI] [PubMed] [Google Scholar]