Abstract
Outbreaks of Escherichia coli O157:H7 infections have been linked increasingly to leafy greens, particularly to lettuce. We present here the first evidence that this enteric pathogen can multiply on the leaves of romaine lettuce plants. The increases in population size of E. coli O157:H7 in the phyllosphere of young lettuce plants ranged from 16- to 100-fold under conditions of warm temperature and the presence of free water on the leaves and varied significantly with leaf age. The population size was consistently ca. 10-fold higher on the young (inner) leaves than on the middle leaves. The growth rates of Salmonella enterica and of the natural bacterial microflora were similarly leaf age dependent. Both enteric pathogens also achieved higher population sizes on young leaves than on middle leaves harvested from mature lettuce heads, suggesting that leaf age affects preharvest as well as postharvest colonization. Elemental analysis of the exudates collected from the surfaces of leaves of different ages revealed that young-leaf exudates were 2.9 and 1.5 times richer in total nitrogen and carbon, respectively, than middle-leaf exudates. This trend mirrored the nitrogen and carbon content of the leaf tissue. Application of ammonium nitrate, but not glucose, to middle leaves enhanced the growth of E. coli O157:H7 significantly, suggesting that low nitrogen limits its growth on these leaves. Our results indicate that leaf age and nitrogen content contribute to shaping the bacterial communities of preharvest and postharvest lettuce and that young lettuce leaves may be associated with a greater risk of contamination with E. coli O157:H7.
Lettuce (Lactuca sativa) was among the fresh produce items most commonly implicated in epidemics of food-borne illness in the United States between 1973 and 1997 (36). Escherichia coli O157:H7 is the most common bacterial etiologic agent of outbreaks associated with this commodity and other leafy greens (2, 34, 36). According to the U.S. Centers for Disease Control and Prevention, 20 outbreaks and 634 cases of illness from E. coli O157:H7 were attributed to lettuce alone during 1998 to 2005 (25). However, lettuce has been linked also to several outbreaks of salmonellosis in the United States (36), Australia (38), Finland (40), and England (see reference 1 and references therein).
Ercolani (11) demonstrated that E. coli and Salmonella enterica serovar Typhimurium survived on lettuce in the field throughout the growing season until harvest. More recent studies by Islam and coworkers (17, 18) provided evidence that E. coli O157:H7 and Salmonella serovar Typhimurium can persist on lettuce and parsley plants in the field from the time of inoculation onto young seedlings with contaminated manure or irrigation water until several days after they normally would be harvested. It remains unclear whether the long-term persistence of enteric pathogens in the studies mentioned above resulted solely from the survival of a low percentage of the inoculum cells or from the outcome of growth and death events in the pathogen cell population on these plants. Previous greenhouse and plant growth chamber studies have provided evidence for the ability of E. coli K-12 and S. enterica to multiply on the leaves of beans and corn (33) and cilantro (3) plants. However, despite growth-conducive conditions of warm temperature and high water availability in the cilantro phyllosphere, S. enterica achieved lower population sizes on the leaf surface than bacterial leaf colonists, thus indicating that the human pathogen had a comparatively reduced fitness in the leaf habitat (3). The nature of the bacterial and plant factors that dictate the fitness of enteric pathogens on leafy crops in the preharvest environment remains largely unexplored.
Leaf surfaces are overall poor in substrates for bacterial cells (22, 24, 27, 41) compared to the nutrient-rich intestines of animals that enteric pathogens colonize. However, studies performed with whole-cell bacterial biosensors for sucrose and fructose revealed heterogeneous distribution of these sugars on leaf surfaces, with few microsites harboring abundant quantities of them (8, 22, 28). In addition, rapid changes in water availability on the leaf surfaces of crops affect the solubility of nutrients that may be used by the plant microflora (15). Periods of dryness on the phylloplane are interrupted by rainfall, dew formation, or crop irrigation, which may benefit bacterial leaf inhabitants by increasing water availability and solubilization of substrates. The distribution of water on the leaf surface upon wetting events also is not homogenous. For example, the enhanced wettability of the leaf veins has been proposed as one of the factors that enable the increased colonization of this area by epiphytic bacteria (21), as well as by S. enterica (3). Thus, the heterogeneous distribution of physicochemical factors on a given leaf and between leaves of the same plant may provide microsites that are hospitable to bacteria, including enteric pathogens such as S. enterica and E. coli O157:H7.
Although several studies have reported on the behavior of E. coli O157:H7 on cut or shredded lettuce leaves (9, 23, 26, 37), the potential of this pathogen to colonize intact lettuce leaves in the pre- and postharvest environments remains largely unknown. The ability of enteric pathogens to multiply on the surface of leafy greens may be a critical factor in the epidemiology of zoonotic diseases linked to this commodity. The main objective of this study was to investigate the growth of E. coli O157:H7 on lettuce leaves in a pre- and postharvest model. Bacterial population dynamics and microscopy were used to study the behavior of E. coli O157:H7 in the complex canopy of young romaine lettuce plants and on harvested leaves. Additionally, elemental analysis of leaf exudates and complementation of bacterial growth with specific nutrients were carried out to provide evidence that leaf age and nitrogen abundance affect the fitness of E. coli O157:H7 in the lettuce phyllosphere.
MATERIALS AND METHODS
Strains and growth conditions.
A spontaneous rifampin-resistant mutant of E. coli O157:H7 strain H1827, a clinical isolate linked to an outbreak of E. coli O157:H7 infections associated with lettuce in Connecticut and Illinois in 1996 (14) and a gift from T. Barrett (U.S. Centers for Disease Control and Prevention), was used in this study. The spontaneous rifampin mutant of E. coli O157:H7 H1827, named herein H1827R, was isolated from Luria-Bertani agar plates containing 100 μg rifampin per ml. The plate had been streaked with the parental strain and incubated at 37°C. For microscopy, E. coli O157:H7 H1827 was transformed with plasmid pGT-KAN, which harbors the gene encoding the green fluorescent protein (GFP) expressed from the kanamycin resistance gene promoter and which was described previously (4). This plasmid was stably maintained in E. coli O157:H7 H1827 and conferred it intrinsic green fluorescence. This GFP-labeled strain is referred to as H1827R pGT-KAN. Also, a spontaneous nalidixic acid-resistant mutant of S. enterica serovar Thompson strain RM1987, designated RM1987N, was used in comparative plant studies with E. coli O157:H7. Strain RM1987 is a clinical isolate from a patient in a cilantro-linked outbreak in California and was described previously (4).
For plant inoculations, all strains were cultured to the early stationary phase of growth on a rotary shaker at 28°C in Luria-Bertani broth amended with rifampin (100 μg/ml) or nalidixic acid (50 μg/ml). Gentamicin (15 μg/ml) was used also for the growth of E. coli O157:H7 H1827R pGT-KAN. For in vitro studies with leaf homogenates or exudates, E. coli O157:H7 strain H1827R inoculum was cultured in minimal M9 with 0.2% glucose and rifampin.
Plant growth conditions.
Romaine lettuce plants (Lactuca sativa cv. Parris Island) were used throughout these studies. The plants were grown to the 10th to 12th leaf stage (Fig. 1) in a Percival plant growth chamber (Percival Scientific, Perry, IA) with a 14-h photoperiod and day and night temperatures of 22°C and 16°C, respectively, before being used in preharvest experiments. Alternatively, the plants were grown to mature heads in a greenhouse with a 16-h photoperiod and day and night temperatures of 24°C and 18°C, respectively, before the leaves were harvested for postharvest experiments (Fig. 1). All plants were grown in Sunshine mix 1 (Sun Gro Horticulture Distribution, Inc., Bellevue, WA) and were fertilized weekly, starting at 2 weeks after emergence, with 1 mg NKP 20:20:20 (Spectrum Brands, Inc., Atlanta, GA) per plant.
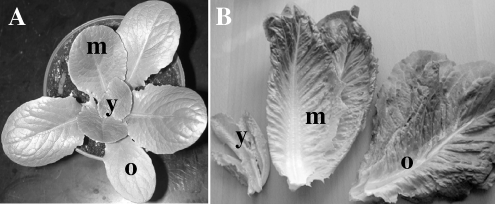
FIG. 1.
Images of (A) a young romaine lettuce plant grown in a growth chamber and (B) romaine lettuce leaves harvested from plants grown in a greenhouse. The plant material is representative of that used in the preharvest (A) and postharvest (B) studies described herein. The leaves were categorized into three age groups: young (y), middle (m), and old (o).
Plant inoculations—preharvest studies.
Cells of E. coli O157:H7 H1827R or S. enterica RM1987N that were cultured as described above were washed twice by centrifugation in 10 mM potassium phosphate buffer, pH 7 (PPB-10 mM), and resuspended in 0.5 mM potassium phosphate buffer, pH 7 (PPB-0.5 mM), at 1 × 105 cells/ml. Potted lettuce plants at ca. the 10th to 12th leaf stage were inoculated with one of the pathogens by inverting the pot and immersing the aerial part of the plant in the bacterial suspension for 3 s. Care was taken to prevent contamination of the inoculum suspension with soil particles from the pot by wrapping the top of the pot around the lettuce plants with plastic film. Following inoculation, the plants were kept inverted for a short time to drain the excess inoculum from their surface and prevent its accumulation at the bottom of the inner rosette. The inoculum concentration resulted in approximately 104 cells of the pathogen per gram of leaf tissue. The plants were then placed at 28°C for 3 days in a randomized design in a chamber that allowed for the presence of free water on the leaf surface. High humidity levels (100% relative humidity) were maintained in the chamber by placing water-saturated paper at its bottom; this high relative humidity combined with minimal airflow in the chamber promoted the formation of dew on the lettuce plants.
For microscopy, lettuce plants were grown to the sixth to eighth leaf stage and inoculated with a suspension of 105 cells of E. coli O157:H7 H1827R pGT-KAN per milliliter. The inoculation and subsequent plant incubation were performed as described above.
Leaf inoculations—postharvest studies.
Leaves of romaine lettuce plants grown to full head in the greenhouse or leaves of field-grown romaine lettuce heads obtained from a distributor were harvested and inoculated in a suspension of the pathogen prepared as described for the preharvest studies and adjusted to a final concentration of 104 cells/ml. For nitrogen and carbon amendment studies on leaves, ammonium nitrate or glucose at a final concentration of 6 mM or 0.1%, respectively, was added to the PPB-0.5 mM used to prepare the inoculum suspension. The cells were suspended at 104 cells per milliliter, and the suspension was inoculated onto the leaves immediately after preparation. For these studies, ammonium nitrate was used instead of ammonium chloride in order to avoid a potential inhibition of bacterial growth caused by chloride on the leaf surface.
Each leaf was inoculated individually by holding it at its base and immersing it for 3 s in the bacterial suspension up to 4 cm from the base to prevent inoculum from penetrating the vascular tissue at the cut end of the leaf. The excess inoculum suspension was drained briefly from the inverted leaves, and each leaf was placed inverted in a plastic bag. The open bags were placed at 28°C for 3 days in a randomized design in a humidity chamber that allowed for the presence of free water on the leaf surface, as described for the preharvest studies.
Measurement of bacterial populations on leaves.
For most experiments, lettuce leaves were sampled from three different age groups. For inoculated potted plants (ca. 10th to 12th leaf stage), old, middle, and young leaves represented, in order of emergence, the first and second leaves, the fifth and sixth leaves, and the inner rosette consisting of all leaves smaller than 4 cm, respectively. For leaves that were harvested from mature lettuce heads grown in the greenhouse, old, middle, and young leaves represented, respectively, the sixth to ninth leaves, the 11th to 15th leaves (not part of the tightly closed head), and the young inner leaves that were 5 to 12 cm long and were enclosed in more-mature leaves. For these experiments, the middle and old leaf samples each consisted of one leaf per replicate sample, whereas three young leaves from the same inner rosette were pooled into one replicate sample.
At each sampling time, each leaf sample from young lettuce plants or from mature heads was placed in a bag in 20 or 100 ml PPB-10 mM, respectively. The leaf sample was sonicated in the buffer in an Astramax Generator sonicator bath (Misonix, Inc., Farmingdale, NY) at 250 W for 1 min and then rubbed vigorously by hand for 1 min on each side to further remove the bacterial cells from the leaves. Rubbing was as efficient in bacterial removal as the use of a stomacher at high power for 1 min, while allowing for the use of smaller buffer volumes and therefore for the detection of smaller bacterial populations. The resulting suspension was shaken to homogenization and plated with an automated plater (Autoplate 4000; Spiral Biotech, Inc., Norwood, MA). Suspensions from leaves inoculated with S. enterica RM1987N and E. coli O157:H7 H1827R were plated onto Luria-Bertani agar containing nalidixic acid and rifampin, respectively. The total culturable aerobic bacterial flora on the leaves was estimated by plating the leaf washing suspensions onto 10% tryptic soy agar. The population size of the indigenous culturable bacteria was calculated by subtraction of the enteric pathogen count on selective plates from that of total counts on tryptic soy agar.
Culture of the pathogens in leaf homogenates.
Homogenates were prepared from leaves sampled from mature heads of lettuce plants grown in the greenhouse. Young, middle, and old leaves, as defined above, were homogenized with a Sorvall Omni-mixer separately on ice at 42 g (wet weight) leaf tissue per 100 ml double-distilled water. The homogenates were passed through a fine-mesh sieve, and the filtrate was diluted 1.25-fold in 1× final modified M9 minimal salts (42 mM Na2HPO4, 24 mM KH2PO4, 9 mM NaCl, and 1 mM MgSO4) alone or containing a final concentration of 19 mM ammonium chloride, 19 mM potassium nitrate, or 0.5% glucose. All homogenate cultures were amended with rifampin to select for growth of E. coli O157:H7 H1827R. Overnight cultures of this pathogen in standard M9-glucose minimal medium were centrifuged, and the cells were washed twice in PPB-10 mM, resuspended in this buffer, and then added to the homogenate solution at 106 cells/ml. Each type of homogenate was tested as three replicate cultures of 5 ml each. The cultures were incubated at 28°C and 250 rpm, and the bacterial concentration was measured after 16 and 22 h of incubation. Homogenates amended with potassium nitrate were incubated as stationary cultures to minimize their oxygen content.
Recovery of leaf exudates, C and N quantification, and in vitro culture tests.
Mature plants from the greenhouse were sprayed lightly with double-distilled water and placed in a humidity chamber (described above) at 4°C overnight in order to promote solubilization of exudates on the surface of the leaves as well as guttation. Young, middle, and old leaves (as defined above) were sampled, both sides of each leaf were sprayed with double-distilled water with an air brush, and the runoff water was collected in a large petri dish. During this process, the leaf was held upside down by the stem over the petri dish and water was prevented from touching the open stem area. About 100 ml aqueous leaf exudate solution was collected in total from 10 to 20 leaves (depending on the age of the leaves) for each age group. The exudate solutions were concentrated in a freeze dryer (Freezemobile 12XL; VirTis, Gardiner, NY), transferred to tin cups, and dried to completion before analysis for total N and C content on a Carlo-Erba elemental analyzer (CE Instruments 1500 elemental analyzer).
To test leaf exudates for their ability to support growth of E. coli O157:H7 H1827R in vitro, the dried exudates from 13 g (wet weight) leaf tissue were mixed with 2 ml PPB-10 mM, supplemented or not with either glucose or ammonium chloride at a final concentration of 0.2% or 10 mM, respectively. E. coli O157:H7 H1827R was cultured in M9 minimal medium as described above, and the cells were washed twice in PPB-10 mM before being resuspended in the same buffer. Two replicate samples of 1 ml filter-sterilized exudate solution were inoculated to a final concentration of 104 cells/ml. The cultures were incubated at 40 rpm and 28°C and sampled at various times after inoculation for measurement of bacterial concentration by plating onto Luria-Bertani agar containing rifampin.
Microscopy.
Five leaves of each age group were sampled from replicate lettuce plants 4 days after inoculation. The localization of E. coli O157:H7 H1827R pGT-KAN was examined on the adaxial surface of four or six 1-cm discs that were sampled from a young or middle leaf, respectively, and were then mounted in AquaPoly/mount (Polysciences, Warrington, PA). Approximately 20 fields of view were observed for each leaf disc. The GFP signal from the bacteria and the red autofluorescence of the plant cells were visualized under a Leica TCS-NT confocal microscope (Leica Microsystems, Wetzlar, Germany) with emission filter sets BP525/50 and LP590, respectively. Observations were confirmed in two additional replicate experiments.
Statistical analyses.
Each experiment was replicated at least twice. All statistical analyses were performed with the program Prism, version 3.0 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Colonization of pre- and postharvest leaves.
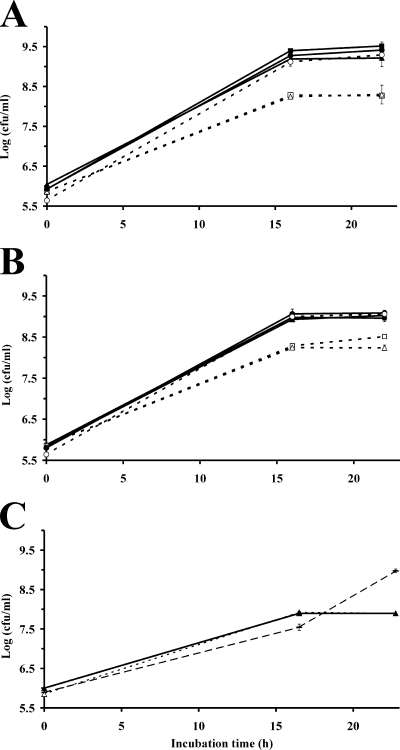
The growth potential of E. coli O157:H7 and S. enterica on lettuce leaves was investigated first by inoculation of young potted plants and their subsequent incubation under conditions of warm temperature and the presence of free water on the plant surface. The population sizes of E. coli O157:H7 and S. enterica on these leaves increased as much as 100- and 155-fold, respectively (Fig. 2A and B). The growth and maximum population sizes of these pathogens in the lettuce phyllosphere varied with the age of the leaves. The growth achieved on the young leaves of the inner rosette was consistently higher than that on the middle leaves, with a sevenfold difference in population size for both E. coli O157:H7 and S. enterica 48 h after inoculation (unpaired t test; t = 9.68, P < 0.0001, and t = 13.20, P < 0.0001, respectively) (Fig. 2A and B). The population sizes of E. coli O157:H7 and S. enterica on the old leaves varied markedly between replicate experiments but rarely exceeded those on young leaves. Similar differences in population sizes on middle and young leaves were observed for inoculated lettuce plants that were grown in the greenhouse to the same developmental stage as the plants grown in growth chambers (data not shown).
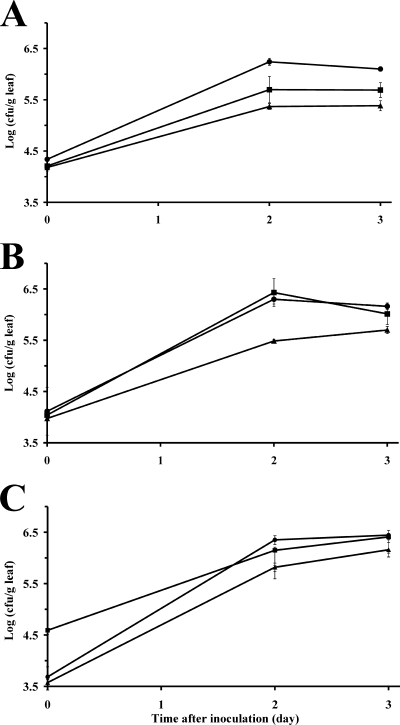
FIG. 2.
Preharvest population dynamics of (A) E. coli O157:H7 strain H1827R and (B) Salmonella enterica serovar Thompson strain RM1987N on young (•), middle (▴), and old (▪) leaves of young romaine lettuce plants. The plants were inoculated with each pathogen separately and were then incubated at 28°C under conditions conducive to the presence of free water on the leaf surface. (C) Population sizes of the indigenous culturable bacterial flora on the leaves of plants inoculated with strain H1827. Each datum point represents the mean of the log-transformed value of the bacterial population size per gram tissue of 10 leaf samples; error bars indicate ±1 standard error of the mean.
The population sizes of the culturable indigenous bacteria on the inoculated leaves showed leaf age-dependent trends that were very similar to those observed for the enteric pathogens (Fig. 2C). Indeed, although differences in population size were not as great as for enteric pathogens, the growth and population sizes of the native bacteria also were higher on the young leaves than on the middle leaves. It is noteworthy that the old leaves harbored 10 times more indigenous bacteria than the middle and young leaves at the time of inoculation. Also, the indigenous bacteria multiplied on the leaves over a longer period of time than the inoculated pathogens. This corroborated our observations from previous studies (3).
In order to better understand the behavior of the pathogens on lettuce leaves contaminated during harvest or processing, the growth of E. coli O157:H7 and S. enterica was quantified on inoculated whole leaves harvested from mature plants grown in the greenhouse and used for our experiments immediately after harvest. The population sizes of E. coli O157:H7 and S. enterica increased 500- and 740-fold on young leaves, respectively (Fig. 3A and B). In contrast, E. coli O157:H7 and S. enterica had a much lower growth rate on middle leaves, with population sizes increasing only 56- and 150-fold, respectively (Fig. 3A and B). Thus, similar trends in the growth of the pathogens in relation to leaf age were observed for both pre- and postharvest lettuce.
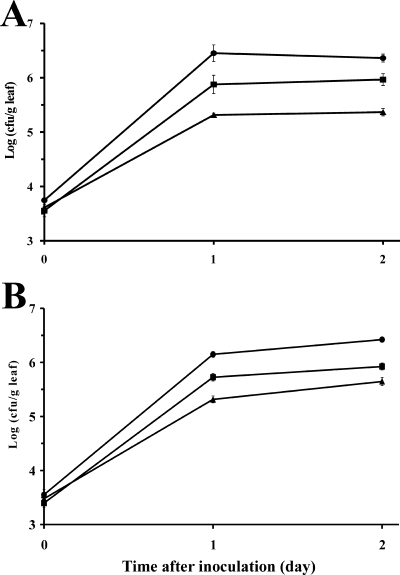
FIG. 3.
Postharvest population dynamics of (A) E. coli O157:H7 strain H1827R and (B) Salmonella enterica serovar Thompson strain RM1987N on young (•), middle (▴), and old (▪) leaves of mature romaine lettuce plants. The leaves were harvested from lettuce heads immediately after harvest and were then inoculated with each pathogen separately before incubation at 28°C under conditions allowing for the presence of free water on the leaf surface. Each datum point represents the mean of the log-transformed value of the bacterial population size per gram leaf tissue for 10 leaf samples; error bars indicate ±1 standard error of the mean.
In order to test the relevance of our experimental system to field-grown lettuce, we measured the growth of E. coli O157:H7 on leaves from lettuce heads that were farm grown and purchased from a distributor in California. The doubling time of E. coli O157:H7 on young leaves (equivalent to romaine heart leaves) was 2.38 h, compared to 3.63 h on middle leaves, and maximum E. coli O157:H7 populations were 10-fold higher on young leaves than on middle leaves 24 h after inoculation. Thus, leaf age-dependent growth of E. coli O157:H7 occurred also on farm-grown lettuce. In addition, the growth rates of the pathogen were similar, although slightly higher on farm-grown lettuce than on our greenhouse-grown plants, since the doubling times on the latter were 2.66 h and 4.23 h on young and middle leaves, respectively.
Localization of E. coli O157:H7 cells on lettuce leaves.
The distributions of E. coli O157:H7 pGT-KAN cells appeared to be very similar on both young and middle leaves immediately after inoculation, as all cells were located singly at distant locations on the leaves. Four days after inoculation, the densities of E. coli O157:H7 cells varied from disc to disc within the same leaf as well as from leaf to leaf for both leaf ages studied (young and middle leaves). However, obvious differences in colonization by the pathogen were observed. In general, the pathogen colonized a larger percentage of the surface of young leaves than that of middle leaves, despite the great similarity in distributions of the pathogen cells on these leaves immediately after inoculation. Although cells of the pathogen were present on the veins and in the areas between the veins on both the middle and the young leaves, they did not occupy as many sites and their density at a given site was not as high on middle leaves as on young leaves (Fig. 4A and B). Nevertheless, large clusters of the pathogen were occasionally observed on middle leaves, which suggested that significant growth could occur at rare microsites (Fig. 4C). Additionally, colonization of the base of the trichomes or of the trichomes themselves was observed frequently on the young lettuce leaves (Fig. 4D).
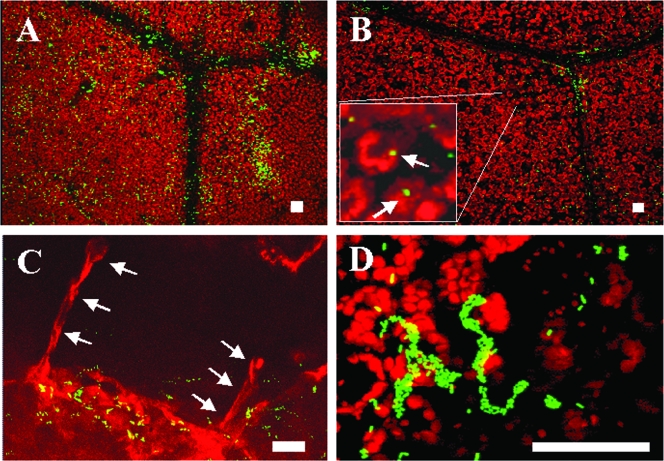
FIG. 4.
Confocal micrographs showing the distribution of cells of GFP-labeled E. coli O157:H7 strain H1827R 4 days after inoculation onto the young (A) and middle (B) leaves of young potted romaine lettuce plants that were incubated at 28°C under conditions promoting the presence of free water on the leaf surface. Note the higher cell densities of the pathogen at colonized sites on the young leaf as well as the broader distribution across the phylloplane (A). On middle leaves, the pathogen was present in interveinal regions, mostly as single cells (arrows) (B, inset). The pathogen often appeared to be associated with trichomes (stacked arrows) on young leaves (C) but also formed large aggregates at some sites on middle leaves (D). Bars, 20 μm.
Growth complementation in leaf homogenates.
The hypothesis that bacterial growth on leaves of different ages was related to the abundance of an essential nutrient was tested first by comparing the levels of growth of E. coli O157:H7 in homogenates of lettuce leaves of different ages. Equal amounts of homogenates of old, middle, and young leaves of mature plants were added separately to M9 minimal medium from which ammonium chloride (nitrogen source) or glucose (carbon source) was left out and inoculated with E. coli O157:H7. The pathogen exhibited considerably faster growth and achieved higher cell concentrations in homogenates of young leaves than in those of middle and old leaves (Fig. 5A and B). Whereas supplementation of the homogenates with ammonium chloride or potassium nitrate did not have any effect on the growth of the pathogen in the homogenates of young leaves, it complemented its growth in the homogenates of middle and old leaves to levels similar to those in the homogenates of young leaves (Fig. 5A and B). In contrast, the addition of glucose to homogenates of middle leaves did not affect growth (Fig. 5C). It is noteworthy that despite supplementation with 0.5% glucose, the homogenates of middle leaves did not support as much growth of E. coli O157:H7 as M9 minimal medium, which also contained 0.5% glucose (Fig. 5C). This indicated again that a nutrient other than glucose was limiting growth in the middle-leaf homogenate cultures.
FIG. 5.
Growth of E. coli O157:H7 strain H1827R in the homogenates of young (circles), middle (triangles), and old (squares) leaves of mature romaine lettuce plants. The homogenates were supplemented (solid lines, filled symbols) or not (dashed lines, open symbols) with (A) 19 mM NH4Cl, (B) 19 mM KNO3, or (C) 0.5% glucose (final concentration). All homogenate cultures were amended with rifampin. The long-dashed line in panel C illustrates the growth of strain H1827R in standard M9 medium with 0.5% glucose. Also in panel C, growth in middle-leaf homogenates with (solid line) or without (short-dashed line) glucose is shown; both lines nearly overlap, indicating that glucose did not affect the growth of strain H1827R in homogenates of middle leaves. Each datum point represents the mean of the log-transformed value of the bacterial cell concentration of three replicate cultures; error bars indicate ±1 standard error of the mean.
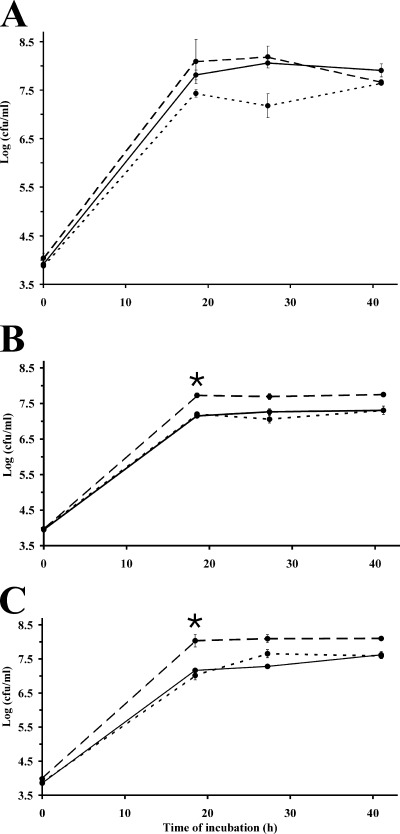
Growth complementation in leaf exudates.
E. coli O157:H7 was cultured in vitro in the exudates collected from leaves of mature lettuce plants grown in the greenhouse. The exudates from equal weights of young, middle, and old leaves were collected, concentrated, and then inoculated with E. coli O157:H7. The effect of supplementation of the exudates with glucose or ammonium chloride on the growth of E. coli O157:H7 in the exudates was tested to determine whether growth was limited by a carbon or nitrogen source. The growth rate and maximum cell concentration of E. coli O157:H7 were higher in the nonsupplemented exudates of young leaves than in those of middle and old leaves (Fig. 6A, B, and C). These results correlated with differences in the population sizes of the pathogen on leaves (Fig. 2 and 3) and in cell concentrations of the pathogen in leaf homogenates (Fig. 5). Comparison of the cell concentrations after 18 h of incubation in the exudates, by which time the exponential growth had occurred, indicated that supplementation with ammonium chloride but not with glucose (Tukey-Kramer multiple comparison test; q = 6.06, P < 0.05, and q = 0.73, P > 0.05, respectively) increased the cell concentration of E. coli O157:H7 in exudates of middle leaves (Fig. 6B) and old leaves (Fig. 6C). On the contrary, supplementation of young leaf exudates with ammonium chloride or glucose did not affect significantly the growth of E. coli O157:H7 (Tukey-Kramer multiple comparison test; P = 0.63), although the addition of glucose appeared to have a slight inhibitory effect at 28 h of incubation (Fig. 6A).
FIG. 6.
Growth of E. coli O157:H7 strain H1827R in the exudates of (A) young, (B) middle, and (C) old leaves of mature romaine lettuce plants. The exudates were dissolved in PPB-10 mM and were not supplemented (solid lines) or supplemented with 10 mM NH4Cl (long-dashed lines) or with 0.2% glucose (short-dashed lines) (final concentration). The asterisk denotes a significant difference in the cell concentrations in nonsupplemented versus NH4Cl-supplemented exudates (Tukey-Kramer test; P < 0.05); growth in the exudates was not affected significantly by supplementation with glucose (P > 0.05). Each datum point represents the mean of the log-transformed value of the bacterial cell concentration of two replicate cultures; error bars indicate ±1 standard error of the mean.
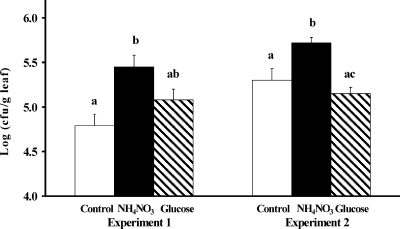
Effect of amendments on epiphytic growth.
The middle leaves of field-grown romaine lettuce heads were sampled and inoculated with E. coli O157:H7 cells suspended in PPB, in PPB with ammonium nitrate, or in PPB with glucose in order to test the effect of nitrogen (N) and carbon (C) amendments on the leaves on E. coli O157:H7 population sizes. In two replicate experiments, E. coli O157:H7 populations on middle leaves were significantly greater (five- and threefold) when ammonium nitrate was added to the leaves than without the amendment (Tukey-Kramer multiple comparison test; P < 0.001) (Fig. 7). In contrast, population sizes of the pathogen on middle leaves were not significantly different with or without glucose amendment (P > 0.05).
FIG. 7.
Effect of nitrogen supplementation on the growth of E. coli O157:H7 strain H1827R on middle leaves sampled from harvested romaine lettuce heads grown in the field. The leaves were inoculated with a suspension of the pathogen in buffer only (white bars) or in buffer containing 6 mM NH4NO3 (black bars) or 0.1% glucose (cross-hatched bars). The results of two replicate experiments are shown. The data represent the means of the log-transformed values of the bacterial population sizes per gram tissue of 16 replicate leaves; error bars indicate ±1 standard error of the mean. Data labeled with the same letter are not significantly different by the Tukey-Kramer multiple comparison test (P < 0.05).
N and C elemental analysis.
The exudates of leaves of the three different age categories were tested for their total contents of N and C by elemental analysis. Table 1 shows that the exudates of young leaves were 2.9- and 1.5-fold richer in N and C, respectively, than the exudates of middle leaves. The exudates of old leaves had the smallest amounts of both N and C. Similarly, the N content of the leaf tissue was higher in young than in middle leaves, but their C contents were the same. Although the absolute amounts of N and C were different in the replicate experiment, the same comparative trend in the N and C contents of the exudates and tissues of middle versus young leaves was observed. Also notable is the overall higher Cexudate/Ctissue ratio than the Nexudate/Ntissue ratio, independent of leaf age, reflecting that more C than N may leach from the tissue onto the surface.
TABLE 1.
Total nitrogen and carbon contents in the exudates and tissue of leaves of various ages sampled from mature romaine lettuce heads
| Leaf age | Total N
|
Total C
|
||
|---|---|---|---|---|
| Exudates (μg/g leafa) | Tissue (mg/g leafb) | Exudates (μg/g leafa) | Tissue (mg/g leafb) | |
| Young | 2.290 | 9.894 | 89 | 92 |
| Middle | 0.782 | 5.956 | 61 | 92 |
| Old | 0.708 | 4.464 | 30 | 67 |
Leaf fresh weight.
Leaf dry weight.
DISCUSSION
Little attention has been given to the hypothesis that enteric pathogens such as E. coli O157:H7 may have the potential to grow on leafy vegetables in the field. On the contrary, the observation of their population decline after their inoculation onto crops in the field has supported the conclusion that the pathogens can persist but lack the ability to multiply in the phyllosphere of agricultural crops. Although survival studies of plants provide information about the populations of the pathogens over broad time scales, they offer little insight into the behavior of enteric pathogens under conditions that may be conducive to their growth on plants over short time scales. Hirano and Upper (16) have demonstrated that a common bacterial colonizer of leaves, Pseudomonas syringae, has very dynamic colonization patterns when monitored over short periods of time in the field. In that study, P. syringae exhibited repeated fluctuations in population size on leaves in as little as a few hours. It remains unknown whether cells of enteric pathogens also may experience similar cycles of growth and death at distinct microsites on leafy vegetables in the field, but the implications of short growth periods to the occurrence of outbreaks caused by preharvest contamination are worth investigating.
The data presented herein provide the first evidence for the ability of E. coli O157:H7 and S. enterica to multiply in the phyllosphere of young lettuce plants and on the harvested leaves of mature plants. This was observed at warm incubation temperatures and with the presence of free water on the leaves, which represent conditions that are conducive to bacterial growth in the presence of nutrient availability. These conditions are likely to occur on lettuce in the field during warm weather and in sites on the leaves where water remains after dew formation, rain, or overhead irrigation or during harvest when the leaves are kept wet in a container before reaching the processing plant.
Although it has been proposed that specific bacterial determinants may underlie the greater association of outbreaks from leafy greens with E. coli O157:H7 than with S. enterica (2), the abilities of the two pathogens to multiply on lettuce leaves were very similar in this study. This similarity was observed for both pre- and postharvest leaves. Therefore, the trend in association of E. coli O157:H7 with leafy vegetables in the United States may instead be related to the prevalence of the pathogen in certain geographical areas where leafy vegetables are grown, as revealed in a recent study by Cooley et al. (6). Nevertheless, the possibility remains that pathogen-specific factors that have a role in the fitness of S. enterica and E. coli O157:H7 on pre- and postharvest leaves were not required under the experimental conditions of our study, thus resulting in a greater similarity in the behaviors of these pathogens than would occur in the field and processing environments.
On the other hand, the results of this study demonstrate that a plant factor, namely, leaf age, has a role in the colonization of romaine lettuce by E. coli O157:H7 and S. enterica. Both pathogens achieved higher growth rates and about 10-fold higher population sizes on young than on middle lettuce leaves. These leaf age-dependent differences were observed for immature plants as well as for leaves sampled from mature lettuce heads. Also, microscopic observations of GFP-labeled E. coli O157:H7 revealed that it colonized young lettuce leaves at higher densities and at more sites than on the middle leaves, hence providing further evidence that an inherent plant factor was associated with these differences in colonization. It is noteworthy that the indigenous bacterial flora of lettuce, as well as a strain of Enterobacter cloacae inoculated onto lettuce (data not shown), showed similar levels of leaf age-dependent growth.
Numerous studies have demonstrated the role of leaf age in the composition and population size of epiphytic microbial communities (19). Gradients of decreasing densities of epiphytic bacteria from the outer to the inner leaves have been reported for rosette-type leafy vegetables at harvest (10, 12, 20, 31). Jacques et al. (20) proposed that decreasing bacterial immigration from the outer, more exposed leaves to the inner, more protected leaves of mature rosette-type plants in the field may contribute to this gradient. On the other hand, several studies have shown that bacteria inoculated onto plants multiply faster on young leaves than on old leaves (see reference 19 and references therein). This suggests that in addition to immigration rates, microbial and physicochemical factors that influence bacterial growth may shape bacterial population densities in the phyllosphere in a leaf age-dependent manner. Several lines of evidence from the present work support a role for nitrogen abundance on the phylloplane in the differential growth of E. coli O157:H7 on leaves of various ages: (i) complementation for growth of E. coli O157:H7 in homogenates and exudates of middle lettuce leaves with nitrogen, but not with glucose, to levels comparable to growth in homogenates and exudates of young leaves; (ii) elemental analysis of exudates and leaf tissue revealing a decreasing gradient of total N content from young to old leaves; and (iii) enhanced growth of E. coli O157:H7 on middle lettuce leaves resulting from amendment with nitrogen but not with glucose.
The above-described observations correlate well with models of nitrogen allocation across the plant canopy, which stipulate that the most metabolically active leaf tissue (young tissue) has the highest nitrogen content (5, 35). Phylloplanes harbor a variety of organic and inorganic substances that are leached or exuded from the leaf tissue (30, 41) and that can serve as nutrient sources to epiphytic bacteria. On lettuce leaves, some of these nutrients may originate from guttation fluids since guttation (the exudation of water through the hydathodes) is considered a common phenomenon in this crop (7). It is noteworthy that the tension of the guttation fluids of lettuce is so low that the fluids appear to spread across the surface of the leaf and may even pool inside the lettuce head (7). Guttation fluids contain various substances, including carbohydrates, amino acids, and numerous inorganic compounds, including significant amounts of NH4 and NO3 ions (13). Considering that young leaf tissue contains more nitrogen than that of middle leaves, one could suggest a scenario in which guttation fluids on young lettuce leaves contribute greatly to increased levels of nitrogen available to bacteria that land on their surface. Also, the very high density of trichomes on young lettuce leaves may have a role in the high levels of nutrients present on their surface. The aggregation of epiphytic bacteria near or on trichomes has been reported, and the role of trichomes in supplying nutrients to the plant microflora has been proposed (29, 32, 43).This is consistent with our observation and that of others (39) that E. coli O157:H7 cells are associated frequently with trichomes.
The results of our study are in contrast with a previous report that carbon, rather than nitrogen, limited the growth of P. syringae in the bean phyllosphere (42). Indeed, addition of glucose failed to enhance significantly the growth of E. coli O157:H7 in exudates of and on middle lettuce leaves. This may be due to inherent differences in the model systems (bacteria and plants) used in the two studies or to experimental conditions that may have affected, among other things, the C:N ratio of nutrients on the phylloplane of the two plant species.
Although less emphasis was placed on the behavior of E. coli O157:H7 on old leaves in our studies, it is apparent that it multiplied more on old leaves than on middle leaves but that its population sizes were in general lower than on young leaves. The E. coli O157:H7 growth complementation in homogenates and exudates of old leaves by the addition of nitrogen, combined with the low levels of nitrogen detected in the exudates of old leaves, may indicate that nitrogen availability has a role in the colonization of old leaves. On the other hand, the observation that the pathogen nevertheless achieves higher population sizes on old leaves than on middle leaves suggests that additional unknown plant or bacterial factors may interact with nitrogen availability to shape its comparative population dynamics on lettuce in relation to leaf age.
Our experimental model relied on lettuce plants that were grown in plant growth chambers and in a greenhouse. Therefore, they probably were not as heavily colonized by microbes as plants that grow in the field and were not fertilized exactly as in a field situation. However, the resulting lower competition from the natural microflora enabled us to deconvolute the effects of microbial competition and leaf age and nutrient availability. One could argue that nitrogen and competitive microbes may not intersect at every microsite on a lettuce leaf and that each factor may play a distinct role at various locations on that leaf. It is important to underline that we observed similar differences in bacterial colonization in relation to leaf age, as well as complementation for growth of E. coli O157:H7 with ammonium nitrate on middle leaves, with leaves from harvested lettuce heads that were farm grown under standard production practices. These observations thus support the relevance of our microcosm model.
The enhanced multiplication of E. coli O157:H7 and S. enterica on young leaves implies that, theoretically, a single pathogen cell could form a colony of 10 cells upon arrival on a young leaf, in contrast to remaining solitary on a middle leaf. Therefore, young lettuce leaves may be more likely to host the pathogens at infectious doses than middle leaves and may represent a higher risk factor for food-borne illness. The pooling of contaminated irrigation water in the inner rosette of young lettuce plants in the field, combined with the hospitable environment to immigrant bacteria due to low population sizes of indigenous bacteria and a high abundance of nutrients compared to other leaves, is a likely scenario of contamination in the field. As these leaves expand over time and become the middle, and later the outer, leaves of the lettuce plant, they may contribute to maintaining inoculum in the field. In view of the role of nitrogen in the colonization of young leaves by enteric pathogens, the modulation of nitrogen fertilization in order to minimize the probability of pathogen amplification on leafy greens is a strategy worth exploring. Finally, if young leaves become contaminated with the enteric pathogens at or after harvest, they may constitute the highest risk factor in contamination of lettuce in the postharvest environment. This should be taken into account in sampling procedures for detection of enteric pathogens on lettuce and in scientific studies on control and sanitization strategies.
Acknowledgments
Thanks are given to Aileen Haxo for technical assistance, Paul Brooks for help with elemental analysis of leaf exudates, and Robert Mandrell for review of the manuscript.
This work was supported with funds from the U.S. Department of Agriculture, Agriculture Research Service CRIS project 5325-42000-044-00D.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Anonymous. 2005. Outbreak of Salmonella Typhimurium DT104 infection in Scotland, England & Wales, January to February 2005 (update)—iceberg lettuce eaten outside the home implicated. CDR Wkly. http://www.hpa.org.uk/cdr/archives/archive05/News/news0905.htm#typhimurium.
- 2.Brandl, M. T. 2006. Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 44:367-392. [DOI] [PubMed] [Google Scholar]
- 3.Brandl, M. T., and R. E. Mandrell. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 68:3614-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandl, M. T., W. G. Miller, A. H. Bates, and R. E. Mandrell. 2005. Production of autoinducer 2 in Salmonella enterica serovar Thompson contributes to its fitness in chickens but not on cilantro leaf surfaces. Appl. Environ. Microbiol. 71:2653-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles-Edwards, D. A., H. Stutzel, R. Ferraris, and D. F. Beech. 1987. An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy. Ann. Bot. 60:421-426. [Google Scholar]
- 6.Cooley, M., D. Carychao, L. Crawford-Miksza, M. T. Jay, C. Myers, C. Rose, C. Keys, J. A. Farrar, and R. E. Mandrell. 2008. Incidence and tracking of Escherichia coli O157:H7 in a watershed associated with a major produce production region in California. PLoS One 2:e1159. doi: 10.1371/journal.pone.0001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis, L. C. 1943. Deleterious effects of guttated fluids on foliage. Am. J. Bot. 30:778-782. [Google Scholar]
- 8.Delaquis, P., S. Bach, and L.-D. Dinu. 2007. Behavior of Escherichia coli O157:H7 in leafy vegetables. J. Food Prot. 70:1966-1974. [DOI] [PubMed] [Google Scholar]
- 9.Delaquis, S., S. Stewart, S. Cazaux, and P. Toivonen. 2002. Survival and growth of Listeria monocytogenes and Escherichia coli O157:H7 in ready-to-eat iceberg lettuce washed in warm chlorinated water. J. Food Prot. 65:459-464. [DOI] [PubMed] [Google Scholar]
- 10.Ercolani, G. L. 1976. Bacteriological quality assessment of fresh marketed lettuce and fennel. Appl. Environ. Microbiol. 31:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ercolani, G. L. 1979. Differential survival of Salmonella typhi, Escherichia coli, and Enterobacter aerogenes on lettuce in the field. Zentbl. Bakteriol. Naturwissensch. 134:402-411. [PubMed] [Google Scholar]
- 12.Geeson, J. D. 1979. The fungal and bacterial flora of stored white cabbage. J. Appl. Bacteriol. 46:189-193. [DOI] [PubMed] [Google Scholar]
- 13.Goatley, J. L., and R. W. Lewis. 1966. Composition of guttation fluids from rye, wheat, and barley seedlings. Plant Physiol. 41:373-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilborn, E. D., J. H. Mermin, P. A. Mshar, J. L. Hadler, A. Voetsch, C. Wojtkunski, M. Swartz, R. Mshar, M. A. Lambert-Fair, J. A. Farrar, M. K. Glynn, and L. Slutsker. 1999. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern. Med. 159:1758-1764. [DOI] [PubMed] [Google Scholar]
- 15.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano, S. S., and C. D. Upper. 1989. Diel variation in population size and ice nucleation activity of Pseudomonas syringae on snap bean leaflets. Appl. Environ. Microbiol. 55:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam, M., M. P. Doyle, S. C. Phatak, P. Millner, and X. Jiang. 2004. Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J. Food Prot. 67:1365-1370. [DOI] [PubMed] [Google Scholar]
- 18.Islam, M., J. Morgan, M. P. Doyle, S. C. Phatak, P. Millner, and X. Jiang. 2004. Persistence of Salmonella enterica serovar Typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog. Dis. 1:27-35. [DOI] [PubMed] [Google Scholar]
- 19.Jacques, M.-A. 1996. The effect of leaf age and position on the dynamics of microbial populations on aerial plant surfaces, p. 233-248. In C. Morris, P. Nicot, and C. Nguyen-The (ed.), Aerial plant surface microbiology. Plenum Press, New York, NY.
- 20.Jacques, M.-A., L. L. Kinkel, and C. E. Morris. 1995. Population sizes, immigration and growth of epiphytic bacteria on leaves of different ages and positions of field-growth endive (Cichorium endiva var. latifolia). Appl. Environ. Microbiol. 61:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leben, C. 1988. Relative humidity and the survival of epiphytic bacteria with buds and leaves of cucumber plants. Phytopathology 78:179-185. [Google Scholar]
- 22.Leveau, J. H. J., and S. E. Lindow. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 98:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Y., R. E. Brackett, J. Chen, and L. R. Beuchat. 2001. Survival and growth of Escherichia coli O157:H7 inoculated onto cut lettuce before or after heating in chlorinated water, followed by storage at 5 or 15 degrees C. J. Food Prot. 64:305-309. [DOI] [PubMed] [Google Scholar]
- 24.Lindow, S. E., and M. T. Brandl. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch, M. 2007. Escherichia coli O157:H7 outbreaks due to raw leafy green vegetables. Memorandum. U.S. Centers for Disease Control and Prevention, Atlanta, GA.
- 26.Mandrell, R. E., L. Gorski, and M. T. Brandl. 2006. Attachment of microorgamisms to fresh produce, p. 33-73. In G. M. Sapers, J. R. Gorny, and A. E. Yousef (ed.), Microbiology of fruits and vegetables. CRC Press, Boca Raton, FL.
- 27.Mercier, J., and S. E. Lindow. 2000. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 66:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, W. G., M. T. Brandl, B. Quinones, and S. E. Lindow. 2001. Biological sensor for sucrose availability: relative sensitivities of various reporter genes. Appl. Environ. Microbiol. 67:1308-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monier, J. M., and S. E. Lindow. 2004. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 70:346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan, J. V., and H. B. Tukey. 1964. Characterization of leachate from plant foliage. Plant Physiol. 39:590-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris, C. E., and T. Lucotte. 1993. Dynamics and variability of bacterial population density on leaves of field-grown endive destined for ready-to-use processing. Int. J. Food Sci. Technol. 28:201-209. [Google Scholar]
- 32.Morris, C. E., and J.-M. Monier. 2003. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41:429-453. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien, R. D., and S. E. Lindow. 1989. Effect of plant species and environmental conditions on epiphytic population sizes of Pseudomonas syringae and other bacteria. Phytopathology 79:619-627. [Google Scholar]
- 34.Rangel, J. M., P. H. Sparling, C. Crowe, P. M. Griffin, and D. L. Swerdlow. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seginer, I., P. Bleyaert, and M. Breugelmans. 2004. Modelling ontogenic changes of nitrogen and water content in lettuce. Ann. Bot. 94:393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sivapalasingam, S., C. R. Friedman, L. Cohen, and R. V. Tauxe. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67:2342-2353. [DOI] [PubMed] [Google Scholar]
- 37.Solomon, E. B., M. T. Brandl, and R. E. Mandrell. 2006. Biology of food-borne pathogens on produce, p. 55-83. In K. R. Matthews (ed.), Microbiology of fresh produce. ASM Press, Washington, DC.
- 38.Stafford, R. J., B. J. McCall, A. S. Neill, D. S. Leon, G. J. Dorricott, C. D. Towner, and G. R. Micalizzi. 2002. A statewide outbreak of Salmonella Bovismorbificans phage type 32 infection in Queensland. Commun. Dis. Intell. 26:568-572. [PubMed] [Google Scholar]
- 39.Takeuchi, K., and J. F. Frank. 2001. Expression of red-shifted green fluorescent protein by Escherichia coli O157:H7 as a marker for the detection of cells on fresh produce. J. Food Prot. 64:298-304. [DOI] [PubMed] [Google Scholar]
- 40.Takkinen, J., U. Nakari, T. Johansson, T. Niskanen, A. Siitonen, and M. Kuusi. 2005. A nationwide outbreak of multiresistant Salmonella Typhimurium va. Copenhagen DT104B infection in Finland due to contaminated lettuce from Spain. Eurosurveill. Wkly. 10:E050630.1. http://www.eurosurveillance.org/ew/2005/050630.asp#1. [DOI] [PubMed] [Google Scholar]
- 41.Tukey, H. B. 1970. The leaching of substances from plants. Annu. Rev. Plant Physiol. 21:305-324. [Google Scholar]
- 42.Wilson, M., and S. E. Lindow. 1994. Ecological similarity and coexistence of epiphytic ice-nucleating (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice−) biological control agent. Appl. Environ. Microbiol. 60:3128-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav, R. K. P., K. Karamanoli, and D. Vokou. 2005. Bacterial colonization of the phyllosphere of Mediterranean perennial species as influenced by leaf structure and chemical features. Microb. Ecol. 50:185-196. [DOI] [PubMed] [Google Scholar]