Abstract
The increased serum survival gene iss has long been recognized for its role in extraintestinal pathogenic Escherichia coli (ExPEC) virulence. iss has been identified as a distinguishing trait of avian ExPEC but not of human ExPEC. This gene has been localized to large virulence plasmids and shares strong similarities with the bor gene from bacteriophage λ. Here, we demonstrate that three alleles of iss occur among E. coli isolates that appear to have evolved from a common λ bor precursor. In addition to the occurrence of iss on the ColV/BM virulence plasmids, at least two iss alleles occur within the E. coli chromosome. One of these alleles (designated type 3) was found to occur in the genomes of all currently sequenced ExPEC strains on a similar prophage element that also harbors the Sit iron and manganese transport system. When the prevalence of the three iss types was examined among 487 E. coli isolates, the iss type 3 gene was found to occur at a high frequency among ExPEC isolates, irrespective of the host source. The plasmid-borne iss allele (designated type 1) was highly prevalent among avian pathogenic E. coli and neonatal meningitis-associated E. coli isolates but not among uropathogenic E. coli isolates. This study demonstrates the evolution of iss in E. coli and provides an additional tool for discriminating among E. coli pathotypes through the differentiation of the three iss allele types and bor.
Extraintestinal pathogenic Escherichia coli (ExPEC) is one of the most diverse Escherichia coli pathotypes, with members that cause a variety of diseases in both humans and animals (50). Because of its diversity, ExPEC is further divided into several subpathotypes, including uropathogenic E. coli (UPEC), neonatal meningitis-associated E. coli (NMEC), and avian pathogenic E. coli (APEC). ExPEC strains contain a variety of virulence factors, and it has been suggested that a mix and match of these traits results in the ability of an ExPEC strain to cause disease (16). In fact, efforts to identify universal ExPEC virulence factors, present among all ExPEC strains but absent in other E. coli pathotypes or commensal strains, have proved mostly futile (2, 9, 30). One promising virulence gene in this regard is the increased serum survival gene, iss, identified as significantly more associated (P < 0.0001) with the APEC strains than with fecal isolates from healthy birds (44, 46) and found to occur in around 60% of UPEC and NMEC strains (21, 46) but present in few of the human fecal commensal E. coli isolates examined (T. J. Johnson, unpublished data). iss was previously localized to pathogenicity islands (PAIs) occurring on colicin-encoding (ColV/BM) plasmids, which commonly occur among APEC strains (33, 35). Since such plasmids are less common among human ExPEC strains (21, 46), the location of iss within the human ExPEC genome is unclear.
The iss gene was first identified in a human septicemic E. coli isolate (6, 7) and was associated with a 20-fold increase in complement resistance and a 100-fold increase in virulence toward 1-day-old chicks (6, 7, 12). The Iss protein from an APEC isolate has been purified and expressed (23), has had monoclonal antibodies made against it (22), and has been shown to protect against homologous and heterologous APEC challenges in birds (38). iss shares nucleotide and predicted protein similarities with the phage λ gene bor, whose product is also an outer-membrane lipoprotein involved in serum resistance (3). Iss and Bor were both found to be surface-exposed proteins (40). Similarly, both of the iss and bor mutant strains were attenuated in their abilities to resist the killing effects of host complement (39). However, unlike iss, bor is present on a cryptic prophage within the chromosome of many types of E. coli, pathogenic and nonpathogenic alike (8, 10, 11, 34, 43, 56). The iss sequence was first described by Chuba et al. and was identified on large transmissible plasmids (6, 7, 12). Recent plasmid genome sequencing efforts have localized iss to the highly conserved region of a ColV/BM-encoded PAI (33, 35), thus making it a useful marker of these loci.
Speculation as to the evolutionary relationship between iss and bor has been presented, but no further studies have explored this relationship (3, 27). Several recent studies have identified iss as occurring commonly among APEC isolates, associated with the presence of a ColV/BM virulence plasmid (19, 21, 28, 44, 47, 57, 58). However, some recent studies involving human E. coli have reported conflicting results regarding the prevalence of iss among different E. coli populations (1, 5, 20, 46, 47, 53). Using iss as a marker for ExPEC, Bekal et al. reported that the false-positive reactions they obtained for iss could be attributed to its homology with bor (5). Anjum et al. (1) recently identified UPEC CFT073 as possessing iss, even though its annotation listed no such presence (56) and the cited GenBank reference actually described iss from an APEC isolate (27). In an effort to resolve these discrepancies and to avoid future errors in the detection of iss, we have performed a more comprehensive comparison of iss and bor.
MATERIALS AND METHODS
Bacterial strains used in this study.
A total of 487 bacterial strains were used in this study. These isolates included 91 NMEC strains (31), 91 UPEC strains (46), 91 APEC strains (44, 46, 47), 30 strains isolated from cases of bovine septicemia (necrotoxigenic E. coli [NTEC]), 92 strains isolated from the feces of healthy chickens and turkeys (44), and 92 strains isolated from the feces of healthy humans. All bacterial isolates were stored in glycerol at −80°C until used.
Analysis of iss- and bor-containing regions.
The genomes of 13 available E. coli sequences were searched for the presence of bor or iss, to determine the number of copies and location(s) in the chromosome. These sequences were extracted in silico from the 13 chromosomes and from available plasmid and phage sequences in the NCBI database. Nucleotide sequences were aligned using a ClustalW algorithm with DNASTAR software (Lasergene, Madison, WI). A phylogenetic tree was constructed based upon the iss or the bor nucleotide sequence. Additionally, a 1,700-bp sequence surrounding iss or bor was extracted from each of the available genomes and plasmids and aligned using a ClustalW algorithm (Lasergene software package). The nucleotide homology of each sequence, compared to the 1,700-bp region from pAPEC-O1-ColBM (33), was determined for every 100 bp within the 1,700-bp sequence. Phage regions were aligned using Mauve software (15).
PCR prevalence of iss and bor.
Sequence analysis revealed that the E. coli genome contains at least three iss alleles. The prevalence of these alleles and the bor gene was determined among 487 isolates, using two multiplex reactions, screening for iss type 1 and bor (panel 1) and iss types 2 and 3 (panel 2) (Table 1). Templates were prepared as previously described (32). Reactions were performed in 25-μl reaction mixtures using 4 mM MgCl2, 0.125 mM of each deoxynucleoside triphosphate, 0.3 μM each primer, and 1.25 U Amplitaq Gold (Applied Biosystems, Inc.). Cycling parameters were 94°C for 5 min, and then 25 cycles of 94°C for 30 s, 94°C for 30 s, and 94°C for 3 min, with a final extension at 72°C for 10 min.
TABLE 1.
Primers used in PCR studies
| Gene | Forward (F) and reverse (R) primers | Amplicon size (bp) | Multiplex allele detection panel |
|---|---|---|---|
| iss type 1 | F 5′-CAGCAACCCGAACCACTTGATG-3′ | 323 | 1 |
| R 5′-TTCTGCCGCTCTGGCAATGCT-3′ | |||
| iss type 2 | F 5′-GGTAACCCTGCGTCTGTCAGCACA-3′ | 581 | 2 |
| R 5′-CCAGCGGAGTATAAATGCCTAAAG-3′a | |||
| iss type 3 | F 5′-GTCCCCAACTTCCTCCAATAGTCT-3′ | 390 | 2 |
| R 5′-CCAGCGGAGTATAAATGCCTAAAG-3′a | |||
| bor | F 5′-CCCGTCAGGGCTGTGGACATAGTT-3′ | 201 | 1 |
| R 5′-GGGCCAGCGCAGTAGCGAGTAG-3′ |
The same reverse primer was used for iss types 2 and 3.
Phylogenetic typing.
All isolates were examined for the presence of three amplicons corresponding to phylogenetic types A, B1, B2, and D, according to the methods described by Clermont et al. (13).
Biostatistics.
Fisher's exact test was used to compare differences in prevalence among the populations studied. A P value of less than 0.05 was considered statistically significant.
RESULTS
At least three iss alleles exist in the E. coli strains.
Our analyses of the regions annotated as Bor in ExPEC strains revealed discrepancies in the annotations (Table 2). In CFT073 and UTI89, the gene is annotated as a 342-base-pair gene with a product description of a Bor protein homolog. The UPEC 536 annotation describes a 294-bp gene as a Bor protein precursor. The APEC O1 annotation describes a 309-bp gene as Bor. However, a reannotation performed here revealed that the best gene prediction and annotation of this coding region, in all cases, is a 309-bp gene that should be described as iss.
TABLE 2.
iss and bor in sequenced genomes
| Organism genome or plasmid | Start position (bp) | Annotated protein description | Annotated gene length (bp) | Reannotated gene size (bp) | Reannotated gene |
|---|---|---|---|---|---|
| E. coli K-12 MG1655 | 578116 | Bacteriophage λ Bor protein | 294 | 294 | bor |
| E. coli O157:H7 | 1359194 | ||||
| EDL933 | 1712116 | Putative Bor protein precursor | 294 | 294 | bor |
| UPEC CFT073 | 1423221 | Bor protein homolog | 342 | 309 | iss type 3 |
| UPEC 536 | 1205810 | Bor protein precursor | 294 | 309 | iss type 3 |
| UPEC UTI89 | 1259756 | Bor-like protein | 342 | 309 | iss type 3 |
| APEC O1 | 1197110 | λ prophage Bor protein | 309 | 309 | iss type 3 |
| pAPEC-O1-ColBM | 126545 | Increased-serum-survival protein | 309 | 309 | iss type 1 |
| pAPEC-O2-ColV | 137931 | Increased-serum-survival protein | 309 | 309 | iss type 1 |
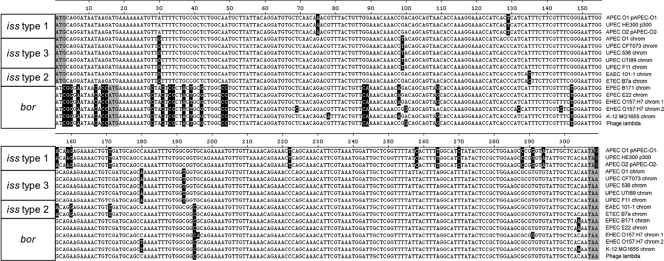
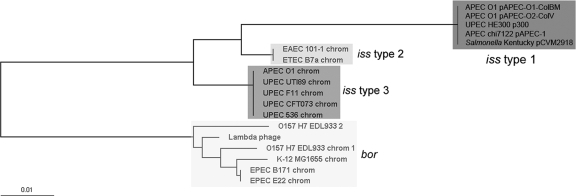
A ClustalW alignment of all of the available iss and bor sequences revealed three genetically distinct alleles of iss, which we have designated iss types 1 to 3 (Fig. 1 and 2). Compared to the plasmid-borne iss type 1 that was described previously in the literature, types 2 and 3 are 94.2% and 95.5% similar, respectively (Table 3). iss type 3, previously annotated as bor, occurs on a prophage in the chromosomes of all sequenced ExPEC strains. iss type 2 was found on the chromosomes of draft genome sequences of enteroaggregative E. coli (EAEC) strain 101-1 and enterotoxigenic E. coli (ETEC) strain B7a (Fig. 3). This the first report of the presence of iss on the bacterial chromosome. The bor gene was identified only within the complete chromosomes of the E. coli K-12 and EHEC O157:H7 strains.
FIG. 1.
Alignment of the iss and bor nucleotide sequences. The three different iss types and bor are identified based upon nucleotide and amino acid differences. Black shading indicates those nucleotides that are different from the consensus sequence, and gray shading indicates the start and stop codons for either bor or iss.
FIG. 2.
Phylogenetic tree based upon iss and bor alignment. Tree displays the three iss types (1, dark gray; 2, light gray; 3, medium gray) and bor based upon nucleotide differences.
TABLE 3.
Similarities between iss and bor
| Gene | Location | Nucleotide differences | % Nucleotide difference | Amino acid differences | % Amino acid difference |
|---|---|---|---|---|---|
| iss type 1 | ColV/BM plasmids | 0 | 100 | 0 | 100 |
| iss type 2 | Chromosome | 18 | 94.2 | 3 | 97.1 |
| iss type 3 | ExPEC chromosome | 14 | 95.5 | 2 | 98.1 |
| bor | Chromosome | 36 | 88.4 | 11 | 88.8 |
FIG. 3.
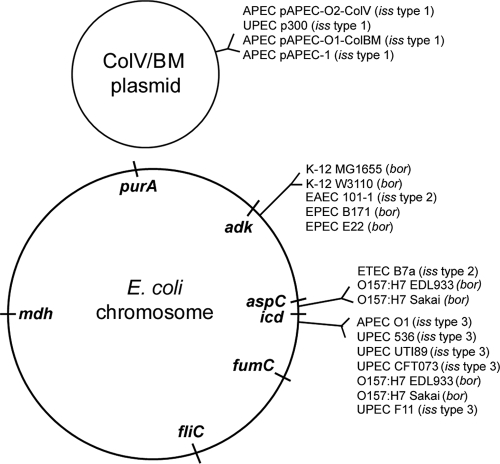
Locations of iss and bor in the E. coli chromosome. Housekeeping genes were used as markers of the locations of bor- and iss-containing prophage in the E. coli chromosome.
iss and bor occur in multiple locations in the E. coli genome.
Within the sequenced E. coli genomes, the iss alleles were identified in three different loci (Fig. 3). Some E. coli genomes instead harbored bor in these same loci. The genomes of the E. coli strains K-12 MG1655 (8) and K-12 W3110 (24), EAEC strain 101-1 (GenBank accession no. NZ_AAMK00000000), EPEC strain B171 (GenBank accession no. NZ_AAJX00000000), and EPEC strain E22 (GenBank accession no. NZ_AAJV00000000) contained bor or iss type 2 on a prophage element near the adk gene. Strains O157:H7 EDL933 (43) and O157:H7 Sakai (25) and ETEC strain B7a (GenBank accession no. NZ_AAJT00000000) contained bor or iss type 2 on a prophage between the aspC and icd genes. The sequenced ExPEC strains examined included APEC O1 (34), UPEC 536 (10), UPEC UTI89 (11), UPEC CFT073 (56), and UPEC F11 (GenBank accession no. NZ_AAJU00000000). These strains and the O157:H7 genomes (EDL933 and Sakai) contained a bor or an iss type 3 on a prophage element between icd and fumC. The location of iss was also analyzed within the extrachromosomal elements. The virulence plasmids pAPEC-O2-ColV (35), p300 (54), pAPEC-O1-ColBM (33), pAPEC-1 (17, 18, 51), and pVM29188 (Salmonella enterica serovar Kentucky strain CVM29188, GenBank accession no. NZ_ABAK00000000) contained iss within a highly conserved region of the ColV/BM PAI, between the salmochelin siderophore system (the iroBCDEN genes) and the repFIB replicon region.
iss occurs on the E. coli chromosome.
Nucleotide and protein alignments were performed between all of the available iss and bor sequences (Fig. 1). Of the 12 analyzed chromosomal sequences thought to be bor, only 6 sequences appeared to be the bor gene upon reannotation. Chromosomes containing bor included those for E. coli K-12 (both MG1655 and W3110), EHEC O157:H7 (both EDL933 and Sakai), and EPEC strains B171 and E22 (draft sequences) (Fig. 3). In the K-12 and EPEC strains, bor was present near the adk gene. E. coli O157:H7 strains EDL933 and Sakai each contained two copies of bor. One copy was found on the BP-933W prophage located between the aspC and icd housekeeping genes, and the second copy was on the DLP12-like prophage located between icd and fumC (25, 43). In the K-12 strains, bor was found within the DLP12 prophage (8, 24).
The remaining six sequences originally thought to be bor were reannotated as iss (Fig. 2), when analyzed for DNA sequence homology and protein sequence homology and the predicted start and stop sequences. Those sequences reannotated as iss types 2 and 3 had predicted protein lengths of 103 amino acids and had 94 to 95% nucleotide homology and 97 to 98% protein homology with the ColV/BM plasmid-borne iss type 1 that was originally described as iss (Table 3) (27). Sequences reannotated as bor had a shorter predicted protein length of 98 amino acids due to nucleotide variations within the first 15 base pairs of the sequences, resulting in an alternative start site. These sequences also had an alternative stop codon (TAA in bor; TAG in iss) and shared approximately 88% nucleotide homology and 89% protein homology with iss type 1 (12, 27).
iss occurs more frequently among E. coli strains than previously reported.
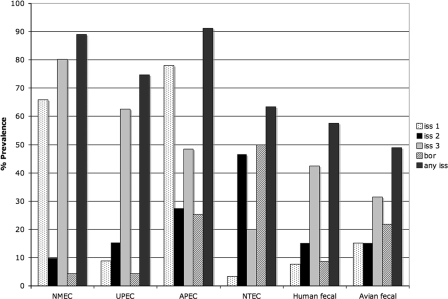
A multiplex panel was designed to detect and differentiate between the different iss alleles and bor gene among E. coli strains. The panel was verified by using several fully sequenced E. coli strains (Table 4) and by sequencing the products obtained for each primer pair. The occurrence of plasmid-borne iss type 1 was highest among populations of NMEC and APEC strains (66% and 78%, respectively), suggesting a high prevalence of the ColV/BM plasmids with a PAI among these populations (Fig. 4 and Table S1 in the supplemental material). The prevalence of type 1 iss was significantly lower (P < 0.05) among the UPEC, NTEC, and fecal strains examined, ranging from 8 to 15%. The occurrence of iss type 2 was lowest among the ExPEC populations and fecal strains examined (15 to 27%) but was significantly higher among NTEC strains (47%). The occurrence of iss type 3 was highest among human ExPEC strains (62 to 80%), lower among APEC (48%) and fecal (31 to 42%) strains, and even lower among NTEC (20%) strains than all other populations. The occurrence of bor was low among the human ExPEC and human fecal strains examined (4 to 9%) but was significantly higher among APEC (25%), NTEC (50%), and avian fecal (22%) strains. The occurrence of at least one iss type ranged from 75 to 91% among the ExPEC strains examined, from 49 to 57% among the fecal strains examined, and in 52% of the NTEC strains examined. Overall, screening for the multiple iss alleles resulted in an enhanced detection of iss sequences compared to that reported previously (44, 46, 47).
TABLE 4.
Comparison of iss prevalence data, using Fisher's exact test
| Gene type | Strain or phylotype | Significance of iss prevalence (P value) among:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli strains
|
E. coli fecal isolates
|
Phylotypes
|
|||||||||
| NMEC | UPEC | APEC | NTEC | Human | Avian | A | B1 | B2 | D | ||
| iss 1 | NMEC | <0.0001 | 0.4926 | <0.0001 | <0.0001 | <0.0001 | |||||
| UPEC | <0.0001 | 0.6856 | 1 | 0.2657 | |||||||
| APEC | <0.0001 | <0.0001 | <0.0001 | ||||||||
| NTEC | 0.6792 | 0.1898 | |||||||||
| Human fecal isolates | 0.1715 | ||||||||||
| Avian fecal isolates | |||||||||||
| iss 2 | NMEC | 0.3801 | 0.0144 | 0.0011 | 0.3818 | 0.3818 | |||||
| UPEC | 0.1161 | 0.0117 | 0.5908 | 0.5908 | |||||||
| APEC | 0.2163 | 0.1145 | 0.1145 | ||||||||
| NTEC | 0.0112 | 0.0112 | |||||||||
| Human fecal isolates | 1 | ||||||||||
| Avian fecal isolates | |||||||||||
| iss 3 | NMEC | 0.3022 | 0.0429 | 0.0023 | 0.0112 | 0.0004 | |||||
| UPEC | 0.322 | 0.0178 | 0.1318 | 0.0126 | |||||||
| APEC | 0.0667 | 0.6916 | 0.1306 | ||||||||
| NTEC | 0.1403 | 0.4942 | |||||||||
| Human fecal isolates | 0.3226 | ||||||||||
| Avian fecal isolates | |||||||||||
| bor | NMEC | 1 | 0.0006 | <0.0001 | 0.3744 | 0.0021 | |||||
| UPEC | 0.0006 | <0.0001 | 0.3744 | 0.0021 | |||||||
| APEC | 0.099 | 0.012 | 0.7355 | ||||||||
| NTEC | 0.0003 | 0.0548 | |||||||||
| Human fecal isolates | 0.0421 | ||||||||||
| Avian fecal isolates | |||||||||||
| Any iss type | UPEC | 0.38 | 0.7407 | 0.2922 | 0.0932 | ||||||
| APEC | 0.3304 | 0.0533 | 0.011 | ||||||||
| NTEC | 0.8645 | 0.486 | |||||||||
| Human fecal isolates | 0.5337 | ||||||||||
| Avian fecal isolates | |||||||||||
| iss 1 | A | 0.5977 | 0.9035 | 0.7584 | |||||||
| B1 | 0.4328 | 0.8521 | |||||||||
| B2 | 0.5085 | ||||||||||
| D | |||||||||||
| iss 2 | A | 0.6336 | 0.3149 | 0.0626 | |||||||
| B1 | 0.1396 | 0.3031 | |||||||||
| B2 | 0.0015 | ||||||||||
| D | |||||||||||
| iss 3 | A | 0.0027 | 0.1047 | 0.1352 | |||||||
| B1 | <0.0001 | 0.0818 | |||||||||
| B2 | 0.0013 | ||||||||||
| D | |||||||||||
| bor | A | 0.4445 | <0.0001 | 0.0003 | |||||||
| B1 | 0.0002 | 0.0239 | |||||||||
| B2 | 0.4772 | ||||||||||
| D | |||||||||||
| Any iss type | A | 0.2859 | 0.4784 | 1 | |||||||
| B1 | 0.076 | 0.2728 | |||||||||
| B2 | 0.4455 | ||||||||||
| D | |||||||||||
FIG. 4.
Prevalence of the three iss types and the bor gene among E. coli populations.
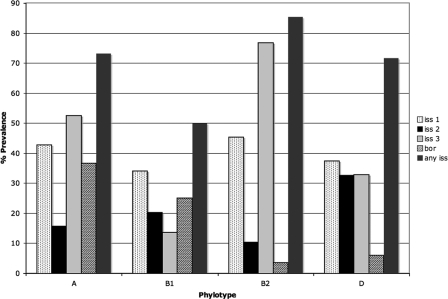
Distribution of the iss types and bor among the E. coli phylotypes was also examined (Fig. 5 and Table 4). The occurrences of iss type 1 were similar (34 to 45%) among all phylotypes examined. The prevalence rate of iss type 2 ranged from 10 to 33% among all populations examined, with group D containing a significantly higher proportion of iss type 2 than the other groups. The occurrences of iss type 3 were different among all populations examined. Its prevalence rate was highest among the B2 phylotype (77%), lower among the A phylotype (52%), and even lower among the B1 (14%) and D (33%) phylotypes. The occurrences of bor were highest among the A (37%) and B1 (25%) phylotypes and significantly lower among the B2 (3%) and D (6%) phylotypes. Overall, the occurrence of at least one type of iss was highest among the B2 (85%), A (73%), and D (72%) phylotypes and slightly lower among the B1 (50%) phylotype.
FIG. 5.
Prevalence of the three iss types and bor among E. coli phylotypes.
Homology between plasmid-borne and chromosomal iss regions accounts for diagnostic difficulties.
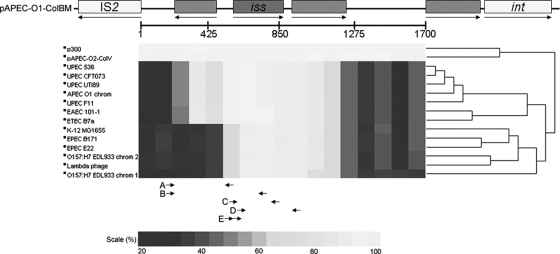
A nucleotide comparison of the 1,700-bp regions surrounding iss and bor in the sequenced E. coli strain revealed that the homology between the plasmid-borne iss and the chromosomal iss types extends beyond the gene itself (Fig. 6). Two predicted genes of unknown function flanked iss type 1 and shared strong homology to the respective regions within the ExPEC chromosome. This extended homology is likely the source of the problems encountered when attempts were made to identify iss and distinguish it from bor (Fig. 6). This is particularly true for hybridization-based techniques, with which it would be virtually impossible to avoid false positives with strains containing bor when using primers within the iss gene. In fact, the PCR primers used previously for identifying iss actually produce an amplicon spanning iss and its adjacent upstream gene (35, 44, 46, 47). Prior to the sequencing of multiple ExPEC genomes, it would have appeared as though these primers would not detect chromosomal iss/bor sequences. However, the ExPEC-borne iss type 3 has more homology with the ColV/BM iss region than do the bor-containing regions of K-12 or O157:H7; therefore, false positives with previous primer sets would have been likely to occur. Table 1 lists primers that are suggested to be specific for the plasmid and chromosomal variants of iss and for bor. These primer sets contain one primer within the sought-for iss or bor sequence and a primer outside of the sequence that is specific for a particular location within the genome. Though the complete E. coli genomic sequences that are available at present do not contain both bor and iss, our PCR identified several isolates that do, as well as every other possible combination of the four traits of interest. Thus, while these primer sets have been verified and used effectively to analyze E. coli populations for the presence of bor and the different iss alleles, it will be necessary to review these regions with care as more E. coli genomes are sequenced so that these primer sets may be refined accordingly.
FIG. 6.
Nucleotide homology comparison of 15 bor- and iss-containing regions to that of pAPEC-O1-ColBM (33). Genes are indicated by rectangles, with their orientation depicted by arrows. The scale below the genes depicts the 1,700-bp region in which BLASTn was performed. Below the homology map, primers are given from several recent studies: A to C for iss in PCR studies (20, 32, 44, 46, 47); D for bor in this PCR study; and E for iss in an oligonucleotide-based microarray study (1).
Proposed evolution of iss and bor.
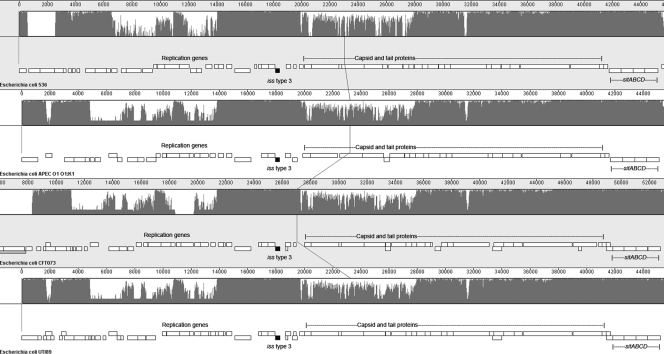
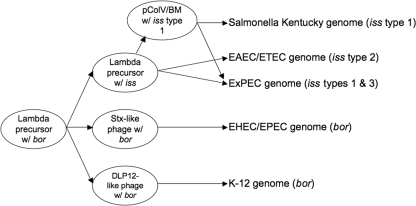
Based upon our analyses of the iss and bor genes and a comparison of the prophage elements on which they reside (Fig. 7), we propose a scheme for the evolution of iss (Fig. 8). It appears that the different iss- and bor-containing prophage elements may have been derived from a common λ phage precursor containing bor. A major evolutionary divergence likely occurred at an early time point when two phage types apparently split from the λ phage ancestor. Subsequently, the bor-containing phage precursor may have inserted into the genomes of K-12 and EHEC/EPEC, possibly at different time points. It is also likely that a further divergence of the bor-containing phage occurred prior to this integration to form the DLP12 phage of K-12 and the Shiga toxin (Stx)-like phage of the EHEC strains. These phage then apparently introduced bor into the E. coli chromosome in separate events. Another important event was the evolution of bor into iss. This likely occurred on a separate phage element after its early divergence from the λ phage ancestor. This iss-containing phage then integrated into multiple E. coli loci, in the ExPEC chromosome at one locus and in EAEC/ETEC chromosomes at other loci. In the ExPEC chromosome, iss type 3 was introduced by a phage that also appears to have carried the Sit iron and manganese transport system (48, 49, 51). The association of both iss types 1 and 3 with Sit provides further evidence that a common phage element harboring iss (and Sit) was responsible for the evolution of the three iss alleles. A final key event in the history of iss was its integration into the ColV/BM plasmid. The significance of this recombinational event, possibly involving IS2 elements, was that iss then became readily transmissible to enteric bacterial recipients in the gut. Overall, these key evolutionary events have shaped the emergence of the three allelic iss variants.
FIG. 7.
Nucleotide alignment of ExPEC strain iss-containing prophage using MAUVE software (15). The iss type 3 gene is shaded black. The overall homology is indicated by spiked lines across sequences. The vertical line indicates the center of each sequence.
FIG. 8.
Proposed evolution of the iss types and bor. Phage types carrying the iss types or bor are thought to have evolved from a common λ phage precursor. The iss variants were likely introduced by a similar phage element at different points in time, with the differences in the three iss alleles due to their independent evolution postintegration.
As mentioned above, in addition to the introduction of iss type 3 on prophage, some ExPEC genomes have also been affected by the acquisition of the ColV/BM plasmids harboring iss type 1. This iss type likely evolved independently of the chromosomally acquired iss types, and the introduction of iss could have involved recombination between an ancestral plasmid type and one of the different phage types harboring iss or bor. A recent study by Jeziorowski and Gordon supported the idea that iss type 1 is significantly associated with the presence of genes of the ColV/BM PAI and that the conserved region of this PAI has arisen in multiple plasmid types (ColV, ColBM, and ColIa) on separate occasions (29). Overall, the incorporation of iss-containing phage and plasmids into the ExPEC genome may have contributed to its evolution toward an extraintestinal lifestyle.
The K-12, EHEC, and EPEC genomes harboring bor on prophage likely arose independently of any iss-containing phage or prophage. The K-12 genome contains bor within the DLP12 prophage, which may be the closest relative of all the sequenced prophage to a λ phage precursor. Similarly, the EHEC and EPEC strains appear to have acquired bor from Stx-like phage arising from a common λ phage precursor. Interestingly, sequenced EHEC strains possess multiple copies of bor on different prophages. One loci of prophage insertion is the same that is occupied by ExPEC prophage harboring iss type 3. The other locus of insertion for EHEC bor-containing prophage is the same as that occupied by an ETEC B7a prophage containing iss type 2. Speculations aside, it is evident that the bor-containing prophage, the iss type 2-containing prophage, and the iss type 3-containing prophage are distinct from one another and appear to have arisen independently of one another via acquisitional events. Also apparent is that there appears to be certain hot spots within the E. coli genome for phage integration, as evidenced here by the apparent integration of multiple phage types within three loci at different points in time.
Finally, there is evidence that the dissemination of iss via horizontal gene transfer continues. The recent emergence of Salmonella serovar Kentucky strains among poultry and the strains' presence among cattle and swine and their presence within retail meats have been reported without plausible explanation (4, 26, 36, 37, 41, 42, 45, 52, 55, 59). However, recent genome sequencing efforts have identified the ColV plasmid as a component of the genome of a multidrug-resistant Salmonella serovar Kentucky isolate (GenBank accession no. NZ_ABAK00000000). This plasmid, harboring a PAI containing iss type 1, was very similar to the plasmids possessed by APEC strains. The spread of this plasmid, its PAI, and the iss gene to non-E. coli strains is of particular concern because these elements encode virulence-related traits, fitness, and multidrug resistance. Perhaps recent transfer events have resulted in the acquisition of ColV plasmids by some Salmonella species, and such an acquisition is responsible for the increased prevalence of Salmonella serovar Kentucky among production animals and within retail meats. The apparent emergence of Salmonella serovar Kentucky strains might have implications for human health. It is therefore tempting to speculate that the introduction of the ColV plasmids might have resulted in an increased pathogenic and zoonotic potential for these strains (14, 37, 55). Such findings emphasize the need for continued monitoring of changes occurring in the food production environments.
While the identification of multiple iss alleles among E. coli genomes is interesting from an evolutionary standpoint, what is its significance? It has been reported that both Iss and Bor confer complement resistance. However, Lynne et al. recently reported that an iss mutant resulted in a significantly greater attenuation of complement resistance capabilities than did a bor mutation in the same strain (39). Therefore, it appears that Iss and Bor confer different biological properties on their hosts. Whether these differences in conferred properties are due to structural differences between Iss and Bor remains to be addressed. An alternative explanation for the differences between Iss and Bor could be their different genomic locations. Perhaps the ColV/BM plasmid-borne iss type 1 undergoes different regulation than the chromosomal iss types, resulting in a differentially expressed protein. Future work may answer these and other related questions.
In sum, this study provides an explanation for the recent discrepancies observed while screening for the presence of iss among E. coli strains. Surprisingly, many ExPEC strains have iss within their chromosomes, and screening for the different iss alleles results in an enhanced detection of iss among E. coli strains. Analysis of these allelic variants helps to shed light on the evolution of iss and perhaps on the evolution of E. coli virulence in general. The different iss types appear to have evolved from a bor-containing phage precursor, with several key events leading to the current iss alleles present on different prophage elements and conjugative plasmids. From these analyses, it also appears that more ExPEC strains possess iss than previously thought. Because iss is highly prevalent among ExPEC and other pathotypes, is transmitted on mobile elements, and has been implicated in E. coli virulence, further analysis of the iss types should be undertaken to explore the possibility that this gene and the protein it encodes are useful diagnostic tools or vaccine targets for the prevention of various ExPEC-caused diseases.
Supplementary Material
Acknowledgments
We thank those who supplied bacterial strains for this study. Bovine NTEC strains were kindly provided by Chitrita Debroy of Penn State University's E. coli Reference Center. Human fecal strains were provided by James Johnson (University of Minnesota and VA Medical Center of Minneapolis) and Catherine Logue (North Dakota State University). The NMEC strains for this study were kindly provided by Kwang Sik Kim (Johns Hopkins University) and Lodwick Spanjaard (Academic Medical Center, Amsterdam, The Netherlands).
Footnotes
Published ahead of print on 15 February 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anjum, M. F., M. Mafura, P. Slickers, K. Ballmer, P. Kuhnert, M. J. Woodward, and R. Ehricht. 2007. Pathotyping Escherichia coli by using miniaturized DNA microarrays. Appl. Environ. Microbiol. 73:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, H. J., and W. B. Gross. 1997. Colibacillosis, p. 131-141. In B. W. Calnek, H. J. Barnes, C. W. Beard, L. R. McDougald, and Y. M. Saif (ed.), Diseases of poultry, 10th ed. Iowa State University Press, Ames.
- 3.Barondess, J., and J. Beckwith. 1995. bor gene of phage λ, involved in serum resistance, encodes a widely conserved outer membrane lipoprotein. J. Bacteriol. 177:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beach, J. C., E. A. Murano, and G. R. Acuff. 2002. Serotyping and antibiotic resistance profiling of Salmonella in feedlot and nonfeedlot beef cattle. J. Food Prot. 65:1694-1699. [DOI] [PubMed] [Google Scholar]
- 5.Bekal, S., R. Brousseau, L. Masson, G. Prefontaine, J. Fairbrother, and J. Harel. 2003. Rapid identification of Escherichia coli pathotypes by virulence gene detection with DNA microarrays. J. Clin. Microbiol. 41:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binns, M., J. Mayden, and R. Levine. 1982. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of colV,I-K94. Infect. Immun. 35:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binns, M. M., D. L. Davies, and K. G. Hardy. 1979. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature 279:778-781. [DOI] [PubMed] [Google Scholar]
- 8.Blattner, F., G. I. Plunkett, C. Bloch, N. Perna, V. Burland, M. Riley, J. Collado-Vides, J. Glasner, C. Rode, G. Mayhew, J. Gregor, N. Davis, H. Kirkpatrick, M. Goeden, D. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 9.Bonacorsi, S., and E. Bingen. 2005. Molecular epidemiology of Escherichia coli causing neonatal meningitis. Int. J. Med. Microbiol. 295:373-381. [DOI] [PubMed] [Google Scholar]
- 10.Brzuszkiewicz, E., H. Bruggemann, H. Liesegang, M. Emmerth, T. Olschlager, G. Nagy, K. Albermann, C. Wagner, C. Buchrieser, L. Emody, G. Gottschalk, J. Hacker, and U. Dobrindt. 2006. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc. Natl. Acad. Sci. USA 103:12879-12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, S. L., C. S. Hung, J. Xu, C. S. Reigstad, V. Magrini, A. Sabo, D. Blasiar, T. Bieri, R. R. Meyer, P. Ozersky, J. R. Armstrong, R. S. Fulton, J. P. Latreille, J. Spieth, T. M. Hooton, E. R. Mardis, S. J. Hultgren, and J. I. Gordon. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. USA 103:5977-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuba, P., M. Leon, A. Banerjee, and S. Palchaudhuri. 1989. Cloning and DNA sequence of plasmid determinant iss, coding for increased serum survival and surface exclusion, which has homology with lambda DNA. Mol. Gen. Genet. 216:287-292. [DOI] [PubMed] [Google Scholar]
- 13.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collard, J. M., S. Place, O. Denis, H. Rodriguez-Villalobos, M. Vrints, F. X. Weill, S. Baucheron, A. Cloeckaert, M. Struelens, and S. Bertrand. 2007. Travel-acquired salmonellosis due to Salmonella Kentucky resistant to ciprofloxacin, ceftriaxone and co-trimoxazole and associated with treatment failure. J. Antimicrob. Chemother. 60:190-192. [DOI] [PubMed] [Google Scholar]
- 15.Darling, A. C., B. Mau, F. R. Blattner, and N. T. Perna. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14:1394-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrindt, U. 2005. (Patho-)genomics of Escherichia coli. Int. J. Med. Microbiol. 295:357-371. [DOI] [PubMed] [Google Scholar]
- 17.Dozois, C. M., F. Daigle, and R. Curtiss III. 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 100:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewers, C., T. Janssen, S. Kiessling, H. C. Phillip, and L. H. Wieler. 2004. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet. Microbiol. 104:91-101. [DOI] [PubMed] [Google Scholar]
- 20.Ewers, C., T. Janben, S. Kiebling, H. C. Philipp, and L. H. Wieler. 2005. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 49:269-273. [DOI] [PubMed] [Google Scholar]
- 21.Ewers, C., G. Li, H. Wilking, S. Kiessling, K. Alt, E. M. Antao, C. Laturnus, I. Diehl, S. Glodde, T. Homeier, U. Bohnke, H. Steinruck, H. C. Philipp, and L. H. Wieler. 2007. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? Int. J. Med. Microbiol. 297:163-176. [DOI] [PubMed] [Google Scholar]
- 22.Foley, S. L., S. M. Horne, C. W. Giddings, T. R. Gustad, E. D. Handegard, M. Robinson, and L. K. Nolan. 2003. Monoclonal antibodies to avian Escherichia coli Iss. Avian Dis. 47:79-86. [DOI] [PubMed] [Google Scholar]
- 23.Foley, S. L., S. M. Horne, C. W. Giddings, M. Robinson, and L. K. Nolan. 2000. Iss from a virulent avian Escherichia coli. Avian Dis. 44:185-191. [PubMed] [Google Scholar]
- 24.Hayashi, K., N. Morooka, Y. Yamamoto, K. Fujita, K. Isono, S. Choi, E. Ohtsubo, T. Baba, B. L. Wanner, H. Mori, and T. Horiuchi. 2006. Highly accurate genome sequences of Escherichia coli K-12 strains MG1655 and W3110. Mol. Syst. Biol. 2:2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez, T., A. Sierra, C. Rodriguez-Alvarez, A. Torres, M. P. Arevalo, M. Calvo, and A. Arias. 2005. Salmonella enterica serotypes isolated from imported frozen chicken meat in the Canary Islands. J. Food Prot. 68:2702-2706. [DOI] [PubMed] [Google Scholar]
- 27.Horne, S. M., S. J. Pfaff-McDonough, C. W. Giddings, and L. K. Nolan. 2000. Cloning and sequencing of the iss gene from a virulent avian Escherichia coli. Avian Dis. 44:179-184. [PubMed] [Google Scholar]
- 28.Jeffrey, J. S., L. K. Nolan, K. H. Tanooka, S. Wolfe, C. W. Giddings, S. M. Horne, S. L. Foley, A. M. Lynne, J. O. Ebert, L. M. Elijah, G. Bjorklund, S. J. Pfaff-McDonough, R. S. Singer, and C. Doetkott. 2002. Virulence factors of Escherichia coli from cellulitis or colisepticemia lesions in chickens. Avian Dis. 46:48-52. [DOI] [PubMed] [Google Scholar]
- 29.Jeziorowski, A., and D. M. Gordon. 2007. Evolution of microcin V and colicin Ia plasmids in Escherichia coli. J. Bacteriol. 189:7045-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, J. R., and T. A. Russo. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int. J. Med. Microbiol. 295:383-404. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, J. R., E. Oswald, T. T. O'Bryan, M. A. Kuskowski, and L. Spanjaard. 2002. Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in the Netherlands. J. Infect. Dis. 185:774-784. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, T. J., S. J. Johnson, and L. K. Nolan. 2006. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J. Bacteriol. 188:5975-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson, T. J., S. Kariyawasam, Y. Wannemuehler, P. Mangiamele, S. J. Johnson, C. Doetkott, J. A. Skyberg, A. M. Lynne, J. R. Johnson, and L. K. Nolan. 2007. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J. Bacteriol. 189:3228-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, T. J., K. E. Siek, S. J. Johnson, and L. K. Nolan. 2006. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J. Bacteriol. 188:745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, X., J. B. Payne, F. B. Santos, J. F. Levine, K. E. Anderson, and B. W. Sheldon. 2007. Salmonella populations and prevalence in layer feces from commercial high-rise houses and characterization of the Salmonella isolates by serotyping, antibiotic resistance analysis, and pulsed field gel electrophoresis. Poult. Sci. 86:591-597. [DOI] [PubMed] [Google Scholar]
- 37.Lo-Ten-Foe, J. R., J. A. van Oers, A. M. Kotsopoulos, and A. G. Buiting. 2007. Pulmonary colonization with Salmonella enterica serovar Kentucky in an intensive care unit. J. Hosp. Infect. 67:105-107. [DOI] [PubMed] [Google Scholar]
- 38.Lynne, A. M., S. L. Foley, and L. K. Nolan. 2006. Immune response to recombinant Escherichia coli Iss protein in poultry. Avian Dis. 50:273-276. [DOI] [PubMed] [Google Scholar]
- 39.Lynne, A. M., J. A. Skyberg, C. M. Logue, C. Doetkott, S. L. Foley, and L. K. Nolan. 2007. Characterization of a series of transconjugant mutants of an avian pathogenic Escherichia coli isolate for resistance to serum complement. Avian Dis. 51:771-776. [DOI] [PubMed] [Google Scholar]
- 40.Lynne, A. M., J. A. Skyberg, C. M. Logue, and L. K. Nolan. 2006. Detection of Iss and Bor on the surface of Escherichia coli. J. Appl. Microbiol. 102:660-666. [DOI] [PubMed] [Google Scholar]
- 41.Maurer, J. J., A. Pedroso, M. D. Lee, H. Sellers, E. Linnemann, and K. Zamperini. 2007. Is Salmonella enterica Kentucky a newly emergent, poultry adapted strain?, abstr. 4420. In Proceedings of the American Veterinary Medical Association. American Veterinary Medical Association, Schaumburg, IL.
- 42.McCrea, B. A., K. S. Macklin, R. A. Norton, J. B. Hess, and S. F. Bilgili. 2006. A longitudinal study of Salmonella and Campylobacter jejuni isolates from day of hatch through processing by automated ribotyping. J. Food Prot. 69:2908-2914. [DOI] [PubMed] [Google Scholar]
- 43.Perna, N. T., G. Plunkett III, V. Burland, B. Maur, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfal, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli 0157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 44.Pfaff-McDonough, S. J., S. M. Horne, C. W. Giddings, J. O. Ebert, C. Doetkott, M. H. Smith, and L. K. Nolan. 2000. Complement resistance-related traits among Escherichia coli isolates from apparently healthy birds and birds with colibacillosis. Avian Dis. 44:23-33. [PubMed] [Google Scholar]
- 45.Quirke, A. M., N. Leonard, G. Kelly, J. Egan, P. B. Lynch, T. Rowe, and P. J. Quinn. 2001. Prevalence of Salmonella serotypes on pig carcasses from high- and low-risk herds slaughtered in three abattoirs. Berl. Munch. Tierarztl. Wochenschr. 114:360-362. [PubMed] [Google Scholar]
- 46.Rodriguez-Siek, K. E., C. W. Giddings, C. Doetkott, T. J. Johnson, M. K. Fakhr, and L. K. Nolan. 2005. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 151:2097-2110. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Siek, K. E., C. W. Giddings, C. Doetkott, T. J. Johnson, and L. K. Nolan. 2005. Characterizing the APEC pathotype. Vet. Res. 36:241-256. [DOI] [PubMed] [Google Scholar]
- 48.Runyen-Janecky, L., E. Dazenski, S. Hawkins, and L. Warner. 2006. Role and regulation of the Shigella flexneri Sit and MntH systems. Infect. Immun. 74:4666-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Runyen-Janecky, L. J., S. A. Reeves, E. G. Gonzales, and S. M. Payne. 2003. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71:1919-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russo, T. A., and J. R. Johnson. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. [DOI] [PubMed] [Google Scholar]
- 51.Sabri, M., S. Leveille, and C. M. Dozois. 2006. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology 152:745-758. [DOI] [PubMed] [Google Scholar]
- 52.Santos, F. B., D. H. Dsouza, L. Jaykus, P. R. Ferket, and B. W. Sheldon. 2007. Genotypes, serotypes, and antibiotic resistance profiles of Salmonella isolated from commercial North Carolina turkey farms. J. Food Prot. 70:1328-1333. [DOI] [PubMed] [Google Scholar]
- 53.Skyberg, J. A., S. M. Horne, C. W. Giddings, R. E. Wooley, P. S. Gibbs, and L. K. Nolan. 2003. Characterizing avian Escherichia coli isolates with multiplex polymerase chain reaction. Avian Dis. 47:1441-1447. [DOI] [PubMed] [Google Scholar]
- 54.Sorsa, L. J., S. Dufke, J. Heesemann, and S. Shubert. 2003. Characterization of an iroBCDEN gene cluster on a transmissible plasmid of uropathogenic Escherichia coli: evidence for horizontal transfer of a chromosomal virulence factor. Infect. Immun. 71:3285-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weill, F. X., S. Bertrand, F. Guesnier, S. Baucheron, A. Cloeckaert, and P. A. Grimont. 2006. Ciprofloxacin-resistant Salmonella Kentucky in travelers. Emerg. Infect. Dis. 12:1611-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, H., S. Chen, D. G. White, S. Zhao, P. McDermott, R. Walker, and J. Meng. 2005. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J. Clin. Microbiol. 42:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao, S., J. J. Maurer, S. Hubert, J. F. De Villena, P. F. McDermott, J. Meng, S. Ayers, L. English, and D. G. White. 2005. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet. Microbiol. 107:215-224. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, S., P. F. McDermott, S. Friedman, J. Abbott, S. Ayers, A. Glenn, E. Hall-Robinson, S. K. Hubert, H. Harbottle, R. D. Walker, T. M. Chiller, and D. G. White. 2006. Antimicrobial resistance and genetic relatedness among Salmonella from retail foods of animal origin: NARMS retail meat surveillance. Foodborne Pathog. Dis. 3:106-117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.