Abstract
An Escherichia coli galactose kinase gene knockout (ΔgalK) strain, which contains the l-arabinose isomerase gene (araA) to isomerize d-galactose to d-tagatose, showed a high conversion yield of tagatose compared with the original galK strain because galactose was not metabolized by endogenous galactose kinase. In whole cells of the ΔgalK strain, the isomerase-catalyzed reaction exhibited an equilibrium shift toward tagatose, producing a tagatose fraction of 68% at 37°C, whereas the purified l-arabinose isomerase gave a tagatose equilibrium fraction of 36%. These equilibrium fractions are close to those predicted from the measured equilibrium constants of the isomerization reaction catalyzed in whole cells and by the purified enzyme. The equilibrium shift in these cells resulted from the higher uptake and lower release rates for galactose, which is a common sugar substrate, than for tagatose, which is a rare sugar product. A ΔmglB mutant had decreased uptake rates for galactose and tagatose, indicating that a methylgalactoside transport system, MglABC, is the primary contributing transporter for the sugars. In the present study, whole-cell conversion using differential selectivity of the cell membrane was proposed as a method for shifting the equilibrium in sugar isomerization reactions.
Recently, the biotransformation of galactose to tagatose, a valuable sweetener (20), has been intensively investigated using many bacterial l-arabinose isomerases, including those from Alicyclobacillus acidocaldarius (19), B. halodurans (17), B. stearothermophilus (26), Escherichia coli (24, 27, 30), Geobacillus stearothermophilus (11, 13, 15, 28), G. thermodenitrificans (2, 14, 25), Thermus sp. (16), Thermoanaerobacter mathranii (10), Thermotoga neapolitana (12), and Thermotoga maritima (18). The reported equilibrium fraction of tagatose from galactose is 32% at 30°C for l-arabinose isomerase purified from E. coli (27), 58% at 65°C for that from G. thermodenitrificans (25), and 68% at 85°C for that from T. neapolitana (12). The equilibrium of the isomerization reaction shifts toward the tagatose product as the temperature increases. The equilibrium fraction of product for an enzyme-catalyzed isomerization reaction, i.e., the equilibrium between substrate and product, cannot be changed simply by protein engineering of the enzyme, since it is controlled by the reaction temperature (4). Compared to conversion using hyperthermophilic enzyme, whole-cell conversion has advantages such as greater resistance to environmental perturbations and a lower effective enzyme cost, while eliminating the enzyme purification and extraction steps. Also, it may not be easy to find a hyperthermophilic enzyme to carry out the reaction at high temperatures.
In the conversion of galactose to tagatose by E. coli expressing l-arabinose isomerase, transport systems of galactose and tagatose through the cell membrane are important because they may affect the conversion rate and yield. Three separate transport systems for galactose are reported for E. coli: (i) a methylgalactoside transport system, which is designated MglABC because it is a member of the ATP-binding cassette (ABC) superfamily of transporters and transports β-methylgalactoside in addition to its natural galactose substrate (29); (ii) a galactose-specific transport system (GalP) (5, 22); and (iii) sugar efflux transporters (SETs, including SetA, -B, and -C) for sugar release, which are believed to have broad substrate specificity to various sugars including galactosides (21). However, an E. coli transport system for tagatose has not previously been reported.
Instead of using temperature to control the enzymatic process in isomerization reactions, whole-cell conversion is proposed as a new alternative method to shift equilibrium. The method uses the cell membrane to separate the substrate and product (extracellular region) from the enzyme reaction (intracellular region). Membrane selectivity can cause differences in the concentrations of substrate and product inside and outside of the cells, resulting in an equilibrium shift. However, direct or kinetic evidence for the equilibrium shift in whole-cell conversions has never been documented. We measure here the equilibrium shift of whole-cell conversions and describe it quantitatively based on enzyme kinetic studies, which unambiguously show that membrane selectivity for the substrates results in an equilibrium shift. Moreover, the uptake and release rates of galactose and tagatose were determined to explain the equilibrium shift, and the related transport systems for the sugars were investigated.
MATERIALS AND METHODS
Plasmids, bacterial strains, and culture conditions.
Plasmids pTrc99A and pTaraA were used as a control and for expression, respectively, of l-arabinose isomerase. E. coli MG1655 and its ΔgalK strain harboring pTaraA were used to investigate whole-cell conversion of galactose to tagatose. The recombinant cells were cultivated at 37°C with shaking at 200 rpm in a 2-liter flask containing 500 ml of Luria-Bertani (LB) medium with 50 μg/ml ampicillin. E. coli BW25113 ΔgalK harboring pTaraA and its ΔsetA, ΔsetB, ΔsetC, ΔgalP, ΔmglB, and ΔgalP ΔmglB mutants were cultured in LB medium containing 50 μg/ml ampicillin and 20 μg/ml kanamycin. When the absorbance of the culture reached 1.0 at 600 nm, the cells were harvested by centrifugation at 15,000 × g for 20 min at 4°C, washed with saline solution, and then resuspended in 50 mM Tris-HCl buffer (pH 7.0) for use in the conversion of galactose to tagatose.
DNA cloning, purification, and quantification of l-arabinose isomerase.
Genomic E. coli DNA was prepared using a Genomic DNA buffer set (Qiagen, Hilden, Germany). The araA gene encoding the enzyme l-arabinose isomerase was isolated from the genomic DNA by PCR using forward (araA-F, 5′-CCCATGGCGATTTTTGATAATTATGAAGTG-3′) and reverse (araA-R, 5′-CTCTAGATTAGCGACGAAACCCGTAATAC-3′) primers designed to introduce NcoI and XbaI restriction sites (underlined). PCR was carried out using Pfu DNA polymerase (Invitrogen, Carlsbad, CA) according to a standard PCR protocol. The E. coli araA gene was inserted into the NcoI and XbaI sites of pTrc99A, resulting in pTaraA.
Cells were harvested from the culture broth by centrifugation at 15,000 × g for 20 min at 4°C, washed with saline solution, and then resuspended in 50 mM Tris-HCl buffer (pH 7.0) containing 1 mM phenylmethylsulfonyl fluoride. The resuspended cells were disrupted by passing them twice through a French press at 15,000 lb/in2. The cell debris was removed by centrifugation at 15,000 × g for 20 min at 4°C, and the resulting supernatant fraction was filtered through a 0.45-μm-pore-size filter and applied to a Hi-trap Q ion-exchange chromatography column (Amersham Biosciences Uppsala, Sweden) equilibrated with 50 mM Tris-HCl buffer (pH 7.0). All chromatographic purification steps were carried out in a cold room using a fast protein liquid chromatography system (Amersham Biosciences). The bound protein was eluted with a linear gradient from 0.15 to 0.5 M NaCl in the same buffer. The active fractions were collected and further purified on a Resource Q ion-exchange chromatography column (Amersham Biosciences) equilibrated with 50 mM Tris-HCl buffer (pH 7.0). The concentrated enzyme was loaded onto the chromatography column and eluted with a gradient from 0.15 to 0.4 M NaCl in the same buffer. The active fractions were pooled in a 50 mM Tris-HCl buffer (pH 7.0). The resulting solution was used as the purified l-arabinose isomerase.
Two-dimensional (2D) polyacrylamide gel electrophoresis (PAGE) was performed to determine the amount of intracellular l-arabinose isomerase, which was used in calculating the turnover number (kcat) of the enzyme. The cells were washed twice with phosphate-buffered saline at 4°C, resuspended in a lysis buffer (7 M urea, 2 M thiourea, 4% [wt/vol] 3-[(3-cholamidopropy) dimethyammonium]-1-propanesulfonate, 1% [wt/vol] dithiothreitol, 2% [vol/vol] Pharmalyte, and 1 mM benzamidine), and disrupted using a Sonopuls instrument (Bandelin Electronic, Berlin, Germany). The lysate was centrifuged at 15,000 × g for 1 h at 4°C to remove cell debris, and the supernatant was used for 2D PAGE.
The dissolved proteins were subjected to isoelectric focusing on an immobilized pH gradient DryStrips system (Amersham Biosciences) for 18 h at 3,500 V. After isoelectric focusing, the strips were equilibrated for 10 min at room temperature in a 50 mM Tris-HCl (pH 6.8) buffer containing 6 M urea, 2% sodium dodecyl sulfate (SDS), and 30% glycerol, and then subjected to sodium dodecyl sulfate-PAGE on a 12% polyacrylamide gel (20 by 24 cm) using a Multiphor II system (Amersham Biosciences). The gel was fixed using a Hoefer Dalt 2D system (Amersham Biosciences), scanned, and processed for image analysis using PDQuest software (version 7.0; Bio-Rad, Hercules, CA). The amount of protein in the target protein spot in the digitized image was calculated and used to determine the proportion of target protein in the total intracellular protein.
Construction of transport system knockout mutants.
E. coli BW25113 strain and its ΔsetA, ΔsetB, ΔsetC, ΔgalP, ΔgalP ΔmglB, and ΔgalP ΔmglB mutants, which were obtained from H. Mori, were developed by Baba et al. (1). Mutants harboring the kanamycin selection marker gene were constructed using the method of Datsenko and Wanner (6). E. coli MG1655 ΔgalK::Km was transferred by P1 transduction into the BW25113 mutants (ΔsetA, ΔsetB, ΔsetC, ΔgalP, and ΔmglB) lacking kanamycin. The ΔgalK::Km transductants were selected by plating on LB agar containing 50 μg/ml kanamycin. Transduction was confirmed by colony-based PCR, and the resulting knockout mutants were designated ΔsetA ΔgalK::Km, ΔsetB ΔgalK::Km, ΔsetC ΔgalK::Km, ΔgalP ΔgalK::Km, ΔmglB ΔgalK::Km, and ΔgalP ΔmglB ΔgalK::Km.
Conversion of galactose to tagatose.
The pTaraA plasmid was transformed into the galK and ΔgalK strains of E. coli MG1655. These transformed cells were cultivated as described above, and then 10 ml of cell suspension (19 mg/ml of dry cell weight) was incubated for 15 h at 37°C in 50 mM Tris-HCl buffer (pH 7.0) containing 50 mM galactose, with shaking at 100 rpm. To investigate the cell growth and galactose consumption for the galK+ and ΔgalK strains of E. coli MG1655 without pTaraA, the strains were cultivated for 20 h at 37°C with shaking in a 500-ml flask containing 100 ml of M9 minimal broth with 50 mM galactose instead of glucose as a carbon source.
The ΔgalK strain harboring a plasmid carrying l-arabinose isomerase (pTaraA) at 19 mg/ml was disrupted by sonication (Sonic Dismembrator Model 100; Fisher Scientific, Pittsburgh, PA). The cell debris was removed by centrifugation at 15,000 × g for 20 min at 4°C, and the supernatant fraction was used as a crude extract. To convert galactose to tagatose, whole cells and crude extract were incubated for 17 h at 37°C in 10 ml of 50 mM Tris-HCl buffer (pH 7.0) containing 50 mM galactose with shaking at 200 rpm. The concentrations of galactose and tagatose in samples withdrawn at various time intervals were measured during the conversion. The activity to produce tagatose was assayed after incubation at 37°C in 50 mM Tris-HCl buffer (pH 7.0) containing 50 mM galactose for 10 min.
E. coli BW25113 ΔgalK and its ΔsetA, ΔsetB, ΔsetC, ΔgalP, ΔmglB, and ΔgalP ΔmglB mutants were transformed with pTaraA and then used in the conversion of galactose to tagatose. Ten milliliters of the cell suspension at 19 mg/ml in 50 mM Tris-HCl buffer (pH 7.0) containing 50 mM galactose was incubated for 16 h at 37°C with shaking at 100 rpm. The relative conversion of galactose to tagatose in cells of the ΔgalK strain and its transport knockout mutants harboring pTaraA was measured after 16 h.
Determination of equilibrium fractions.
To determine the extracellular concentrations of galactose and tagatose, 10 ml of a 19 mg/ml cell suspension was incubated at 37°C for 22 h in a 10 mM concentration of sugars at three different initial ratios of galactose to tagatose (0:100, 50:50, and 100:0), with shaking at 100 rpm. The suspension was centrifuged at 15,000 × g for 10 min at 4°C, and the resulting supernatant was analyzed. To determine the intracellular concentrations of galactose and tagatose, the cell pellet from the above step was resuspended in saline solution and then centrifuged at 15,000 × g for 20 min at 4°C. Lysis solution (Turbolytic cell lysis reagent; Genofocus, Daejeon, Korea) was added to the washed cells, which were then incubated at 70°C for 15 min. After cellular debris was removed by centrifugation at 15,000 × g for 10 min at 4°C, a premixed solution of phenol, chloroform, and isoamyl alcohol (Amresco, Parkway Solon, OH) was added to the supernatant to remove soluble protein. The mixture was centrifuged at 15,000 × g for 10 min at 4°C, and the concentrations of galactose and tagatose in the cell- and protein-free supernatant were determined.
Purified l-arabinose isomerase was incubated at 20°C for 4 h with 1 mM Mn2+ and then dialyzed against 50 mM Tris-HCl buffer (pH 7.0) at 4°C for 16 h. The equilibrium fraction of tagatose was determined as the average of the fractions obtained after incubation of the enzyme solution at 37°C for 16 h in 50 mM Tris-HCl buffer (pH 7.0) containing a 10 mM concentration of the sugars at five different initial ratios of galactose to tagatose (0:100, 25:75, 50:50, 75:25, and 100:0). The enzyme concentration used was 10 mg/ml.
Determination of kinetic parameters.
Substrate was mixed with whole cells (19 mg/ml) or purified l-arabinose isomerase (3 mg/ml) in 50 mM Tris-HCl buffer (pH 7.0) and incubated for 1 h at 37°C. The galactose concentration used ranged from 50 to 1,000 mM. The tagatose concentration used ranged from 50 to 500 mM for whole cells and from 5 to 100 mM for the purified enzyme. The Michaelis-Menten constant (Km) and turnover number (kcat) of the purified enzyme and cells were calculated using Lineweaver-Burk transformations of the concentration and velocity data. The equilibrium constants of the whole cells and purified enzyme were calculated using the Haldane equation (7).
Measurement of transport rates for uptake and release.
The rates of galactose and tagatose uptake into cells were determined using the ΔgalK strain without pTaraA. Ten milliliters of cells suspended in 50 mM Tris-HCl (pH 7.0) at 19 mg/ml was incubated with 5, 10, 15, 20, or 25 mM galactose or tagatose at 37°C for 5 min. The cell suspension was then rapidly washed twice at 15,000 × g for 30 s at 4°C to remove extracellular sugar. The concentrations of intracellular galactose and tagatose were measured after the cells were lysed with Turbolytic lysis solution (Genofocus).
To determine the cellular release rates of galactose and tagatose, 5 ml of cell suspension (19 mg/ml) was incubated for 1 h with 5, 10, 15, or 20 mM galactose and 2, 4, 6, or 8 mM tagatose. The cells were rapidly washed twice as described above and divided into two portions. One portion was lysed and used to determine the intracellular concentrations of galactose and tagatose, and the other portion was resuspended in 50 mM Tris-HCl buffer solution without the sugars. This cell suspension was incubated for 5 min and then centrifuged at 15,000 × g for 30 s at 4°C. The sugar concentrations of the supernatant were measured, and the intracellular concentrations were calibrated using the reported intracellular volume of 7.3 μl per 1010 E. coli cells (8). One milligram of cells used in this study corresponded to 1.9 × 109 cells.
Analytical methods.
Cell mass was determined using a calibration curve of absorbance at 600 nm versus dry cell weight. Protein concentrations were determined by the Bradford method using bovine serum albumin as a standard (3). The concentrations of galactose and tagatose were determined by high-performance liquid chromatography (SCL-10A; Shimadzu, Kyoto, Japan) with a refractive index detector (RID-10A, Shimadzu) on a BP-100 Ca2+ column (Benson Polymeric, Reno, NV). The column was eluted at 80°C with distilled water at a flow rate of 0.4 ml/min. Sugar concentrations of less than 10 mM were determined using an high-performance liquid chromatography instrument (Agilent 1100 series; Agilent, Palo Alto, CA) fitted with a Corona charged aerosol detector (ESA Biosciences, Chelmsford, MA) on a Shodex column (Showa Denko, Kawasaki, Japan). The column was eluted at 30°C with 70% acetonitrile at a flow rate of 1.0 ml/min.
RESULTS
Conversion of galactose to tagatose by the galK and ΔgalK strains.
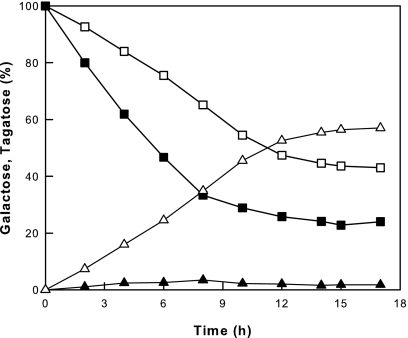
The ΔgalK strain harboring pTaraA did not grow in the minimal medium without glucose, and it converted little galactose to tagatose due to initially low cell mass. Thus, concentrated suspensions of E. coli MG1655 galK and ΔgalK strains harboring pTaraA were used in 50 mM Tris-HCl buffer (pH 7.0) containing 50 mM galactose and compared with respect to their ability to convert galactose to tagatose. The E. coli MG1655 galK+ strain gave a very low conversion yield of tagatose from galactose, whereas the ΔgalK strain gave a high yield owing to the lack of galactose degradation by galactose kinase (Fig. 1). Galactose kinase normally catalyzes the conversion of galactose to galactose-1-phosphate, which is subsequently converted to glucose-6-phosphate, a starting material for glycolysis (cell materials), by a two-step reaction of epimerase and phosphoglucomutase (23).
FIG. 1.
Conversion of galactose to tagatose by the galK and ΔgalK strains of E. coli MG1655 harboring pTaraA. The graph shows data for galactose and tagatose of the galK+ (▪ and ▴, respectively) and ΔgalK (□ and ▵, respectively) strains.
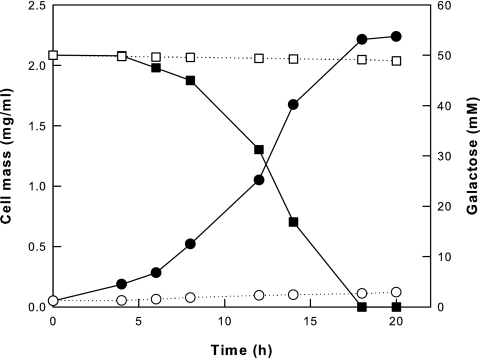
To investigate the utilization of galactose by the galK+ and ΔgalK strains, these strains without pTaraA were cultivated in M9 minimal broth containing 50 mM galactose as a carbon source rather than glucose. The galK+ strain grew well and completely consumed the galactose, whereas the ΔgalK strain grew poorly and consumed little galactose because of the absence of galactose kinase (Fig. 2).
FIG. 2.
Cell growth and galactose utilization by the galK and ΔgalK strains of E. coli MG1655 without pTaraA. Data are shown for the cell mass and galactose utilization of the galK+ (• and ▪, respectively) and ΔgalK (○ and □, respectively) strains.
Conversion of galactose to tagatose by whole cells and crude extract.
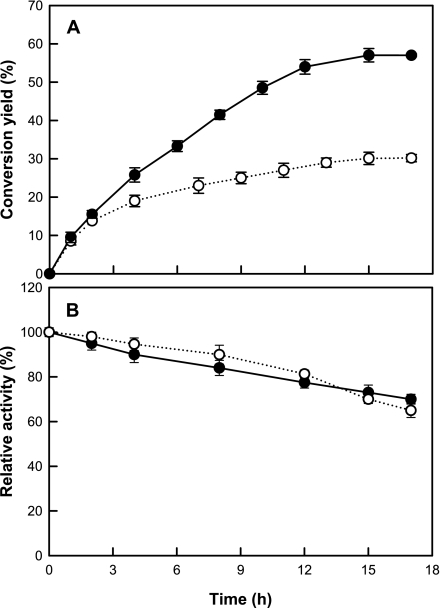
To investigate the effect of the cell membrane as a selective barrier on the conversion of galactose to tagatose, the isomerization reaction yields of whole cells and a crude extract were compared (Fig. 3A). The conversion yield of galactose to tagatose at 37°C was 57.0% in the ΔgalK strain harboring pTaraA and 30.2% in a crude extract of this strain. The stability, meaning the decrease of activity during the conversion, was similar for whole cells and the crude extract (Fig. 3B), suggesting that the difference in the conversion yield between the two was not due to a difference in stability. The high tagatose yield in whole cells (57%) compared with crude extract (30%) and purified enzyme (36%) suggests that the presence of the cell membrane affected the shift of equilibrium toward tagatose.
FIG. 3.
(A) Conversion of galactose to tagatose by whole cells and crude extract of the ΔgalK strain harboring pTaraA. (B) Activity decrease during conversion of galactose to tagatose by whole cells and crude extract of the ΔgalK strain harboring pTaraA. Data represent the means of three separate experiments. •, whole cells; ○, crude extract.
The intra- and extracellular equilibrium fractions of galactose and tagatose were measured using whole cells of the ΔgalK stain harboring pTaraA incubated in 10 mM sugars at three different initial ratios of galactose to tagatose (100:0, 50:50, and 0:100) (Fig. 4). The whole cells did not grow due to the absence of galactose kinase. The intra- and extracellular equilibrium fractions of tagatose converged at 25% and 68%, respectively, reflecting an extracellular equilibrium shift toward tagatose but an intracellular equilibrium shift toward galactose.
FIG. 4.
Intra- and extracellular equilibrium fractions of tagatose to total sugar in the ΔgalK strain harboring pTaraA. Three different initial ratios of galactose to tagatose were used: 100:0 (10 mM galactose; ○), 50:50 (5 mM each; ◑), and 0:100 (10 mM tagatose; •).
Equilibrium constants for conversion of galactose to tagatose by whole cells and purified enzyme.
The target spot on a 2D gel was identified as l-arabinose isomerase by mass analysis and comparison with purified l-arabinose isomerase (data not shown). The amount of intracellular l-arabinose isomerase in the ΔgalK strain harboring pTaraA, as determined by image analysis of a 2D gel, was 3.46 mg/g of cells. The cellular kcat value was calculated from the maximum velocity of the conversion reaction and the amount of intracellular l-arabinose isomerase. The kinetic parameters were measured using galactose concentrations ranging from 50 to 1,000 mM and tagatose concentrations ranging from 50 to 500 mM for whole cells and from 5 to 100 mM for the purified enzyme. High concentrations of galactose and tagatose were used for determining Km values because the authentic substrate and product for l-arabinose isomerase are not d-galactose and d-tagatose but l-arabinose and l-ribulose, respectively. The equilibrium constant (Keq) for whole cells and purified enzyme was determined using the Haldane equation: Keq = [T]eq/[G]eq = (kcat/Km)G/(kcat/Km)T, where G is galactose and T is tagatose (7) (Table 1). In whole cells, the Keq was 2.30, which corresponds to a predicted tagatose equilibrium fraction of 69.7%; the Keq for the purified enzyme was 0.53, which corresponds to a predicted tagatose equilibrium fraction of 34.6%. These predicted equilibrium fractions are within 2% of the experimental values obtained for whole cells (67.9%) and the purified enzyme (36.0%). The equilibrium shift toward tagatose associated with conversion in whole cells resulted primarily from increased catalytic efficiency (kcat/Km) for galactose owing to an increase in the kcat for galactose.
TABLE 1.
Equilibrium constants and kinetic parameters of cells and purified enzyme for galactose and tagatosea
| Catalyst and substrate | Km (mM) | kcat (min−1) | kcat/Km (min−1 mM−1) | Keq |
|---|---|---|---|---|
| Whole cells | ||||
| Galactose | 256 ± 3.8 | 253 ± 4.3 | 0.99 ± 0.022 | 2.30 ± 0.090 |
| Tagatose | 114 ± 3.2 | 49 ± 0.88 | 0.43 ± 0.014 | |
| Purified enzyme | ||||
| Galactose | 365 ± 9.3 | 62 ± 1.05 | 0.17 ± 0.005 | 0.53 ± 0.024 |
| Tagatose | 11 ± 0.28 | 3.5 ± 0.08 | 0.32 ± 0.011 |
Data represent the means of three separate experiments.
Uptake and release rates of galactose and tagatose.
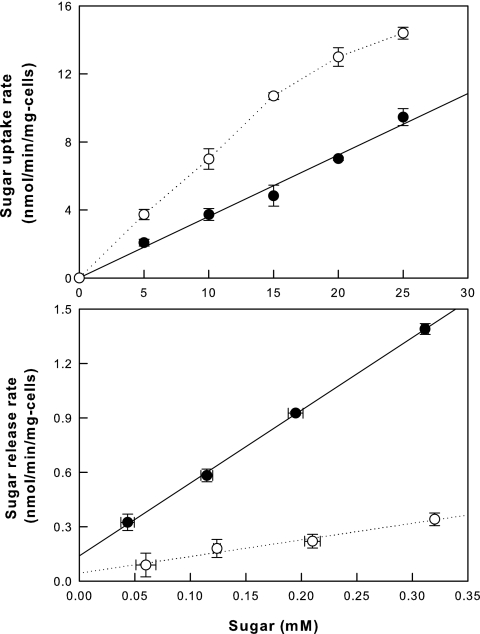
The uptake and release rates for galactose and tagatose can be measured in the ΔgalK strain without pTaraA. In this strain, the intra- and extracellular concentrations of the sugars are affected only by the sugar transport rates; no interconversion occurs between the two sugars in the absence of pTaraA, and the galK knockout prevents the metabolism of galactose and tagatose via galactose. The slopes of the linear regressions for uptake at 0 to 15 mM sugar and for release at 0 to 0.32 mM sugar were compared between galactose and tagatose in cells. The galactose uptake rate into cells was 2-fold the tagatose uptake rate, whereas the tagatose release rate was 4.4-fold the galactose release rate (Fig. 5). Thus, the extracellular equilibrium shift toward tagatose outside the cells was the result of its lower cellular uptake rate and higher cellular release rate relative to the rates of galactose.
FIG. 5.
Uptake and release rates of galactose (○) and tagatose (•) by the ΔgalK strain without pTaraA. Data represent the means of three separate experiments.
Transport systems for galactose and tagatose in cells.
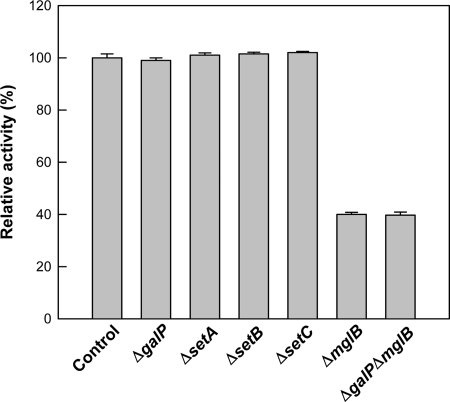
Deletions of SETs (ΔsetA, ΔsetB, and ΔsetC) and galactose transporters (ΔmglB and ΔgalP) were combined with the E. coli BW25113 ΔgalK strain harboring pTaraA. For the conversion reaction of galactose to tagatose, the resulting transporter knockout strains were examined. The conversion yields of galactose to tagatose in the ΔgalP, ΔsetA, ΔsetB, and ΔsetC mutants were almost the same as those in the original strain (BW25113 ΔgalK strain harboring pTaraA), which was used as the control (Fig. 6). The ΔmglB and ΔgalP ΔmglB mutants showed reduced conversion of galactose to tagatose (40%) compared with the control strain. The conversion yield in a ΔgalP ΔmglB double mutant was almost the same as that in the ΔmglB mutant, suggesting that GalP is not a critical transport system for galactose and tagatose. In the ΔmglB mutant without pTaraA, the uptake rates for galactose and tagatose were 26% and 25%, respectively, of those in the original strain without the transporter knockout (Fig. 5). Since the ΔmglB mutant exhibited significant decreases in conversion yield and uptake of galactose to tagatose, we conclude that the primary transport system for galactose and tagatose uptake is MglABC.
FIG. 6.
Relative conversion yields of galactose to tagatose in the E. coli BW25113 ΔgalK strain harboring pTaraA (control) and its ΔsetA, ΔsetB, ΔsetC, ΔgalP, ΔmglB, and ΔgalP ΔmglB mutants.
DISCUSSION
An increase in reaction temperature combined with use of a hyperthermophilic enzyme resulted in an equilibrium shift toward a tagatose product, resulting in a higher conversion yield (12). To shift the equilibrium position of an isomerization reaction toward tagatose using an alternative method, we exploited the membrane selectivity of cells resulting in intra- and extracellular concentration changes for a galactose substrate and tagatose product at equilibrium. The equilibrium shift toward tagatose was caused by the compartmentalization of intra- and extracellular regions of the cell membrane, with a lower cellular uptake rate relative to that of galactose and the higher cellular release rate for tagatose relative to that of galactose.
In whole cells of the ΔgalK strain, the isomerase-catalyzed reaction exhibited an equilibrium shift toward tagatose, producing a tagatose fraction of 68% at 37°C, whereas the purified l-arabinose isomerase gave a tagatose equilibrium fraction of 36%. These equilibrium fractions are close to those predicted from the measured equilibrium constants of the isomerization reaction catalyzed in whole cells and by the purified enzyme. The values of the equilibrium constant Keq were determined from the kinetic parameters for galactose and tagatose using the Haldane equation. Although the kinetic parameters were dependent on the sugar concentrations, the Keq values appear to be reasonable because the relative values of the kinetic parameters are used. The predicted equilibrium fractions obtained from the Keq values accorded well with the experimental values of equilibrium fractions.
MglABC is a β-methylgalactoside transport system that is a member of the ABC superfamily of transporters. The mglA, mglB, and mglC genes encode the ATP-binding, galactose-binding, and integral membrane components of the ABC transporter, respectively (9). Insertion mutations in each gene indicate that all three components are necessary for galactose transport function. Therefore, the ΔmglB mutant is functionally the same with respect to galactose transport as a mutant lacking the MglABC transporter. A different transporter, GalP, is a member of the major facilitator superfamily of transporters, which act as a major route for galactose transport (22), and SetA, SetB, and SetC are probable efflux transporters for sugars (21). The finding that the knockouts of GalP and SET transporters did not affect the cellular conversion of galactose to tagatose suggests that the MglABC transporter plays a major role in galactose uptake and that SET transporters have nothing to do with the release of galactose and tagatose. However, these results may be due to the continued operation of other unidentified transporters, similar to the MglABC transporter, in the mutants, thereby maintaining transport rates in the transporter knockout cells. The effect of the mglB gene knockout on both the galactose and tagatose uptake rates suggests that the MglABC transporter is a primary transport system for not only galactose but also tagatose, although its uptake rate for galactose is higher. The higher selectivity of the ATP-binding MglABC transporter for galactose over tagatose may be attributable to a higher preference for a common sugar over a rare sugar in cells using ATP.
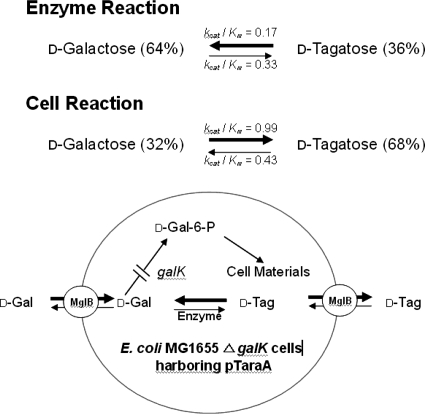
In the present study, the equilibrium shift was identified based on direct experimental evidence; it was also explained theoretically using equilibrium constants for a galactose substrate and tagatose product derived from the kinetic parameters of whole cells and the purified enzyme. Moreover, this study also demonstrated that MglABC is a primary transporter for tagatose as well as galactose in E. coli. The conversion of galactose to tagatose by whole cells compared with the purified enzyme was summarized as a schematic diagram (Fig. 7). Whole-cell conversion is proposed as a new alternative method for shifting the equilibrium of the isomerization reaction instead of using temperature to control enzymatic processes. If other sugar isomerization reactions converting a common sugar to a rare sugar were also to be shifted by changing membrane selectivity, the production of rare sugars could be improved.
FIG. 7.
A schematic diagram of the conversion from galactose to tagatose by the purified enzyme and the ΔgalK strain harboring pTaraA. For cells, the galactose uptake rate was 2.0-fold the tagatose uptake rate, whereas the tagatose release rate was 4.4-fold the galactose release rate.
Acknowledgments
This study was supported by the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory Program funded by the Ministry of Science and Technology (R0A-2007-000-20015-0) and MOST/KOSEF (Environmental Biotechnology National Core Research Center) (grant no. R15-2003-012-02001-0).
We are grateful to H. Mori (NAIST, Japan) for E. coli BW25113 transporter knockout mutants and to F. R. Blattner for the E. coli MG1655 ΔgalK mutant.
Footnotes
Published ahead of print on 8 February 2008.
REFERENCES
- 1.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Systems Biol. 2:2006.2008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek, D. H., Y. Lee, H. S. Sin, and D. K. Oh. 2004. A new thermophile strain of Geobacillus thermodenitrificans having l-arabinose isomerase activity for tagatose production. J. Microbiol. Biotechnol. 14:312-316. [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Chang, C., B. C. Park, D. S. Lee, and S. W. Suh. 1999. Crystal structure of thermostable xylose isomerases from Thermus caldophilus and Thermus thermophilus: possible structural determinants of thermostability. J. Mol. Biol. 288:623-634. [DOI] [PubMed] [Google Scholar]
- 5.Daruwalla, K. R., A. T. Paxton, and P. J. Henderson. 1981. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem. J. 200:611-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fersht, A. 1999. Structure and mechanism in protein science: a guide to enzyme catalysis and protein folding. W. H. Freeman and Co., New York, NY.
- 8.Gunsalus, R. P., A. G. Miguel, and G. L. Gunsalus. 1986. Intracellular Trp repressor levels in Escherichia coli. J. Bacteriol. 167:272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harayama, S., J. Bollinger, T. Iino, and G. L. Hazelbauer. 1983. Characterization of the mgl operon of Escherichia coli by transposon mutagenesis and molecular cloning. J. Bacteriol. 153:408-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgensen, F., O. C. Hansen, and P. Stougaard. 2004. Enzymatic conversion of d-galactose to d-tagatose: heterologous expression and characterisation of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 64:816-822. [DOI] [PubMed] [Google Scholar]
- 11.Jung, E. S., H. J. Kim, and D. K. Oh. 2005. Tagatose production by immobilized recombinant Escherichia coli cells containing Geobacillus stearothermophilus l-arabinose isomerase mutant in a packed-bed bioreactor. Biotechnol. Prog. 21:1335-1340. [DOI] [PubMed] [Google Scholar]
- 12.Kim, B. C., Y. H. Lee, H. S. Lee, D. W. Lee, E. A. Choe, and Y. R. Pyun. 2002. Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiol. Lett. 212:121-126. [DOI] [PubMed] [Google Scholar]
- 13.Kim, H. J., J. H. Kim, H. J. Oh, and D. K. Oh. 2006. Characterization of a mutated Geobacillus stearothermophilus l-arabinose isomerase that increases the production rate of d-tagatose. J. Appl. Microbiol. 101:213-221. [DOI] [PubMed] [Google Scholar]
- 14.Kim, H. J., and D. K. Oh. 2005. Purification and characterization of an l-arabinose isomerase from an isolated strain of Geobacillus thermodenitrificans producing d-tagatose. J. Biotechnol. 120:162-173. [DOI] [PubMed] [Google Scholar]
- 15.Kim, H. J., S. A. Ryu, P. Kim, and D. K. Oh. 2003. A feasible enzymatic process for d-tagatose production by an immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor. Biotechnol. Prog. 19:400-404. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. W., Y. W. Kim, H. J. Roh, H. Y. Kim, J. H. Cha, K. H. Park, and C. S. Park. 2003. Production of tagatose by a recombinant thermostable l-arabinose isomerase from Thermus sp. IM6501. Biotechnol. Lett. 25:963-967. [DOI] [PubMed] [Google Scholar]
- 17.Lee, D. W., E. A. Choe, S. B. Kim, S. H. Eom, Y. H. Hong, S. J. Lee, H. S. Lee, D. Y. Lee, and Y. R. Pyun. 2005. Distinct metal dependence for catalytic and structural functions in the l-arabinose isomerases from the mesophilic Bacillus halodurans and the thermophilic Geobacillus stearothermophilus. Arch. Biochem. Biophys. 434:333-343. [DOI] [PubMed] [Google Scholar]
- 18.Lee, D. W., H. J. Jang, E. A. Choe, B. C. Kim, S. J. Lee, S. B. Kim, Y. H. Hong, and Y. R. Pyun. 2004. Characterization of a thermostable l-arabinose (d-galactose) isomerase from the hyperthermophilic eubacterium Thermotoga maritima. Appl. Environ. Microbiol. 70:1397-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, S. J., D. W. Lee, E. A. Choe, Y. H. Hong, S. B. Kim, B. C. Kim, and Y. R. Pyun. 2005. Characterization of a thermoacidophilic l-arabinose isomerase from Alicyclobacillus acidocaldarius: role of Lys-269 in pH optimum. Appl. Environ. Microbiol. 71:7888-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin, G. V. 2002. Tagatose, the new GRAS sweetener and health product. J. Med. Food 5:23-36. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J. Y., P. F. Miller, J. Willard, and E. R. Olson. 1999. Functional and biochemical characterization of Escherichia coli sugar efflux transporters. J. Biol. Chem. 274:22977-22984. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson, A. J. S., M. C. Jones-Mortimer, P. Horne, and P. J. Henderson. 1983. Identification of the GalP galactose transport protein of Escherichia coli. J. Biol. Chem. 258:4390-4396. [PubMed] [Google Scholar]
- 23.Nelson, D. L., and M. M. Cox. 2004. Lehninger principles of biochemistry, 4th ed. Freeman, New York, NY.
- 24.Oh, D. K., H. J. Kim, S. A. Ryu, and P. Kim. 2001. Development of an immobilization method of l-arabinose isomerase for industrial production of tagatose. Biotechnol. Lett. 23:1859-1862. [Google Scholar]
- 25.Oh, H. J., H. J. Kim, and D. K. Oh. 2006. Increase in d-tagatose production rate by site-directed mutagenesis of l-arabinose isomerase from Geobacillus thermodenitrificans. Biotechnol. Lett. 28:145-149. [DOI] [PubMed] [Google Scholar]
- 26.Rhimi, M., and S. Bejar. 2006. Cloning, purification and biochemical characterization of metallic ion independent and thermoactive l-arabinose isomerase from the Bacillus stearothermophilus US100 strain. Biochim. Biophys. Acta 1760:191-199. [DOI] [PubMed] [Google Scholar]
- 27.Roh, H. J., P. Kim, Y. C. Park, and J. H. Choi. 2000. Bioconversion of d-galactose into d-tagatose by expression of l-arabinose isomerase. Biotechnol. Appl. Biochem. 31:1-4. [DOI] [PubMed] [Google Scholar]
- 28.Ryu, S. A., C. S. Kim, H. J. Kim, D. H. Baek, and D. K. Oh. 2003. Continuous d-tagatose production by immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor. Biotechnol. Prog. 19:1643-1647. [DOI] [PubMed] [Google Scholar]
- 29.Wu, L. F., and M. A. Mandrand-Berthelot. 1995. A family of homologous substrate-binding proteins with a broad of substrate specificity and dissimilar biological functions. Biochimie 77:744-750. [DOI] [PubMed] [Google Scholar]
- 30.Yoon, S. H., P. Kim, and D. K. Oh. 2003. Properties of l-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World J. Microbiol. Biotechnol. 19:47-51. [Google Scholar]