Abstract
Agrobacterium-mediated gene transfer is widely used for plant molecular genetics, and efficient techniques are required. Recent studies show that ethylene inhibits the gene transfer. To suppress ethylene evolution, we introduced 1-aminocyclopropane-1-carboxylate (ACC) deaminase into Agrobacterium tumefaciens. The ACC deaminase enhanced A. tumefaciens-mediated gene transfer into plants.
Agrobacterium-mediated gene transfer is widely used for plant molecular genetics and its applications (14). In particular, efficient systems of genetic transformation are required for plant functional genomics and molecular breeding to improve traits (20, 21). Recent studies showed that ethylene is one of the factors that inhibits Agrobacterium-mediated gene transfer (1, 3, 5). Therefore, if Agrobacterium tumefaciens has the ability to decrease the ethylene level in the host plant, the frequency of gene transfer will increase. To suppress ethylene evolution in plant cells during cocultivation, we introduced the 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene from Pseudomonas sp. strain ACP (7, 18) into A. tumefaciens. ACC deaminase cleaves ACC (the immediate ethylene precursor) into α-ketobutyrate and ammonia, and as a result, the ethylene level is decreased (4, 12, 16).
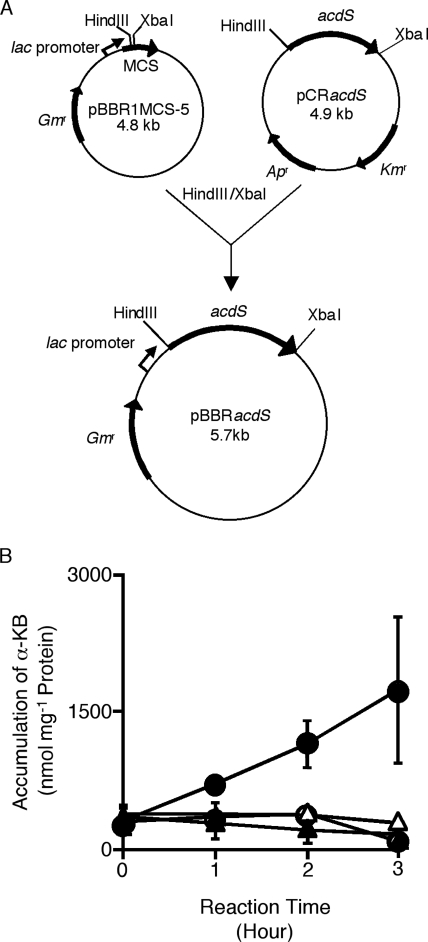
The ACC deaminase gene was amplified and cloned into pBBR1MCS-5 (10), a broad-host-range plasmid, to generate a lacZ::acdS translational fusion (Fig. 1A). The resulting plasmid was designated pBBRacdS and was introduced into A. tumefaciens C58 (17) or C58C1RifR (2) by electroporation (19). The binary vector pIG121-Hm, involved in T-DNA transfer (6), was also harbored in A. tumefaciens C58C1RifR(pBBRacdS, pIG121-Hm). The ACC deaminase activity in A. tumefaciens C58C1RifR (pBBRacdS, pIG121-Hm) was assayed according to the method of Honma and Shimomura (7). The amount of α-ketobutyrate in the reaction buffer was estimated from a standard curve based on a dilution of 10 to 400 μM (detected at 340 nm). The controls for this experiment were C58C1RifR(pBBRMCS-5, pIG121-Hm) and samples in reaction buffer without the substrate 200 μM ACC. The accumulation of α-ketobutyrate was observed only in the lysate from A. tumefaciens C58C1RifR(pBBRacdS, pIG121-Hm) in the presence of the substrate (Fig. 1B). Therefore, we succeeded in conferring ACC deaminase activity on A. tumefaciens.
FIG. 1.
Construction of the ACC deaminase-producing A. tumefaciens strain. (A) Plasmid construction for expression of ACC deaminase in A. tumefaciens. An HindIII and XbaI fragment (1 kb) containing the ACC deaminase gene from Pseudomonas sp. strain ACP was ligated into the HindIII and XbaI sites of the broad-host-range plasmid pBBR1MCS-5, resulting in pBBRacdS. The expression of the ACC deaminase gene acdS was under the control of the lac promoter. MCS stands for multiple cloning sites. (B) Detection of ACC deaminase activity in A. tumefaciens. The α-ketobutirate (α-KB) accumulation in the reaction buffer was measured according to the method of Honma and Shimomura (7). The triangles and circles indicate the lysates from A. tumefaciens C58C1RifR(pBBR1MCS-5, pIG121-Hm) and C58C1RifR(pBBRacdS, pIG121-Hm), respectively. Closed and open symbols represent samples with and without ACC in the reaction buffer, respectively. Bars indicate standard deviations (n = 3).
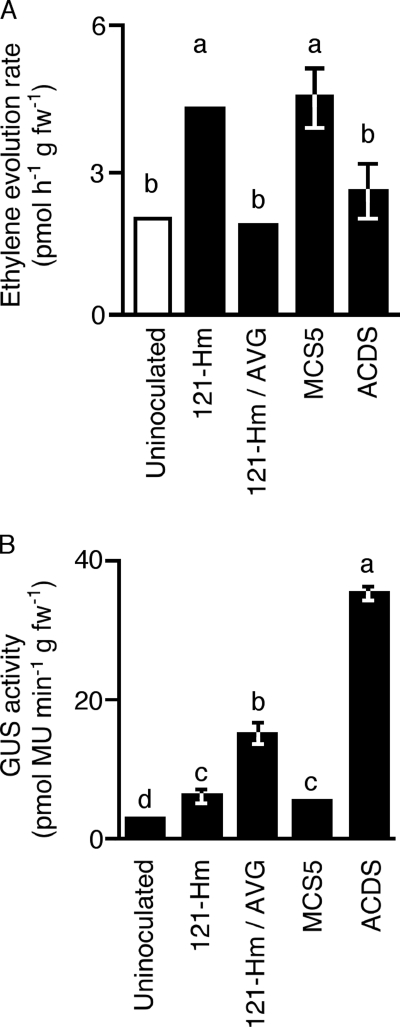
Surface-sterilized melon (Cucumis melo L. var. cantaloupensis cv. Vedrantais) seeds were sown on a half-strength preparation of Murashige and Skoog's medium (MS) (13) and incubated at 25°C with 16 h of light per day for 5 days. Cotyledons from the germinated seedlings were transversely sectioned by hand into five pieces, and among these five, three internal pieces were inoculated. The segments were soaked in an A. tumefaciens cell suspension of 107 cells ml−1 for 20 min and then placed on cocultivation medium (MS containing 1.0 mg of 6-benzylamino-purine liter−1, 2% glucose, and 0.4% Gelrite [Wako, Tokyo, Japan], pH 5.5) in a gas vial with 16 h of light per day. Thirty melon cotyledon segments were inoculated with A. tumefaciens C58C1RifR (pBBRacdS, pIG121-Hm) per experiment. The experiments were repeated three times. After 24 h of incubation, ethylene evolution in melon cotyledon segments was measured by gas chromatography (Fig. 2A). Compared to that in the uninoculated controls, ethylene evolution in the melon segments inoculated with A. tumefaciens C58C1RifR(pIG121-Hm) and C58C1RifR(pBBRMCS-5, pIG121-Hm) was enhanced. The application of 1 μM aminoethoxyvinylglycine (AVG), an ethylene biosynthesis inhibitor, reduced ethylene evolution in the inoculated segments. Inoculation with A. tumefaciens C58C1RifR(pBBRacdS, pIG121-Hm) suppressed ethylene evolution in melon cotyledon segments, and the ethylene accumulation rate was the same as that in the control and AVG-treated samples. These results indicated that A. tumefaciens with ACC deaminase activity reduced ethylene evolution in plants (Fig. 2A).
FIG. 2.
Effect of ACC deaminase activity on ethylene evolution and gene transfer. (A) Measurement of ethylene evolution. The accumulation of ethylene in the headspace was measured on a gas chromatograph. 121-Hm, 121-Hm/AVG, MCS-5, and ACDS indicate samples inoculated with A. tumefaciens C58C1RifR(pIG121-Hm), those inoculated with A. tumefaciens C58C1RifR(pIG121-Hm) and treated with the addition of 1 μM AVG to the cocultivation medium, and samples inoculated with C58C1RifR(pBBR1MCS-5, pIG121-Hm) and C58C1RifR(pBBRacdS, pIG121-Hm), respectively. Bars represent standard deviations (n = 3). The characters a and b show statistically significant differences (t test; P < 0.05). fw, fresh weight. (B) Quantification of gene transfer by GUS assay. Melon cotyledon segments were cocultivated with three different A. tumefaciens strains for 3 days. 121-Hm, 121-Hm/AVG, MCS-5, and ACDS indicate samples inoculated with A. tumefaciens C58C1RifR (pIG121-Hm), those inoculated with A. tumefaciens C58C1RifR(pIG121-Hm) and treated with the addition of 1 μM AVG to the cocultivation medium, and samples inoculated with C58C1RifR(pBBR1MCS-5, pIG121-Hm) and C58C1RifR(pBBRacdS, pIG121-Hm), respectively. Bars represent standard deviations (n = 3). Different letters indicate statistically significant differences (t test; P < 0.05).
Three days after inoculation, the level of gene transfer was estimated (Fig. 2B). The pIG121-Hm plasmid has a reporter gene (35S-uidA intron) in the T-DNA region. Because the uidA reporter gene possesses an intron sequence, it can produce active protein only in plant cells, thereby making it a marker for gene transfer (15). Gene transfer was evaluated using a fluorometric β-glucuronidase (GUS) assay according to the method of Jefferson et al. (8). Melon segments inoculated with C58C1RifR(pIG121-Hm) and C58C1RifR(pBBR1MCS-5, pIG121-Hm) showed higher levels of GUS activity than controls. These higher levels of GUS activity indicated that the gene was transferred. The addition of AVG (1 μM) increased GUS activity two times over that in the absence of AVG. Inoculation with A. tumefaciens C58C1RifR(pBBRacdS, pIG121-Hm) yielded approximately six-times-higher levels of GUS activity than inoculation with C58C1RifR(pIG121-Hm). Thus, ACC deaminase enhanced the ability for gene transfer from A. tumefaciens (Fig. 2B).
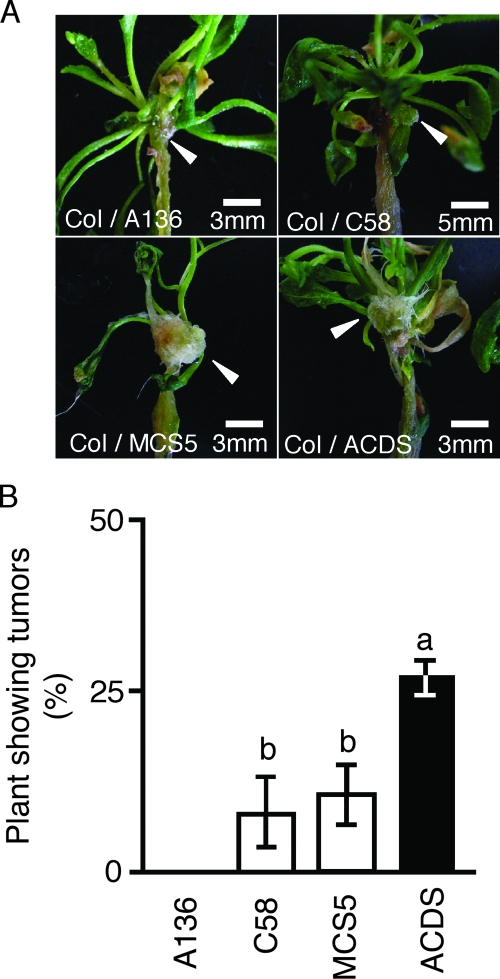
Seeds of Arabidopsis thaliana (Columbia) were sterilized and grown at 22°C for 7 days with 16 h of light per day after 4 days of vernalization. Intact A. thaliana plants were dipped into a suspension of A. tumefaciens C58 or A136 (107 cells ml−1). A136 lacks the Ti plasmid and the T-DNA region and was used as a control. The inoculated seedlings were blotted onto sterile filter paper to remove excess suspension material and cocultivated on MS for 7 days with 16 h of light per day. After cocultivation, to eliminate the bacteria, the plants were washed in sterilized water and then incubated on MS containing 375 mg of amoxicillin-clavulanic acid (Augmentin) liter−1 for 3 weeks. Four weeks after inoculation with C58, C58(pBBR1MCS-5), and C58(pBBRacdS), green tumors had formed on the stems (Fig. 3A). The sizes of tumors among the different infections were almost the same (Fig. 3A). There were no tumors observed on plants inoculated with A136 (Fig. 3). This result indicated that the tumor formation was induced by stable transformation (22). To estimate the genetic transformation efficiency, the numbers of A. thaliana plants forming green tumors were determined and the percentages were calculated. Fifteen intact A. thaliana seedlings were used in each experiment, and there were three independent repetitions. The percentages of plants that formed tumors were 8.1% ± 2.3%, 10.6% ± 4.1%, and 27.2% ± 2.4%, respectively, of those inoculated with A. tumefaciens C58, C58(pBBR1MCS-5), and C58(pBBRacdS) (Fig. 3B). The tumor incidence was higher among plants inoculated with the ACC deaminase-producing strain. This result indicated that ACC deaminase activity increased the ability for stable transformation with A. tumefaciens (Fig. 3B).
FIG. 3.
Estimation of the frequency of genetic transformation by acdS-expressing A. tumefaciens. (A) Photographs of tumor formation on A. thaliana Columbia (Col) stems. (B) Frequency of genetic transformation. A136, C58, MCS5, and ACDS indicate samples inoculated with A. tumefaciens strains A136, C58, C58(pBBRMCS-5), and C58(pBBRacdS), respectively. Bars indicate standard deviations (n = 3). The characters a and b represent statistically significant differences based on chi-square testing (P < 0.05).
Genetic transformation is a key technology for plant molecular breeding. Among several techniques of genetic transformation, Agrobacterium-mediated gene transfer is the most frequently used. Although great efforts have been made to establish efficient protocols of genetic transformation for plants of interest, species and genotypes recalcitrant to genetic transformation, such as cotton (11) and soybeans (9), still exist. We succeeded in producing an Agrobacterium strain with improved potential for gene transfer by providing the ability to reduce the ethylene level of the plant during cocultivation. The knowledge obtained in this study will provide a clue to overcome such problems in plant molecular breeding as producing transgenic plants of recalcitrant species and genotypes.
Acknowledgments
We thank the members of the Ezura laboratory for helpful discussions. We are grateful to Shohael Abdullah for his advice on the English in this article.
This work was supported by the 21st Century Centers of Excellence Program and a grant-in-aid for scientific research, category B (no. 15380002), from the Ministry of Education, Science, Sports, and Technology of Japan to H.E.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Davis, M. E., A. R. Miller, and R. D. Lineberger. 1992. Studies on the effects of ethylene on transformation of tomato cotyledons (Lycopersicon esculentum Mill.) by Agrobacterium tumefaciens. J. Plant Physiol. 139:309-312. [Google Scholar]
- 2.Deblaere, R., B. Bytebier, H. De Greve, F. Deboeck, J. Schell, M. van Montagu, and J. Leemans. 1985. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 13:4777-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezura, H., K. Yuhashi, T. Yasuta, and K. Minamisawa. 2000. Effect of ethylene on Agrobacterium tumefaciens-mediated gene transfer to melon. Plant Breed. 119:75-79. [Google Scholar]
- 4.Glick, B. R., D. M. Penrose, and J. Li. 1998. A model of the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 190: 63-68. [DOI] [PubMed] [Google Scholar]
- 5.Han, J. S., C. K. Kim, S. H. Park, K. D. Hirschi, and I. G. Mok. 2005. Agrobacterium-mediated transformation of bottle gourd (Lagenaria siceraria Standl.). Plant Cell Rep. 23:692-698. [DOI] [PubMed] [Google Scholar]
- 6.Hiei, Y., S. Ohta, T. Komari, and T. Kumashiro. 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6:271-282. [DOI] [PubMed] [Google Scholar]
- 7.Honma, S., and T. Shimomura. 1978. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 42:1825-1831. [Google Scholar]
- 8.Jefferson, R. K., T. A. Kavanagh, and M. W. Bevan. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko, T. S., S. S. Korban, and D. A. Somers. 2006. Soybean (Glycine max) transformation using immature cotyledon explants. Methods Mol. Biol. 343:397-405. [DOI] [PubMed] [Google Scholar]
- 10.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 11.Leelavathi, S., V. G. Sunnichan, R. Kumria, G. P. Vijaykanth, R. K. Bhatnagar, and V. S. Reddy. 2004. A simple and rapid Agrobacterium-mediated transformation protocol for cotton (Gossypium hirsutum L.): embryogenic calli as a source to generate large numbers of transgenic plants. Plant Cell Rep. 22:465-470. [DOI] [PubMed] [Google Scholar]
- 12.Madhaiyan, M., S. Poonguzhali, J. Ryu, and T. Sa. 2006. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta 224:268-278. [DOI] [PubMed] [Google Scholar]
- 13.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15:473-497. [Google Scholar]
- 14.Newell, C. A. 2000. Plant transformation technology. Developments and applications. Mol. Biotechnol. 16:53-65. [DOI] [PubMed] [Google Scholar]
- 15.Ohta, S., S. Mira, T. Hattori, and K. Nakamura. 1990. Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol. 31:805-813. [Google Scholar]
- 16.Penrose, D. M., and B. R. Glick. 2001. Levels of 1-aminocyclopropane-1-carboxylic acid (ACC) in exudates and extracts of canola seeds treated with plant growth-promoting bacteria. Can. J. Microbiol. 47:368-372. [DOI] [PubMed] [Google Scholar]
- 17.Sciaky, D., A. L. Montoya, and M. D. Chilton. 1978. Fingerprints of Agrobacterium Ti plasmids. Plasmid 1:238-253. [DOI] [PubMed] [Google Scholar]
- 18.Sheehy, R. E., M. Honma, M. Yamada, T. Sasaki, B. Martineau, and W. R. Hiatt. 1991. Isolation, sequence, and expression in Escherichia coli of the Pseudomonas sp. strain ACP gene encoding 1-aminocyclopropane-1-carboxylate deaminase. J. Bacteriol. 173:5260-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen, W. J., and B. G. Forde. 1989. Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res. 17:8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun, H. J., S. Uchii, S. Watanabe, and H. Ezura. 2006. A highly efficient transformation protocol for Micro-Tom, a model cultivar of tomato functional genomics. Plant Cell Physiol. 47:426-431. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka, Y., Y. Katsumoto, F. Brugliera, and J. Mason. 2005. Genetic engineering in floriculture. Plant Cell Tissue Organ Cult. 80:1-24. [Google Scholar]
- 22.Yuan, Z. C., M. P. Edlind, P. Liu, P. Saenkham, L. M Banta, A. A. Wise, E. Ronzone, A. N. Binns, K. Kerr, and E. W. Nester. 2007. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc. Natl. Acad. Sci. USA 104:1179-11795. [DOI] [PMC free article] [PubMed] [Google Scholar]