Abstract
A single-chamber microbial fuel cell (MFC) was used to reduce 10 chemicals associated with odors by 99.76% (from 422 ± 23 μg/ml) and three volatile organic acids (acetate, butyrate, and propionate) by >99%. The MFC produced a maximum of 228 mW/m2 and removed 84% of the organic matter in 260 h. MFCs were therefore effective at both treatment and electricity generation.
Swine wastewater treatment and odor control are important components for sustainable animal production. Stricter regulations of the livestock industry that require both effective treatment and odor control are being enacted (24). Aerobic treatment of animal wastewaters can be quite costly and does not generate useful products. Anaerobic treatment can be used to generate methane gas, but ammonia and odor-producing chemicals are not fully removed. Thus, while such treatment methods can be effective, they can still be energy demanding or result in generation of nuisance odors (4, 13).
It was recently shown that volatile organic acids (VAs), commonly associated with odors from animal wastewaters, could be controlled by stimulating dissimilatory iron reduction (3, 7). The addition of ferric iron supported anaerobic respiration, increased the reduction rate of VAs (used as indicators of malodorous chemicals), and enhanced methane production by 200% (7). The dominant bacterial groups were identified as Desulfitobacterium spp. based on 16S rRNA gene amplicons from DNA sequences in one study, while in the other study, several isolates that were all members of the Geobacteraceae family were obtained (3, 7). Bioaugmentation with Geobacter sp. strain NU, obtained from a waste lagoon, and ferric iron accomplished nearly complete odor removal compared to what was found for unamended controls (7). While the use of Fe(III) may be an effective approach for odor removal, the stimulation of methane production (a potent greenhouse gas) in lagoons and the need to achieve adequate concentrations of poorly dissolving iron in these wastewaters may limit the application of this approach.
Bacteria capable of dissimilatory metal reduction have been shown to generate electricity in microbial fuel cells (MFCs), likely a result of the fact that in both processes electrons are transported to a solid surface (1, 20). It was therefore hypothesized that an MFC could be an effective method of odor control and electricity generation. It was previously demonstrated that electricity could be produced using swine wastewater in an MFC (21), but the removal of specific chemicals associated with odors was not previously examined using actual wastewaters. In an MFC, bacteria oxidize organic substrates and transfer electrons to the anode, resulting in current flow to the cathode, where electrons and protons combine with oxygen to produce water. VAs and different types of organic matter have been shown to support electricity generation in MFCs (18). Analysis of the anodic biofilms has shown that many of the exoelectrogenic bacteria in an MFC are of dissimilatory iron-reducing strains (3, 7), and MFC tests have shown that Shewanella oneidensis MR-1 and several Geobacter spp. can generate electricity in an MFC (1, 2, 9, 12). Thus, bacteria capable of iron reduction that have been found to be important for odor removal from swine wastewater are also the same types shown to be capable of electricity production.
In this study, we examined the removal of several different aliphatic and aromatic hydrocarbons, including several VAs, phenolic compounds, and indoles, all of which are known to contribute to nuisance chemical odors from swine wastewater. Removal of these chemicals was compared to that in the same MFC operated in an open-circuit mode (no current generation) as well as in a completely sealed reactor (anaerobic control) over the same period of time.
Swine manure wastewater was obtained from the Swine Research Facility at Penn State University (University Park, PA). The raw wastewater was passed through a sieve (0.25-mm mesh) and stored in a refrigerator at 4°C prior to use. The wastewater was used as both the inoculum and the substrate for all MFC tests without any modifications, such as pH adjustment or addition of nutrients, trace metals, or buffers. The bacteria in the MFC were first enriched for 10 days by using full-strength wastewater. The feed was then switched to wastewater diluted with ultrapure water (1:1) (Milli-Q system; Millipore Corp., New Bedford, MA) in order to reduce the time needed for a complete cycle of power generation in the reactor.
All tests except the sealed-bottle control were conducted using cube-shaped air cathode MFCs constructed as previously described (16). The anode (carbon paper; E-Tek Inc., NJ) and cathode (carbon paper with 0.35 mg Pt/cm2) were connected via an external circuit containing a single resistor (external resistance, 1,000 Ω; closed-circuit operation). This resistance was the same as that used in a previous MFC study using swine wastewater, and at this resistance, power production was close to the maximum (21). The cathode was coated with polytetrafluoroethylene (Sigma-Aldrich, St. Louis, MO) to limit water evaporation as previously described (21). Additional MFCs were operated in open-circuit mode (i.e., without an external circuit) to directly investigate the effect of current flow on the change in chemical concentrations. A sample was incubated in a sealed serum bottle (165 ml, no headspace) for the same period of time as a fully anaerobic control. All experiments were conducted in a temperature-controlled room at 30°C.
Seven VAs (propionic, butyric, isobutyric, valeric, isovaleric, caproic, and isocaproic acids), three phenolic compounds (p-cresol, p-ethylphenol, and phenol), and two indoles (skatole and indole) were used as odor indicators (3, 10, 24, 25). Samples were obtained for chemical analysis at the end of a fed-batch cycle (i.e., when the voltage decreased to <120 mV). Samples (10 ml of each) were acidified with 2.0 ml of 1.0 M HCl, and a layer of diethyl ether (2.5 ml; Sigma-Aldrich, St. Louis, MO) was gently placed on the surface before incubating the samples at 4°C for 4 h for liquid-liquid extraction. The aliquots of diethyl ether extracts were analyzed using a gas chromatograph (Hewlett-Packard 5890 Series II with an HP G1030A ChemStation controller) equipped with a flame ionization detector (10). Acetate, butyrate, and propionate in liquid were separately analyzed using a different gas chromatograph (Agilent 6890) equipped with a flame ionization detector and a DB-FFAP fused-silica capillary column (14). Soluble chemical oxygen demand (sCOD) was measured as previously described (21).
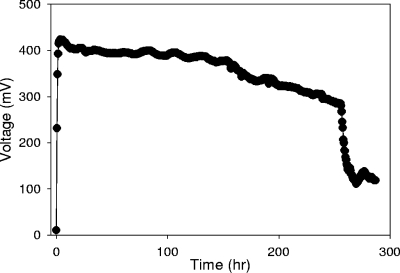
Power production in the MFC reached a maximum of 228 mW/m2 (maximum of 0.4 V) over a typical cycle (Fig. 1). The end of a treatment cycle is indicated by the sharp decrease in voltage at 250 h. There was 84% removal of sCOD for the wastewater originally containing 8,270 ± 120 mg/liter of COD in the electricity-producing MFC (final concentration, 1,320 ± 30 mg/liter; sCOD removal rate, 0.023 kg sCOD/liter/day), compared to 53% removal for the open-circuit MFC (3,860 ± 50 mg/liter, 0.015 kg sCOD/liter/day) and 5.7% removal in the sealed-bottle control (7,800 ± 140 mg/liter, 0.002 kg sCOD/liter/day).
FIG. 1.
Voltage generation of single-chamber MFCs using 1/2-diluted animal wastewater.
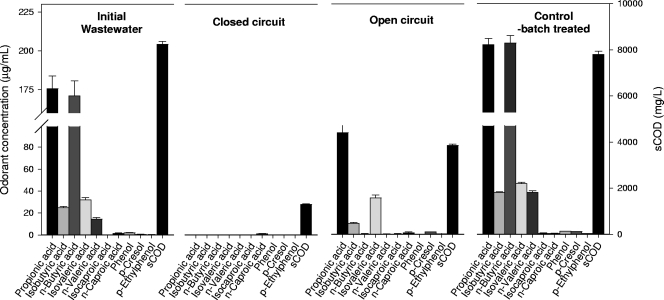
The main odor-producing compounds initially present in the wastewater based on mass concentrations were propionic acid (176 ± 8 μg/ml), isobutyric acid (25 ± 8 μg/ml), n-butyric acid (171 ± 10 μg/ml), and n-valeric acid (14 ± 1 μg/ml), with a total odorant concentration of all species of 422 ± 23 μg/ml. Skatole and indole were not detected. The other compounds were found to be relatively low in concentration (<2 μg/ml) (Fig. 2).
FIG. 2.
Odor component and sCOD removals in single-chamber MFCs with swine wastewater after 260 h of treatment. Error bars represent standard deviations based on analysis of samples in triplicate. Indole and skatole were not detected.
Following a complete cycle of treatment (260 h), the total odorant concentration was reduced by 99.76%, to 1 ± 0 μg/ml, in the closed-circuit MFC. In contrast, the odorant concentration in the completely sealed bottle reactor increased by 28%, to 539 ± 13 μg/ml, over the same period of time. This sealed bottle reactor shows the outcome produced when the sample is kept under completely anaerobic conditions. The increase in odorant concentration was likely a result of fermentation of organic matter to VAs (11, 26). The odorant concentration decreased to 141 ± 12 μg/ml in the open-circuit MFC, likely as a result of aerobic biochemical degradation sustained by the diffusion of oxygen into the wastewater sample through the air cathode.
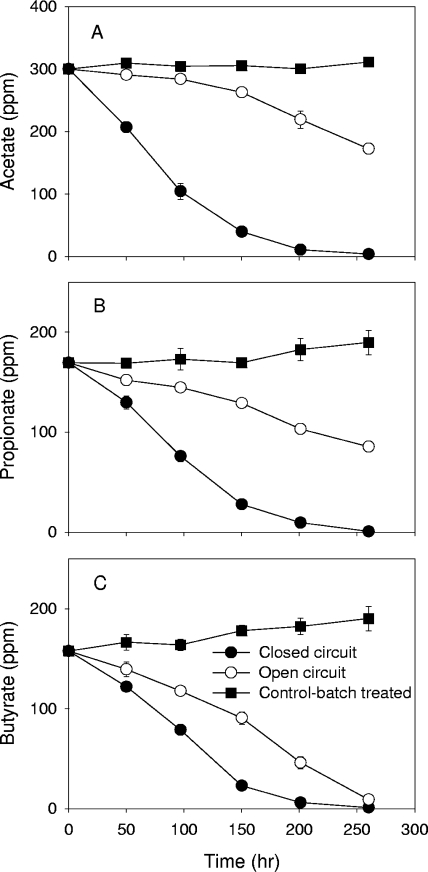
Consumption of low-molecular-weight VAs, including acetate, butyrate, and propionate, was investigated over a complete cycle of operation as shown in Fig. 3. Acetate degradation in the closed-circuit MFC was faster than that in the open-circuit MFC, while it was essentially constant in the closed-bottle control. The concentrations of the other two VAs (butyrate and propionate) increased in the closed-bottle control and were removed more slowly in the open-circuit MFC.
FIG. 3.
Acetate (A), propionate (B), and butyrate (C) consumption in single-chamber MFCs with 1/2-diluted swine wastewater. Error bars represent standard deviations based on analysis of samples in triplicate.
Acetate has been known to be the primary electron donor for Fe(III) in anoxic environments (7, 19, 20), and therefore, it is expected to be a preferable substrate in MFCs compared to other VAs. MFCs using acetate as a substrate have been studied by several groups (1, 6, 15, 17), and in general, power output using acetate is higher than that achieved with other substrates in the same physical system (i.e., in MFCs with the same architecture and inoculum) as long as the system's internal resistance is low enough to observe these differences, which is true for the system examined here (18). Thus, our observation that consumption of long-chain VAs was more rapid than that of the other VAs examined is consistent with these results. Acetate accumulation can inhibit acetate degradation due to end product inhibition, however, if acetate is not removed at a sufficient rate by acetate-consuming bacteria (8, 11, 22, 23).
These results demonstrate that it is possible to accelerate the rate of odor removal using MFCs, while at the same time removing organic matter and producing electricity. Further optimization of MFCs is needed, however, to increase the removal rates of odorants as well as to increase treatment efficiency (e.g., sCOD removal). In addition, the bioaugmentation of MFCs by use of specific strains of iron-reducing bacteria, such as strain NU, may help increase treatment efficiency. Recent advances made in increasing power output in MFCs (5), as well as using scalable architectures (17, 27), will be helpful in improving performance and allowing the development of larger systems.
Acknowledgments
We thank David W. Jones for the gas chromatography analysis of acetate, propionate, and butyrate.
This research was supported by grant 68-3A75-3-150 from the Natural Resources Conservation Service of the United States Department of Agriculture and the Paul L. Busch Award to B.E.L.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretschger, O., A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B.-H. Kim, J. K. Fredrickson, and K. H. Nealson. 2007. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73:7003-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo-Gonzalez, H. A., and M. A. Bruns. 2005. Dissimilatory iron reduction and odor indicator abatement by biofilm communities in swine manure microcosms. Appl. Environ. Microbiol. 71:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, M., J.-H. Kim, N. Kishida, O. Nishimura, and R. Sudo. 2004. Enhanced nitrogen removal using C/N load adjustment and real-time control strategy in sequencing batch reactors for swine wastewater treatment. Water Sci. Technol. 49:309-314. [PubMed] [Google Scholar]
- 5.Cheng, S., and B. E. Logan. 2007. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 9:492-496. [Google Scholar]
- 6.Clauwaert, P., K. Rabaey, P. Aelterman, L. DeSchamphelaire, T. H. Pham, P. Boeckx, N. Boon, and W. Verstraete. 2007. Biological denitrification in microbial fuel cells. Environ. Sci. Technol. 41:3354-3360. [DOI] [PubMed] [Google Scholar]
- 7.Coates, J. D., K. A. Cole, U. Michaelidou, J. Patrick, M. J. McInerney, and L. A. Achenbach. 2005. Biological control of hog waste odor through stimulated microbial Fe(III) reduction. Appl. Environ. Microbiol. 71:4728-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuzaki, S., N. Nishio, M. Shobayashi, and S. Nagai. 1990. Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl. Environ. Microbiol. 56:719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorby, Y. A., S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govere, E. M., M. Tonegawa, M. A. Bruns, E. F. Wheeler, P. H. Heinemann, K. B. Kephart, and J. Dec. 2005. Deodorization of swine manure using minced horseradish roots and peroxides. J. Agric. Food Chem. 53:4880-4889. [DOI] [PubMed] [Google Scholar]
- 11.Horn, M. A., C. Matthies, K. Kusel, A. Schramm, and H. L. Drake. 2003. Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat. Appl. Environ. Microbiol. 69:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, B. H., H. J. Kim, M. S. Hyun, and D. H. Park. 1999. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 13.Kim, J.-H., M. Chen, N. Kishida, and R. Sudo. 2004. Integrated real-time control strategy for nitrogen removal in swine wastewater treatment using sequencing batch reactors. Water Res. 38:3340-3348. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. R., B. Min, and B. E. Logan. 2005. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl. Microbiol. Biotechnol. 68:23-30. [DOI] [PubMed] [Google Scholar]
- 15.Liu, H., S. Cheng, and B. E. Logan. 2005. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 39:658-662. [DOI] [PubMed] [Google Scholar]
- 16.Liu, H., and B. E. Logan. 2004. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 38:4040-4046. [DOI] [PubMed] [Google Scholar]
- 17.Logan, B., S. Cheng, V. Watson, and G. Estadt. 2007. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 41:3341-3346. [DOI] [PubMed] [Google Scholar]
- 18.Logan, B. E., B. Hamelers, R. Rozendal, U. Schroeder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete, and K. Rabaey. 2006. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40:5181-5192. [DOI] [PubMed] [Google Scholar]
- 19.Lovley, D. R. 2000. Fe(III) and Mn(IV) reduction, p. 3-30. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, DC.
- 20.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min, B., J. R. Kim, S. Oh, J. M. Regan, and B. E. Logan. 2005. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 39:4961-4968. [DOI] [PubMed] [Google Scholar]
- 22.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voolapalli, R. K., and D. C. Stuckey. 1999. Relative importance of trophic group concentrations during anaerobic degradation of volatile fatty acids. Appl. Environ. Microbiol. 65:5009-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahn, J. A., J. L. Hatfield, Y. S. Do, A. A. DiSpirito, D. A. Laird, and R. L. Pfeiffer. 1997. Characterization of volatile organic emissions and wastes from a swine production facility. J. Environ. Qual. 26:1687-1696. [Google Scholar]
- 25.Zhu, J., G. L. Riskowski, and M. Torremorell. 1999. Volatile fatty acids as odor indicators in swine manure—a critical review. Trans. Am. Soc. Agric. Eng. 42:175-182. [Google Scholar]
- 26.Zou, B.-Z., K. Takeda, A. Tonouchi, S. Akada, and T. Fujita. 2003. Characteristics of an anaerobic, syntrophic, butyrate-degrading bacterium in paddy field soil. Biosci. Biotechnol. Biochem. 67:2059-2067. [DOI] [PubMed] [Google Scholar]
- 27.Zuo, Y., S. Cheng, D. Call, and B. E. Logan. 2007. Tubular membrane cathodes for scalable power generation in microbial fuel cells. Environ. Sci. Technol. 41:3347-3353. [DOI] [PubMed] [Google Scholar]