Abstract
Spore formation by Clostridium difficile is a significant obstacle to overcoming hospital-acquired C. difficile-associated disease. Spores are resistant to heat, radiation, chemicals, and antibiotics, making a contaminated environment difficult to clean. To cause disease, however, spores must germinate and grow out as vegetative cells. The germination of C. difficile spores has not been examined in detail. In an effort to understand the germination of C. difficile spores, we characterized the response of C. difficile spores to bile. We found that cholate derivatives and the amino acid glycine act as cogerminants. Deoxycholate, a metabolite of cholate produced by the normal intestinal flora, also induced germination of C. difficile spores but prevented the growth of vegetative C. difficile. A model of resistance to C. difficile colonization mediated by the normal bacterial flora is proposed.
Clostridium difficile, a gram-positive spore-forming bacterium, is the leading cause of antibiotic-associated diarrhea, pseudomembranous colitis, and toxic megacolon (12). Perturbation of the colonic flora, a side effect of treatment with broad-spectrum antibiotics, potentiates sensitivity to C. difficile infection (18). The normal flora is thought to protect against C. difficile by occupying potential sites of colonization (35, 45). Once established in the intestinal tract, C. difficile elicits disease upon the secretion of two toxins, TcdA and TcdB (38). Due to the prevalence of C. difficile-associated disease in hospitals and relapse in patients treated for C. difficile infection, it has been estimated that the total financial burden on the health care system for the treatment of C. difficile-associated disease in the United States is approximately $3.2 billion per year (24).
Vegetative cells of C. difficile are exquisitely sensitive to oxygen. To survive outside the anaerobic environment of the large bowel, the bacterium has to be in the spore form. Thus, it is generally accepted that the spore form of C. difficile, acquired from the environment, initiates disease. Since toxins are produced by cells, not spores, the spores presumably germinate in the gastrointestinal tract, grow out as vegetative cells, and produce toxin. Any C. difficile bacteria that are excreted by the host, however, have to be in the spore form to survive for long periods (15). Although the morphological changes during sporulation are very similar in Clostridium and Bacillus species, sporulation and germination in Clostridium species are not as well studied as those in the model organism Bacillus subtilis. In brief, sporulation is initiated under conditions of nutrient limitation and leads to formation of an asymmetrically placed division septum that divides the cell into two unequal compartments, each of which contains one copy of the chromosome. The larger, mother cell compartment then engulfs the forespore and helps the forespore mature (11). The addition of a peptidoglycan cortex and several layers of coat proteins precedes release into the environment by lysis of the mother cell (9).
Once released from the mother cell, the spore is metabolically dormant but highly resistant to many types of environmental insult. When conditions become suitable for growth, the spores germinate and grow out as vegetative cells. In B. subtilis, germination can be induced by l-alanine or by a mixture of asparagine, glucose, fructose, and potassium ions. Receptors involved in sensing these environmental cues are GerA, GerB, and GerK (14, 21). After the germinant is sensed, a large depot of calcium dipicolinate (Ca2+-DPA) is released, the core hydrates, the cortex is degraded, and metabolism begins (34). Homologs of GerA, GerB, and GerK exist in several Bacillus species as well as in many Clostridium species but are absent in C. difficile, suggesting that C. difficile responds to different kinds of environmental cues (26, 33). In fact, spore germination in different species is induced by a variety of germinants. For instance, for Bacillus megaterium spores, l-proline is a germinant (31), while purine ribonucleosides and amino acids act as cogerminants for Bacillus anthracis spores (13). Germination and outgrowth of C. difficile spores have not been studied in depth, due in part to the absence of genetic tools. Specifically, the germination step, classically defined as the change in the optical density caused by spore rehydration and Ca2+-DPA release, has not been studied as an independent phenomenon. Previous work showed that taurocholate, a bile salt, enhances colony formation by C. difficile spores recovered from environmental surfaces and stool (2, 40, 44). Similarly, treatment of C. difficile spores with lysozyme and thioglycolate enhances colony formation (16, 43). These effects on colony formation are clear, but it is difficult to discern what specific effects the treatments might have on germination.
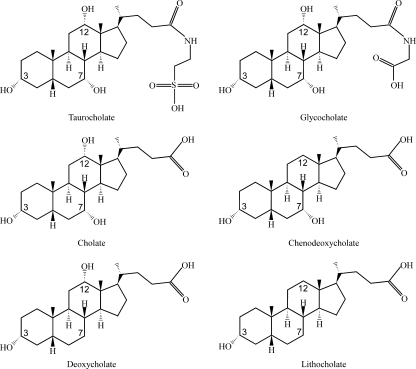
Bile is produced by the liver and stored in the gall bladder. To aid in digestion, the gall bladder secretes bile into the duodenum, where it helps to absorb fat and cholesterol. The primary bile produced by the liver consists mainly of cholate and chenodeoxycholate conjugated with either taurine or glycine (Fig. 1) (30). During passage through the distal ileum, bile is actively reabsorbed and recycled to the liver. However, 400 to 800 mg of bile passes daily from the ileum into the cecum, where it becomes a substrate for biotransforming reactions by the normal, benign bacterial flora (36, 37). Many different species of bacteria, including Clostridium perfringens (6), express on their cell surfaces bile salt hydrolases (BSHs), which remove the conjugated amino acid from the primary bile salt. This hydrolysis reaction appears to proceed to completion, inasmuch as conjugated primary bile salts are essentially undetectable in the human cecum (7, 23). Though some Clostridium species express BSHs, none have been described for C. difficile, and no open reading frame product with homology to BSHs in other species is present.
FIG. 1.
Structures of common primary and secondary bile acids. The primary bile salts cholate and chenodeoxycholate typically are conjugated with taurine or glycine (only taurocholate and glycocholate are shown). The normal intestinal microbial flora deconjugates the tauryl and glycyl group from cholate and chenodeoxycholate. The deconjugated primary bile salts are further metabolized by the microbial flora to deoxycholate and lithocholate, respectively.
Unconjugated primary bile salts are taken up by a small percentage of bacterial species in the colon (30). One of these species, Clostridium scindens, actively transports unconjugated, primary bile salts into the cytosol and, through a series of enzymatic reactions, converts cholate and chenodeoxycholate to the secondary bile salts deoxycholate and lithocholate, respectively (Fig. 1) (20, 41, 42). These secondary bile salts are secreted from the bacteria into the extracellular environment and are eventually excreted by the host.
We report here that C. difficile spores germinate rapidly in vitro when exposed to taurocholate but only in a rich medium that does not by itself induce efficient germination. Using a defined medium, we found that glycine and taurocholate act as cogerminants and are sufficient to induce germination of C. difficile spores. Secondary bile salts induced germination but also inhibited outgrowth of the germinated spores. These results form the basis for a new model to explain the role of the normal flora in preventing C. difficile infection.
MATERIALS AND METHODS
Strains and growth conditions.
C. difficile CD196 and C. perfringens SM101 were described previously (27, 46). C. difficile UK14 was obtained from Dale Gerding's laboratory and was isolated during the epidemic C. difficile outbreak at Stoke-Mandeville Hospital, Aylesbury, Buckinghamshire, England (Meridian Biosciences strain number SM8-6865). All strains were grown in BHIS (brain heart infusion [Difco] supplemented with yeast extract [5 mg/ml] and l-cysteine [0.1%]) at 37°C under anaerobic conditions in a Coy Laboratory anaerobic chamber.
Preparation of C. difficile spores.
Sporulation of C. difficile was induced on BHIS agar as described previously (8). Briefly, an overnight C. difficile culture in BHIS medium was diluted in fresh medium to an optical density at 600 nm (OD600) of 0.2. A 150-μl portion of this suspension was spread on 5 ml BHIS agar in each well of a six-well tissue culture dish. The culture was incubated anaerobically for 4 to 7 days. To determine spore colony formation, samples from the plates containing mixed populations of spores and vegetative cells were resuspended in BHIS and heated to 60°C for 20 min to kill vegetative cells before cooling, diluting, and plating on BHIS medium. For use in germination assays, spores were purified by a method similar to that used by Akoachere and colleagues (1). The vegetative-cell-spore mixture was collected by flooding each well of the six-well dish with ice-cold sterile water. After five washes with ice-cold water, the bacteria were suspended in 20% (wt/vol) HistoDenz (Sigma, St. Louis, MO). This suspension was layered onto a 50% (wt/vol) HistoDenz solution in a centrifuge tube, and the tube was centrifuged at 15,000 × g for 15 min to separate spores from vegetative cells. The purified spores, collected at the bottom of the centrifuge tube, were washed twice with ice-cold water to remove traces of HistoDenz and resuspended in water.
In vitro response of C. difficile spores to bile salts.
To determine the response time of C. difficile spores to taurocholate, spores were produced as described above. Vegetative bacteria were heat killed by incubation for 20 min at 60°C. The heat-treated spores were washed three times in water to remove traces of nutrients and returned to the anaerobic chamber to allow subsequent colony formation. Spores were resuspended in water, and either taurocholate, glycocholate, cholate, deoxycholate, ursodeoxycholate, or chenodeoxycholate (Sigma, St. Louis, MO) was added to 0.1%. At various times, samples were removed, serially diluted, and plated on BHIS agar. One sample was removed prior to the addition of taurocholate and spread on BHIS and BHIS(TA) (BHIS plus 0.1% taurocholate). The latter platings served as the negative and positive controls for colony formation, respectively. After overnight growth, colonies were enumerated (CFU), and the number was compared to that obtained on BHIS(TA).
To determine the amount of taurocholate needed to induce colony formation by C. difficile spores, spores were produced, heated, and washed as described above. Heat-treated C. difficile spores were resuspended in water containing various concentrations of taurocholate. After a 10-minute incubation, the suspensions were serially diluted in BHIS and plated on BHIS agar. After overnight growth, colonies were enumerated, and the number was compared to that obtained by overnight growth on BHIS(TA).
Germination of C. difficile spores.
Germination of C. difficile spores was measured by diluting purified C. difficile spores to an OD600 of 0.3 to 0.4 in BHIS alone or BHIS containing 1% bile salts (taurocholate, glycocholate, cholate, or deoxycholate). For experiments in complete defined medium, a mixture of salts [0.3 mM (NH4)2SO4, 6.6 mM KH2PO4, 15 mM NaCl, 59.5 mM NaHCO3, and 35.2 mM Na2HPO4] was used to buffer the spores and putative germinants (17). The OD600 was determined immediately (time zero) and at various time points during incubation at room temperature. The ratios of the optical densities at the various time points to the optical density at time zero were plotted against time.
Statistical analysis.
All assays listed above were performed in triplicate, and data are reported as means and standard deviations from three independent experiments.
RESULTS
Time of in vitro taurocholate exposure required to enhance colony formation by C. difficile spores.
There have been reports over the past 25 years that the bile salt taurocholate enhances the recovery of C. difficile spores from environmental surfaces and stool (2, 28, 44). We confirmed that inclusion of 0.1% taurocholate in BHIS agar plates enhances the recovery of C. difficile spores approximately 105-fold. To know how long an exposure to taurocholate is required to increase colony formation, spores and vegetative bacteria were heated at 60°C for 20 min and washed three times with water to remove traces of nutrients that may affect germination. Spores were then returned to the anaerobic chamber and treated with 0.1% taurocholate in water (Fig. 2). At the indicated times, samples were removed, diluted in BHI medium, and plated on BHIS agar (without taurocholate). One sample was not incubated in vitro with taurocholate but was plated directly on BHIS(TA). This sample served as a reference for 100% recovery. As shown in Fig. 2, as little as 1 minute of exposure to taurocholate resulted in an increase in spore recovery from 0.0025% to about 1%. Further incubation did not significantly enhance colony formation by C. difficile spores. These results demonstrate that C. difficile spores respond very rapidly to taurocholate, suggesting that taurocholate may be a germinant.
FIG. 2.
Rate of response of C. difficile spores to taurocholate. C. difficile spores were suspended in water containing 0.1% taurocholate. At 1, 5, 10, 30, and 60 min, spores were serially diluted and plated on BHIS agar in the absence of taurocholate. Colonies were enumerated after overnight growth, and data were compared to those for spores spread on BHIS(TA). Data are means from three independent experiments, and error bars represent 1 standard deviation from the mean.
Effects of taurocholate concentration on spore colony formation.
Because colony-forming ability in response to 0.1% taurocholate did not reach that of spores plated on BHIS(TA) directly, we tested the in vitro recovery of spores in response to different concentrations of taurocholate. Spores were prepared as described above, washed with water to remove traces of nutrients, incubated for 10 min with taurocholate at concentrations ranging from 0.001% to 10%, and plated on BHIS agar without taurocholate. Spore colony-forming ability was compared to that of spores plated directly on BHIS(TA). Incubation of spores with 0.001% taurocholate resulted in colony formation by approximately 0.0002% of the total number of spores (Fig. 3). This efficiency of colony formation was routinely seen in the absence of any taurocholate and can vary approximately 10-fold (Fig. 2). Increasing the concentration of taurocholate increased the plating efficiency of C. difficile spores (Fig. 3). Incubation for 10 min in 10% taurocholate resulted in a plating efficiency that was 60% of that seen with continuous exposure to 0.1% taurocholate (Fig. 3). This result suggests that continuous exposure to a low concentration of taurocholate significantly enhances colony formation even further or that the effect of taurocholate is enhanced when spores are in contact with a solid surface or both.
FIG. 3.
Amount of taurocholate required for efficient recovery of C. difficile spores. C. difficile spores were incubated in water containing increasing concentrations of taurocholate, serially diluted, and plated on BHIS agar in the absence of taurocholate. Colonies were enumerated after overnight growth, and data were compared to those for spores spread on BHIS(TA). Data points are means from three independent experiments, and error bars represent 1 standard deviation from the mean.
Germination in response to primary bile salts.
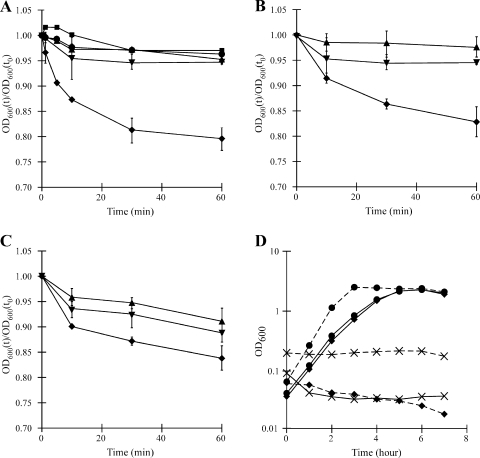
In B. subtilis and other species, the addition of a germinant to spores results in a change from phase bright (refractile) spores to phase dark spores due to the release of Ca2+-DPA and rehydration of the spore (22). This transition is the first stage of germination, can be measured as a decrease in the optical density of the culture, and is typically used to define germinants. To see if taurocholate enhances colony formation by increasing the rate or extent of spore germination, C. difficile spores were incubated in phosphate buffer (pH 7.2) with 1% taurocholate or in buffer alone. One percent taurocholate was chosen because this concentration enabled colony formation by about 30% of the total number of spores during in vitro exposure (Fig. 3). At regular intervals, the OD600 was measured and plotted against time. By this measure, taurocholate did not induce germination of C. difficile spores (Fig. 4A). To see if spores germinate in BHIS medium but become arrested before acquiring the ability to form colonies, spores were suspended in BHIS medium, and the optical density of the culture was monitored. No significant decrease in optical density was seen (Fig. 4A), indicating that spores do not germinate in BHIS alone. This result is in agreement with earlier observations that C. difficile spores do not efficiently form colonies in standard media without additional reagents (44). The addition of 1% taurocholate to BHIS resulted in a rapid decrease in the optical density to about 85% of the starting value, with a continued decrease to about 80% of the starting value (Fig. 4A). This is similar to what is seen for germination of Clostridium botulinum spores; the rate of germination appears to be higher in C. difficile (3). These results suggest that taurocholate and an unknown component of BHIS medium are cogerminants of C. difficile spores; neither cogerminant activates germination by itself.
FIG. 4.
Effect of primary bile salts on the germination and growth of C. difficile. (A) C. difficile spores were purified and suspended in BHIS alone (•), in 1% taurocholate in buffer (▾), or in BHIS containing 1% taurocholate (⧫), 1% glycocholate (▴), or 1% cholate (▪). The ratios of the optical densities at various time points to the starting optical density are plotted against time. (B) C. difficile CD196 spores were purified and suspended in buffered glycine (1.3 mM) (▴), buffered 1% taurocholate (▾), or buffered glycine plus taurocholate (⧫). Germination was measured as for panel A. (C) C. difficile UK14 spores were purified and suspended in buffered glycine (1.3 mM) (▴), buffered 1% taurocholate (▾), or buffered glycine plus taurocholate (⧫). Germination was measured as for panel A. (D) Vegetative C. difficile (solid lines) and vegetative C. perfringens (dashed lines) were grown in BHIS alone (•), BHIS(TA) (⧫), or BHIS plus 0.1% chenodeoxycholate (×). Error bars represent 1 standard deviation from the mean.
Spore germination and colony formation in response to primary bile salts.
As taurocholate is a primary bile salt produced by the liver and secreted to aid in digestion, we hypothesized that other primary bile salts might also induce germination of C. difficile spores. To test the effect of other primary bile salts on colony formation, spores were plated on BHIS agar containing 0.1% cholate, glycocholate, chenodeoxycholate, ursodeoxycholate, or taurocholate. Interestingly, only cholate derivatives (cholate, glycocholate, and taurocholate) stimulated efficient colony formation by C. difficile spores (Table 1). Chenodeoxycholate and ursodeoxycholate, the 7β epimer of chenodeoxycholate, were not effective in this assay (Table 1). The primary bile salts cholate and glycocholate were then compared to taurocholate for germination-inducing ability. Incubation of C. difficile spores in BHIS with glycocholate or cholate did not result in any significant decrease in the optical density of the culture even when the assay was carried out to 6 h (Fig. 4A and data not shown). Thus, glycocholate and cholate enhance colony formation by spores on plates but do not stimulate germination per se by the assay used.
TABLE 1.
Cholate derivatives induce colony formation by C. difficile spores
| Cholate derivative added to BHISa | CFU recovery (%)b
|
|
|---|---|---|
| Mean | SD | |
| TA | 100 | |
| GA | 86.3 | 17.2 |
| CA | 75.1 | 10.3 |
| CDCA | <0.0001c | |
| UA | <0.0001c | |
Spores were serially diluted and spread on BHIS agar containing 0.1% taurocholate (TA), glycocholate (GA), cholate (CA), chenodeoxycholate (CDCA), or ursodeoxycholate (UA).
Data are percentages relative to the value obtained with BHIS(TA) and are means of three independent experiments.
CFU for CDCA and UA were below the limit of detection for this experiment.
Glycine is a cogerminant for C. difficile spores.
To identify the component of BHIS that induces germination with taurocholate, the medium was divided into BHI and yeast extract. In the presence of taurocholate, both BHI and yeast extract induced germination of C. difficile spores (data not shown). We used the defined medium described by Karlsson and colleagues (17) to identify the specific compound or compounds that induce germination. When spores were suspended in complete defined medium with taurocholate, the optical density of the culture decreased to the same extent as in BHIS(TA) (data not shown). When this medium was divided into it constituents and subconstituents, we found that spores suspended in buffer containing glycine germinated in the presence of taurocholate but not in its absence (Fig. 4B). These results indicate that glycine and taurocholate are cogerminants.
To test whether glycine and taurocholate act as cogerminants for another strain of C. difficile, we tested a strain, UK14, isolated during an outbreak at Stoke-Mandeville Hospital in the United Kingdom. When C. difficile UK14 spores were suspended in buffer containing glycine or taurocholate alone, a small decrease in the optical density of the culture was observed (Fig. 4C). When both glycine and taurocholate were present, the efficiency of germination was enhanced (Fig. 4C). These results confirmed our initial results in C. difficile CD196 that taurocholate and glycine act as cogerminants for C. difficile spores.
Effect of primary bile salts on the growth of C. difficile.
We next tested whether vegetative cells of C. difficile are able to grow in the presence of the primary bile salts. As expected, C. difficile was able to grow in BHIS containing 0.1% taurocholate, glycocholate, or cholate to the same extent as in BHIS medium alone (Fig. 4D and data not shown). C. difficile was unable to grow in the presence of chenodeoxycholate. Therefore, the absence of growth demonstrated by the data in Table 1 can be explained by growth inhibition by chenodeoxycholate. In contrast to C. difficile, C. perfringens SM101 was unable to grow in the presence of either 0.1% taurocholate or 0.1% chenodeoxycholate (Fig. 4D) (10). C. perfringens SM101 was able to grow to wild-type levels in the presence of glycocholate and cholate (data not shown). These results suggest that C. difficile may have evolved a mechanism to resist the toxic effects of taurocholate in addition to sensing taurocholate as a germinant.
Chenodeoxycholate inhibited the growth of vegetative cells of C. difficile and C. perfringens. To test whether transient exposure to chenodeoxycholate induces colony formation by C. difficile spores, spores were suspended in water containing 0.1% chenodeoxycholate for 10 min, serially diluted in BHIS medium, and plated on BHIS agar in the absence of chenodeoxycholate. Exposure to chenodeoxycholate did not induce colony formation by C. difficile spores (Table 2). These results suggest that C. difficile spores only germinate in BHIS and form colonies in response to cholate derivatives (taurocholate, glycocholate, and cholate).
TABLE 2.
Colony formation of C. difficile spores
Spores were treated in vitro with 0.1% taurocholate (TA), chenodeoxycholate (CDCA), or deoxycholate (DCA), serially diluted, and spread on BHIS agar.
Data are percentages relative to the value obtained with BHIS(TA) and are means of three independent experiments.
Deoxycholate induces colony formation but prevents the growth of C. difficile.
In the cecum, the primary bile salts cholate and chenodeoxycholate are metabolized by the normal bacterial flora to the secondary bile salts deoxycholate and lithocholate, respectively (Fig. 1) (30, 36). During the passage of C. difficile through the intestinal tract, spores and vegetative cells undoubtedly come into contact with secondary bile salts. We tested whether deoxycholate can induce the germination or recovery of C. difficile spores. Lithocholate could not be tested, as it is insoluble in water. When C. difficile spores were incubated in vitro with 0.1% deoxycholate, serially diluted, and plated on BHIS agar, colony-forming ability was indistinguishable from that of spores incubated with taurocholate (Table 2). These results suggest that deoxycholate, like other cholate derivatives, induces colony formation by C. difficile spores (Tables 1 and 2). Incubation of C. difficile spores in BHIS with 1% deoxycholate resulted in a small drop in OD600 that after 60 min was not significantly more than the change in OD of spores in BHIS alone (Fig. 5A).
FIG. 5.
Effect of deoxycholate on the germination and growth of C. difficile. (A) C. difficile spores were purified and suspended in BHIS alone (•) or in BHIS(TA) (⧫) or 1% deoxycholate (▴). (B) Vegetative C. difficile (solid lines) and vegetative C. perfringens (dashed lines) were grown in BHIS alone (•) or BHIS plus 0.1% deoxycholate (▴). Error bars represent 1 standard deviation from the mean.
C. difficile does not grow in the presence of deoxycholate (43). To quantify this effect, we measured the growth of C. difficile and C. perfringens in BHIS containing deoxycholate. Although C. difficile grew well in medium containing taurocholate, neither C. difficile nor C. perfringens grew in the presence of deoxycholate (Fig. 5B).
DISCUSSION
The initiation of germination is characterized by a change from a phase bright spore to a phase dark spore (39), and this criterion is often used for defining germinants. Interaction of a germinant with its germination receptor triggers a series of enzymatic reactions that leads to the release of Ca2+-DPA, core rehydration, cortex degradation, and the eventual outgrowth of a vegetative cell (34). The absence in C. difficile of germinant receptors homologous to those in B. subtilis raises the possibility that C. difficile spores respond to unique germinants. We have shown that C. difficile spores germinate rapidly in rich medium in the presence of taurocholate. When germination is measured spectrophotometrically, only C. difficile spores that are incubated in rich medium containing taurocholate transition from phase bright spores to phase dark spores, a hallmark of germination (Fig. 4A). The ability to germinate and form colonies on plates requires only a brief exposure to taurocholate, but continuous exposure during colony formation is more effective than transient exposure (Fig. 2). When C. difficile spores are plated on BHIS agar containing cholate or glycocholate, they form colonies with the same efficiency as spores plated on BHIS agar containing taurocholate (Table 1). Further, when spores are incubated briefly with the secondary bile salt deoxycholate, a compound that inhibits the growth of C. difficile, spores are subsequently able to form colonies on BHIS agar in the absence of any germinant. All of these results indicate that cholate conjugates and metabolites are cogerminants for C. difficile spores. However, they must activate germination at different rates, because only taurocholate was capable of inducing rapid germination (loss of refractility) while glycocholate, cholate, and deoxycholate induced colony formation with the same efficiency as taurocholate. Northfield and McColl have shown that postprandial concentrations of bile acids along the ileum range from 10 mM (0.41% to 0.52%) in the upper ileum to 2 mM (0.082% to 0.1%) in the lower (23). Therefore, the concentrations of bile that stimulate colony formation that are used in this study are likely to be physiologically relevant.
If exposure to either glycocholate, cholate, or deoxycholate in liquid medium stimulates subsequent colony formation by C. difficile spores on agar medium, why do these compounds not induce germination as detected spectrophotometrically? When C. difficile spores are treated in vitro with 0.1% taurocholate, only about 1% of the total spores present germinate and resume vegetative growth when serially diluted and plated on agar medium without taurocholate (Fig. 2 and 3). However, the same spore preparation plated on agar medium containing 0.1% taurocholate induces the germination and growth of approximately 100-fold more spores. This suggests either that spores must encounter both cogerminants simultaneously or that the germination of C. difficile spores is enhanced by contact with a solid support. The latter hypothesis is interesting because germination in the host likely involves a semisolid support in the cecal environment. The spore morphology of C. difficile CD196 is not known. However, an exosporial layer is present on the surface of spores of C. difficile strain ATCC 43594 (25). Upon the initiation of germination, the exosporium of C. difficile spores produces small filaments that attach to the solid medium and prevent the spores from being washed away (25). This type of surface attachment may facilitate the contact between taurocholate, the nutrient-rich medium, and the spore, thus enhancing germination in the laboratory environment.
Chenodeoxycholate, a primary bile salt, did not induce colony formation by C. difficile spores (Table 2). As chenodeoxycholate has limited solubility in water, it is possible that not enough was dissolved to induce germination. An alternative hypothesis is that the 12α-hydroxyl group of cholate derivatives is an important side chain that C. difficile spores recognize; chenodeoxycholate derivatives lack the hydroxyl group at the 12α position (Fig. 1 and Table 2). If this hypothesis is correct, the 12α position could be exploited to find potential inhibitors of C. difficile spore germination.
Neither glycine nor taurocholate has been previously described as a germinant in the strict sense for spores of Bacillus or Clostridium species. The only other known approach to inducing germination of C. difficile in the laboratory is to treat spores with high concentrations of a reducing agent and lysozyme (16). This method of germination is not likely to be biologically relevant.
In bile, glycine is conjugated to cholate (Fig. 1). When glycocholate passes into the lower bowel, the glycyl group is deconjugated from cholate by the normal bacterial flora (5). The products of deconjugation are cholate and glycine. When deconjugated by the normal flora, the production of both cholate and glycine from glycocholate results in two compounds that are sufficient to stimulate germination and outgrowth of C. difficile spores.
The results presented here lead to a possible adjunct to the current model for colonization by C. difficile and the protective effect of the normal flora. When a normal, healthy host ingests C. difficile spores, the spores survive the passage through the stomach and pass through the duodenum and into the jejunum, where the concentrations of primary bile salts and nutrients are high (29, 30). C. difficile spores germinate in response to cholate derivatives and glycine. The germinated spores then pass through the ileum and finally into the anaerobic environment of the cecum. Here, certain members of the normal microbial flora metabolize the cholate derivatives that escape enterohepatic circulation to deoxycholate, a predominant bile salt in the feces of healthy humans (4, 19, 36). The deoxycholate produced prevents vegetative growth of C. difficile, and the host remains uncolonized. Spores that did not germinate during passage through the upper part of the digestive system may germinate in response to deoxycholate but are then unable to grow in the toxic environment. Upon antibiotic treatment, the normal microbial flora is perturbed and the species that are capable of 7α-dehydroxylation of primary bile salts are significantly reduced (32). This reduction would lead to an increase in the concentration of primary bile salts (cholate derivatives) and a decrease in the concentration of secondary bile salts (deoxycholate) in the cecum (32). This decrease in secondary bile acids may provide an environment in which C. difficile can grow and colonize. Moreover, the abnormal presence of primary bile acids in the large bowel may contribute to the extent of C. difficile colonization by providing a nontoxic germinant for spores that remain within the colon. Therefore, an important protective role the normal microbial flora plays may be that of metabolizing cholate derivatives to deoxycholate, an inhibitor of C. difficile growth. Standard therapy for C. difficile infection is treatment with vancomycin or metronidazole. It is not surprising that patients suffer relapses after completing treatment regimens with these drugs, since the microbial flora needs time to repopulate the colon and restore the normal balance of primary and secondary bile acids. We are currently testing this hypothesis and are aware of the possibility that chenodeoxycholate, a primary bile salt that inhibits growth, may act to protect against C. difficile colonization. If this model withstands further scrutiny, it would have significant therapeutic potential. For example, patients undergoing antibiotic therapy may benefit from supplementing their normal diets with deoxycholate or with probiotics containing bacterial species that are capable of removing the 7α-hydroxyl group from cholate.
Acknowledgments
This project was supported in part by funding from the National Institutes of Health under contract no. N01-AI-30050 (S. Tzipori, principal investigator). J.A.S. acknowledges support through the NIH Federal Training in Education and Critical Research Skills (TEACRS) fellowship K12 GM074869-02.
We thank Sean Dineen and Boris Belitsky for helpful discussions and critical reading of the manuscript and Dale Gerding for the generous gift of C. difficile UK14.
Footnotes
Published ahead of print on 1 February 2008.
REFERENCES
- 1.Akoachere, M., R. C. Squires, A. M. Nour, L. Angelov, J. Brojatsch, and E. Abel-Santos. 2007. Identification of an in vivo inhibitor of Bacillus anthracis spore germination. J. Biol. Chem. 28212112-12118. [DOI] [PubMed] [Google Scholar]
- 2.Bliss, D. Z., S. Johnson, C. R. Clabots, K. Savik, and D. N. Gerding. 1997. Comparison of cycloserine-cefoxitin-fructose agar (CCFA) and taurocholate-CCFA for recovery of Clostridium difficile during surveillance of hospitalized patients. Diagn. Microbiol. Infect. Dis. 291-4. [DOI] [PubMed] [Google Scholar]
- 3.Broussolle, V., F. Alberto, C. A. Shearman, D. R. Mason, L. Botella, C. Nguyen-The, M. W. Peck, and F. Carlin. 2002. Molecular and physiological characterisation of spore germination in Clostridium botulinum and C. sporogenes. Anaerobe 889-100. [Google Scholar]
- 4.Carey, J. B., and C. J. Watson. 1955. Isolation of deoxycholic acid from normal human feces. J. Biol. Chem. 216847-850. [PubMed] [Google Scholar]
- 5.Corzo, G., and S. E. Gilliland. 1999. Bile salt hydrolase activity of three strains of Lactobacillus acidophilus. J. Dairy Sci. 82472-480. [DOI] [PubMed] [Google Scholar]
- 6.Gopal-Srivastava, R., and P. B. Hylemon. 1988. Purification and characterization of bile salt hydrolase from Clostridium perfringens. J. Lipid Res. 291079-1085. [PubMed] [Google Scholar]
- 7.Hamilton, J. P., G. Xie, J.-P. Raufman, S. Hogan, T. L. Griffin, C. A. Packard, D. A. Chatfield, L. R. Hagey, J. H. Steinbach, and A. F. Hofmann. 2007. Human cecal bile acids: concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 293G256-G263. [DOI] [PubMed] [Google Scholar]
- 8.Haraldsen, J. D., and A. L. Sonenshein. 2003. Efficient sporulation in Clostridium difficile requires disruption of the σK gene. Mol. Microbiol. 48811-821. [DOI] [PubMed] [Google Scholar]
- 9.Henriques, A. O., and C. P. J. Moran. 2007. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 61555-588. [DOI] [PubMed] [Google Scholar]
- 10.Heredia, N. L., R. G. Labbe, M. A. Rodriguez, and J. S. Garcia-Alvarado. 1991. Growth, sporulation and enterotoxin production by Clostridium perfringens type A in the presence of human bile salts. FEMS Microbiol. Lett. 8415-22. [DOI] [PubMed] [Google Scholar]
- 11.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley, B. W., and C. C. Nguyen. 2002. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch. Intern. Med. 1622177-2184. [DOI] [PubMed] [Google Scholar]
- 13.Ireland, J. A. W., and P. C. Hanna. 2002. Amino acid- and purine ribonucleoside-induced germination of Bacillus anthracis ΔSterne endospores: gerS mediates responses to aromatic ring structures. J. Bacteriol. 1841296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irie, R., Y. Fujita, and M. Kobayashi. 1996. Nucleotide sequence and gene organisation of the gerK spore germination locus of Bacillus subtilis 168. J. Gen. Appl. Microbiol. 42141-153. [Google Scholar]
- 15.Jump, R. L. P., M. J. Pultz, and C. J. Donskey. 2007. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob. Agents Chemother. 512883-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamiya, S., K. Yamakawa, H. Ogura, and S. Nakamura. 1989. Recovery of spores of Clostridium difficile altered by heat or alkali. J. Med. Microbiol. 28217-221. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson, S., L. G. Burman, and T. Akerlund. 1999. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 1451683-1693. [DOI] [PubMed] [Google Scholar]
- 18.Lusk, R. H., R. Fekety, J. Silva, R. A. Browne, D. H. Ringler, and G. D. Abrams. 1978. Clindamycin-induced enterocolitis in hamsters. J. Infect. Dis. 137464-475. [DOI] [PubMed] [Google Scholar]
- 19.Makita, M., and W. W. Wells. 1963. Quantitative analysis of fecal bile acids by gas-liquid chromatography. Anal. Biochem. 5523-530. [DOI] [PubMed] [Google Scholar]
- 20.Mallonee, D. H., and P. B. Hylemon. 1996. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 1787053-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moir, A., E. Lafferty, and D. A. Smith. 1979. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J. Gen. Microbiol. 124165-180. [DOI] [PubMed] [Google Scholar]
- 22.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44531-553. [DOI] [PubMed] [Google Scholar]
- 23.Northfield, T., and I. McColl. 1973. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut 14513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien, J. A., B. J. Lahue, J. J. Caro, and D. M. Davidson. 2007. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect. Control Hosp. Epidemiol. 281219-1227. [DOI] [PubMed] [Google Scholar]
- 25.Panessa-Warren, B. J., G. T. Tortora, and J. B. Warren. 1997. Exosporial membrane plasticity of Clostridium sporogenes and Clostridium difficile. Tissue Cell 29449-461. [DOI] [PubMed] [Google Scholar]
- 26.Paredes, C. J., K. V. Alsaker, and E. T. Papoutsakis. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 3969. [DOI] [PubMed] [Google Scholar]
- 27.Popoff, M. R., E. J. Rubin, D. M. Gill, and P. Boquet. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 562299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raibaud, P., R. Ducluzeau, F. Dubos, S. Hudault, H. Bewa, and M. C. Muller. 1980. Implantation of bacteria from the digestive tract of man and various animals into gnotobiotic mice. Am. J. Clin. Nutr. 332440-2447. [DOI] [PubMed] [Google Scholar]
- 29.Rao, A., R. L. Jump, N. J. Pultz, M. J. Pultz, and C. J. Donskey. 2006. In vitro killing of nosocomial pathogens by acid and acidified nitrite. Antimicrob. Agents Chemother. 503901-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridlon, J. M., D. Kang, and P. B. Hylemon. 2006. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47241-259. [DOI] [PubMed] [Google Scholar]
- 31.Rossignol, D. P., and J. C. Vary. 1979. Biochemistry of l-proline-triggered germination of Bacillus megaterium spores. J. Bacteriol. 138431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel, P., C. M. Holtzman, E. Meilman, and I. Sekowski. 1973. Effect of neomycin and other antibiotics on serum cholesterol levels and on 7α-dehydroxylation of bile acids by the fecal bacterial flora in man. Circ. Res. 33393-402. [DOI] [PubMed] [Google Scholar]
- 33.Sebaihia, M., B. W. Wren, P. Mullany, N. F. Fairweather, N. Minton, R. Stabler, N. R. Thomson, A. P. Roberts, A. M. Cerdeno-Tarraga, H. Wang, M. T. G. Holden, A. Wright, C. Churcher, M. A. Quail, S. Baker, N. Bason, K. Brooks, T. Chillingworth, A. Cronin, P. Davis, L. Dowd, A. Fraser, T. Feltwell, Z. Hance, S. Holroyd, K. Jagels, S. Moule, K. Mungall, C. Price, E. Rabbinowitsch, S. Sharp, M. Simmonds, K. Stevens, L. Unwin, S. Whithead, B. Dupuy, G. Dougan, B. Barrell, and J. Parkhill. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38779-786. [DOI] [PubMed] [Google Scholar]
- 34.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550. [DOI] [PubMed] [Google Scholar]
- 35.Surawicz, C., and L. McFarland. 1999. Pseudomembranous colitis: causes and cures. Digestion 6091-100. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, L. A., M. J. Veysey, G. French, P. B. Hylemon, G. M. Murphy, and R. H. Dowling. 2001. Bile acid metabolism by fresh human colonic contents: a comparison of caecal versus faecal samples. Gut 49835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlahcevic, Z. R., D. M. Heuman, and P. B. Hylemon. 1996. Physiology and pathophysiology of enterohepatic circulation of bile acids, p. 376-417. In D. Zakim and T. Boyer (ed.), Hepatology: a textbook of liver disease, 3rd ed. W. B. Saunders Company, Philadelphia, PA.
- 38.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wax, R., E. Freese, and M. Cashel. 1967. Separation of two functional roles of l-alanine in the initiation of Bacillus subtilis spore germination. J. Bacteriol. 94522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weese, J. S., H. R. Staempfli, and J. F. Prescott. 2000. Isolation of environmental Clostridium difficile from a veterinary teaching hospital. J. Vet. Diagn. Invest. 12449-452. [DOI] [PubMed] [Google Scholar]
- 41.Wells, J. E., and P. B. Hylemon. 2000. Identification and characterization of a bile acid 7α-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7α-dehydroxylating strain isolated from human feces. Appl. Environ. Microbiol. 661107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, B. A., R. L. Lipsky, R. J. Fricke, and P. B. Hylemon. 1980. Bile acid induction specificity of 7-alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids 35103-109. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, K. H. 1983. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J. Clin. Microbiol. 181017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson, K. H., M. J. Kennedy, and F. R. Fekety. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson, K. H., and F. Perini. 1988. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect. Immun. 562610-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao, Y., and S. B. Melville. 1998. Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J. Bacteriol. 180136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]