Abstract
Type IV pili (T4P) are dynamic surface structures that undergo cycles of extension and retraction. T4P dynamics center on the PilB and PilT proteins, which are members of the secretion ATPase superfamily of proteins. Here, we show that PilB and PilT of the T4P system in Myxococcus xanthus have ATPase activity in vitro. Using a structure-guided approach, we systematically mutagenized PilB and PilT to resolve whether both ATP binding and hydrolysis are important for PilB and PilT function in vivo. PilB as well as PilT ATPase activity was abolished in vitro by replacement of conserved residues in the Walker A and Walker B boxes that are involved in ATP binding and hydrolysis, respectively. PilB proteins containing mutant Walker A or Walker B boxes were nonfunctional in vivo and unable to support T4P extension. PilT proteins containing mutant Walker A or Walker B boxes were also nonfunctional in vivo and unable to support T4P retraction. These data provide genetic evidence that both ATP binding and hydrolysis by PilB are essential for T4P extension and that both ATP binding and hydrolysis by PilT are essential for T4P retraction. Thus, PilB and PilT are ATPases that act at distinct steps in the T4P extension/retraction cycle in vivo.
Type IV pili (T4P) are versatile, filamentous surface structures found in many gram-negative bacteria. In Myxococcus xanthus, Pseudomonas aeruginosa, and Neisseria gonorrhoeae T4P mediate surface motility (27). T4P also mediate attachment and microcolony formation by human pathogens such as Escherichia coli, N. gonorrhoeae, P. aeruginosa, and Vibrio cholerae on eukaryotic host cells (6). Moreover, T4P have important functions in biofilm formation (22, 34) and DNA uptake by natural transformation (9). A hallmark of T4P compared to other filamentous surface structures is their dynamic nature; i.e., T4P undergo cycles of extension and retraction, and it is during the retraction step that a force sufficiently large to pull a bacterial cell forward is generated (29, 51, 52).
T4P are thin (5- to 8-nm), flexible, helical filaments several micrometers in length, with high tensile strength (>100 pN) and typically composed only of the PilA pilin subunit (6). The protein machinery required for T4P biogenesis and function is highly conserved and encompasses 17 proteins as defined for T4P in Neisseria meningitidis (4). These proteins localize to the cytoplasm, inner membrane, periplasm, and outer membrane (35). In vitro analyses and genetic analyses of T4P in N. meningitidis suggest that these proteins interact extensively and form a trans-envelope complex (4). Many of the proteins involved in T4P biogenesis and function share similarity with proteins found in type II secretion systems (T2SS) and archaeal flagellum systems (35). Several of the proteins are phylogenetically related, suggesting that the three machineries may share functional characteristics (35). Indeed, overexpression of pseudopilins from the T2SS in Klebsiella oxytoca, Xanthomonas campestris, and P. aeruginosa results in the formation of pilin-like structures (10, 16, 45).
T4P dynamics includes two steps: (i) extension by polymerization in a process that involves the addition of pilin subunits from a reservoir in the inner membrane (31) to the base of the pilus (7) and (ii) retraction by depolymerization in a process that involves the removal of pilin subunits from the base and with the pilin subunits being transferred to the inner membrane (29, 31, 51, 52). The dynamic extension/retraction cycle of T4P centers on two members of the superfamily of secretion ATPases, PilB and PilT, which have been identified in all T4P systems. With the exception of the PilT protein, all T4P proteins analyzed, including PilB, are required for T4P extension (27, 55), whereas the PilT protein is specifically required for T4P retraction (29). The T2SS contains only one ATPase, which is an ortholog of the PulE protein in K. oxytoca and closely related to PilB (35, 36).
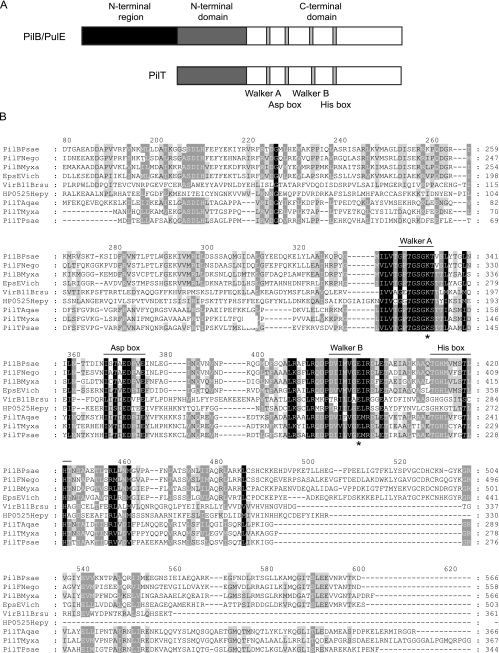
PilB, PilT, and PulE belong to distinct subfamilies of the superfamily of secretion ATPases (35, 36). In addition to T4P systems and T2SS, secretion ATPases have been identified in T4SS as well as in archaeal flagellum systems (35, 36). PilB and PulE orthologs contain a relatively well-conserved N-terminal region of 160 to 175 amino acids that is not present in PilT orthologs (35) (Fig. 1A). Structural analyses of six secretion ATPases (HP0525, which is part of the T4SS of Helicobacter pylori [47, 61]; EpsE, which is part of the T2SS in V. cholerae [40]; XpsE, which is part of the T4SS of X. campestris [5]; VirB11 of the Brucella suis T4SS [12]; afGspE, which functions in protein secretion in Archaeoglobus fulgidus [60]; and PilT from Aquifex aeolicus [44]) have shown that these 160 to 175 residues are followed by a region of 110 to 130 amino acids (Fig. 1A and B), which is relatively well conserved in secretion ATPases and folds into a structurally conserved domain referred to as the N-terminal domain. The N-terminal domain is followed by a highly conserved region of 190 to 240 amino acids (Fig. 1A and B), which also folds into a structurally conserved domain, referred to as the C-terminal domain, encompassing the sequences associated with ATP binding and hydrolysis and including four conserved sequence motifs: the Walker A box with the P loop GX4GK(S/T), the atypical Walker B box motif Dh4GE (h stands for hydrophobic residue), the His box, and the Asp box (Fig. 1A and B) (12, 40, 44, 46, 47, 60, 61).
FIG. 1.
Domain structure of secretion ATPases. (A) Scheme of domain structure of PulE, PilB, and PilT proteins. The conserved N-terminal region in PulE and PilB proteins, the N-terminal domain conserved in all secretion ATPases, and the C-terminal domain conserved in all secretion ATPases are indicated. Vertical gray bars in the C-terminal domain indicate the four conserved sequence motifs in secretion ATPases: Walker A box, Asp box, Walker B box, and His box. (B) Alignment of PilB and PilT of M. xanthus with secretion ATPases. PilB (PilBMyxa) and PilT (PilTMyxa) of M. xanthus were aligned with PilB of P. aeruginosa (PilBPsae), PilF (a PilB ortholog) of N. gonorrhoeae (PilFNego), EpsE of V. cholerae (EpsEVich), VirB11 of B. suis (VirB11Brsu), PilT of A. aeolicus (PilTAqae), and PilT of P. aeruginosa (PilTPsae). The conserved Walker A, Asp, Walker B, and His boxes are indicated. The conserved lysine in the Walker A box and the conserved glutamate in the Walker B box that were replaced in this study are indicated with asterisks. White-on-black residues are 100% conserved, white-on-gray residues are 80% conserved, and black-on-gray residues are 60% conserved. Note that the N-terminal extensions of PilB, EpsE, VirB11, and HP0525 are not included.
The unifying picture emerging from structural analyses of secretion ATPases is that they are dynamic hexameric assemblies that bind and hydrolyze ATP and with ATP binding and hydrolysis inducing major conformational changes that could facilitate the formation of macromolecular complexes (46). Several lines of evidence support this picture. Generally, Walker A box substitutions in PulE orthologs interfere with the normal functioning of these proteins in vivo (37, 38, 43, 53). Specifically, the PulE orthologs EpsE of V. cholerae (3) and XpsE of X. campestris (50) have been shown to have ATPase activity and to form oligomers in vitro. Moreover, replacement of the conserved lysine residue in the Walker A box leads to a reduction in ATPase activity in both proteins (3, 50) and to mutants that are unable to support secretion (43, 50). For the PulE ortholog afGspE in A. fulgidus, replacement of the lysine residue in the Walker A box abolishes ATP binding (60). For PilB orthologs, PilQ, which is required for formation of thin conjugative pili in E. coli (41), and BfpD, which is required for formation of bundle-forming pili in E. coli, were reported to have ATPase activity in vitro and to form homo-octamers and homohexamers, respectively, in vitro (8). Moreover, in PilQ, replacement of the conserved lysine residue in the Walker A box abolishes ATPase activity in vitro and results in the inability to assemble pili (41). For PilT orthologs, ATPase activity was reported in vitro for PilT of Synechocystis sp. strain PCC 6803 (33), for PilT of Microcystis aeruginosa (32), and for hexameric PilT from A. aeolicus (13). Moreover, for the last protein, replacement of the conserved lysine residue in the Walker A box abolishes ATP hydrolysis (13). However, the relevance of the PilT ATPase activity in vivo remains to be demonstrated.
In order to systematically address biochemically and genetically the function of a pair of PilB and PilT proteins acting in the same T4P system, we analyzed PilB and PilT of the T4P system in M. xanthus. In M. xanthus T4P are unipolarly localized (20) to the leading cell pole of the rod-shaped cells (30), and S motility, which is the equivalent of twitching motility in P. aeruginosa and N. gonorrhoeae (27), depends on T4P (20, 58). Deletions of pilB and pilT in M. xanthus abolish S motility (58, 59). We report that PilB and PilT of M. xanthus display ATPase activity in vitro. Using a structure-based approach to replace key residues predicted to be involved in ATP binding and ATP hydrolysis, we show that ATPase activity in both proteins depends on intact Walker A and Walker B boxes and that both motifs are required for PilB and PilT function in vivo. Our data show that PilB and PilT are ATPases that act at distinct steps in the T4P extension/retraction cycle, and they suggest that ATP binding and ATP hydrolysis are essential for PilB and PilT function in vivo.
MATERIALS AND METHODS
Cell growth and motility assays.
The bacterial strains and plasmids used are listed in Table 1. Sequences of primers used in this work are available from the authors upon request. M. xanthus was grown in Casitone-Tris (CTT) medium in liquid cultures or on CTT-1.5% agar plates as described previously (14). Kanamycin was used for selective growth at a concentration of 40 μg/ml. E. coli strains were grown in LB broth in the presence of relevant antibiotics (42). Strains to be tested for motility were grown in CTT to a density of 5 × 108 cells/ml, harvested, and resuspended in MC7 (10 mM MOPS [morpholinepropanesulfonic acid] [pH 7.0], 1 mM CaCl2) to a calculated density of 5 × 109 cells/ml. Ten-microliter aliquots of cell suspensions were spotted on a thin layer of 0.5% agar supplemented with 0.5% CTT (15, 49). After 24 h, colony morphology and colony edges were observed visually in a Leica MZ8 stereomicroscope and visualized using a Leica DFC280 charge-coupled device camera.
TABLE 1.
M. xanthus strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| DK1622 | wild type | 20 |
| DK1622GFP | Wild type, attB::pilA-gfp | 18 |
| DK10409 | ΔpilT | 59 |
| DK10410 | ΔpilA | 59 |
| DK10416 | ΔpilB | 59 |
| SA2020 | ΔpilT attB::pSL104 Kmr | This work |
| SA2021 | ΔpilB attB::pSL105 Kmr | This work |
| SA2412 | ΔpilT attB::pSL104TWalkerA Kmr | This work |
| SA2413 | ΔpilT attB::pSL104TWalkerB Kmr | This work |
| SA2414 | ΔpilT attB::pSL105BWalkerA Kmr | This work |
| SA2415 | ΔpilT attB::pSL105BWalkerB Kmr | This work |
| Plasmids | ||
| pSW105 | Mx8 attP, pilA promoter, Kmr | S. Weiss |
| pMS421 | lacIq Strr | 11 |
| pUHE24-2 | PA1/O4/O3, Ampr | 24 |
| pSL104 | pSW105 with pilT | This work |
| pSL104TWalkerA | pSL104 with Walker A box mutation in pilT | This work |
| pSL104TWalkerB | pSL104 with Walker B box mutation in pilT | This work |
| pSL105 | pSW105 with pilB | This work |
| pSL105BWalkerA | pSL105 with Walker B box mutation in pilB | This work |
| pSL105BWalkerB | pSL104 with Walker B box mutation in pilB | This work |
| pSL4 | pUHE24-2 with His6-pilT | This work |
| pSL4TWalkerA | pSL4 with Walker A box mutation in pilT | This work |
| pSL4TWalkerB | pSL4 with Walker B box mutation in pilT | This work |
| pSL5 | pUHE24-2 with His6-pilB | This work |
| pSL5BWalkerA | pSL5 with Walker A box mutation in pilB | This work |
| pSL5BWalkerB | pSL5 with Walker B box mutation in pilB | This work |
Strain and plasmid construction.
Plasmids were propagated in E. coli Top10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 arsD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG] (Invitrogen) unless otherwise stated. All plasmids generated by PCR were verified by sequencing. To generate pSL105, which contains pilB cloned downstream of the pilA promoter, the primers opilB1 and opilB2 were used to amplify pilB. Subsequently, the PCR product was cloned in the plasmid pSW105, which contains the phage Mx8 attP site. To generate pSL104, which contains pilT downstream of the pilA promoter, the primers opilT1 and opilT2 were used to amplify pilT. Subsequently, the PCR product was cloned in the plasmid pSW105. To generate mutations in the Walker A and Walker B boxes of pilT and pilB, one of the four mutagenic primers (opilTWalkerA, opilTWalkerB, opilBWalkerA, and opilBWalkerB) was used in a first round of PCR with opilT4 or opilB4 and pSL104 or pSL105 as templates. Products from the first PCRs were used as primers in a second PCR with the primers opilT1 or opilB1 and pSL104 or pSL105 as templates. PCR products were cloned in pSW105 to create pSL104TWalkerA, pSL104TWalkerB, pSL105BWalkerA, and pSL105BWalkerB. Plasmids were electroporated into wild-type M. xanthus and ΔpilT or ΔpilB mutant strains (21) and correct clones verified by PCR. To construct pSL5, which encodes the His6-pilB allele expressed from the tac promoter, pilB was amplified using the primers opilB3 (with an EcoRI site followed by six histidines codons and the first 22 nucleotides of pilB) and opilB4 (containing the last 19 nucleotides of pilB and a HindIII site). The PCR product was cloned in pUHE24-2 (24) to give pSL5. To construct pSL4, which contains the His6-pilT allele expressed from the tac promoter, pilT was cloned in pUHE24-2 as described for pilB except that the primers opilT3 (EcoRI site followed by codons for six histidines and the first 18 nucleotides of pilT) and opilT4 (HindIII site and the last 19 nucleotides of pilT) were used. Constructs overexpressing His6-PilB with substitutions in either the Walker A or Walker B box were created by EcoRV/HindIII excising a fragment containing the mutation from pSL105BWalkerA or pSL105BWalkerB, followed by cloning in pSL5 to obtain pSL5BWalkerA and pSL5BWalkerB. The same strategy was used to generate pSL4TWalkerA and pSL4TWalkerB, which encode His6-PilT proteins containing substitutions in either the Walker A or Walker B box. Expression plasmids were propagated in JM109 [F′ traD36 proA+B+ lacIq ΔlacZM15/Δ(lac-proAB) glnV44 e14− gyrA96 recA1 relA1 endA1 thi hsdR17] (New England Biolabs) with the plasmid pMS421 (11).
Cell fractionation and Western blots.
Biochemical fractionation of cells was done as described previously (57). Briefly, 50 ml of exponentially growing M. xanthus cells of DK1622GFP, which expresses the green fluorescent protein (GFP) from the pilA promoter, was grown in CTT medium to a cell density of 5 × 108 cells per ml, harvested, and resuspended in 2.5 ml of buffer containing 20% sucrose (wt/vol) and 50 mM Tris-HCl (pH 7.6) supplemented with Complete Mini protease inhibitor cocktail (Roche). After incubation for 10 min at 25°C, cells were harvested and osmotically shocked by resuspension in 0.5 ml of 4°C 50 mM Tris-HCl (pH 7.6) with the protease inhibitor cocktail. After a short incubation at 4°C, spheroplast formation was verified microscopically. The supernatant (containing periplasmic proteins) was separated from spheroplasts by centrifugation at 4,400 × g for 10 min at 4°C. Spheroplasts were lysed by sonication and cleared from nonlysed cells by centrifugation at 16,000 × g for 10 min at 4°C. The cleared lysate was centrifuged at 100,000 × g for 1 h at 4°C to separate soluble, cytoplasmic proteins from the insoluble, membrane-enriched fraction. Proteins from all collected fractions (periplasmic, cytoplasmic, and membrane) were precipitated with acetone at −20°C overnight, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, separated by SDS-PAGE, and analyzed in Western blots. Western blotting was performed using standard procedures (42) with polyclonal anti-PilT and anti-PilB antibodies and peroxidase-conjugated goat anti-rabbit immunoglobulin G as secondary antibodies as recommended by the manufacturer (Roche). As controls for proper fractionation, fractions were tested with antibodies against GFP in the cytoplasm, full-length CsgA protein in the outer membrane (23, 25), and the inner membrane protein PilC (V. Jakovljevic and L. Søgaard-Andersen, unpublished). For GFP, monoclonal anti-GFP mouse antibody (Roche) and peroxidase-conjugated rabbit anti-mouse secondary antibody (DakoCytomation) were used. Blots were developed using the Supersignal West Pico chemiluminescence reagent (Pierce).
Purification of His6-PilB.
JM109/pMS421 containing pSL5 was grown in 2× YT at 37°C (42) and His6-PilB expression induced by addition of 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Cells were harvested at 5,000 × g for 20 min, resuspended in NLT buffer (300 mM NaCl, 50 mM Tris-HCl, 10% glycerol, 5 mM β-mercaptoethanol, 10 mM imidazole, pH 8.0), and lysed by sonication. The cell lysate was cleared at 13.000 × g for 30 min at 4°C and His6-PilB in the supernatant purified using Ni-nitrilotriacetic acid agarose (Qiagen) according to the recommendations of the supplier. Protein concentrations were determined using the Bradford assay (Bio-Rad). If necessary, purified His6-PilB was concentrated by ultrafiltration through an Microcon filter with a 30-kDa molecular-mass cutoff (Millipore). Mutant His6-PilB proteins were purified using the same procedure.
Purification of His6-PilT.
JM109/pMS421 containing pSL4 was grown in 2× YT at 37°C. Cells were harvested and lysed as described for PilB. After clearing of the cell lysate, the pellet containing His6-PilT was resuspended in DTB buffer (8 M urea, 0.1 M Tris-HCl, pH 8.0), incubated for 1 h at room temperature with shaking to dissolve inclusion bodies, and centrifuged for 30 min at 13,000 × g at 4°C. The supernatant was incubated with Ni-nitrilotriacetic acid agarose (Qiagen) and denatured His6-PilT purified according to the recommendations of the supplier. Protein concentrations were determined using the Bradford assay. To refold His6-PilT, 4 mg of denatured His6-PilT protein was added dropwise to 80 ml of refolding buffer A (0.5 M l-arginine, 1 mM dithiothreitol, 0.1 M Tris-HCl, pH 8.0) and gently stirred overnight at 4°C. The next day, 20 ml of the mixture was concentrated by ultrafiltration through an Amicon filter (Millipore) with a 30-kDa molecular-mass cutoff (Millipore). Buffer A was exchanged by addition of 10 ml of NLT buffer to the same filter and subsequent centrifugation at 4,000 × g to a final volume of 0.5 to 1 ml. Mutant His6-PilT proteins were purified using the same procedure.
Determination of ATPase activity of His6-PilB.
Phosphate release during ATP hydrolysis was measured using the EnzChek phosphate assay kit (Molecular Probes). Briefly, each reaction mixture contained 5 mM Tris-HCl, 10 mM MgCl2, 200 μM EnzChek MESG substrate (2-amino-6-mercapto-7-methyl-purine riboside), and 0.1 unit of purine nucleoside phosphorylase. In the presence of inorganic phosphate, MESG is converted via purine nucleoside phosphorylase to ribose-1-phosphate and 2-amino-6-mercapto-7-methylpurine, leading to an increase in A360. To determine the specific ATPase activity of His6-PilB, A360 was measured at 1-min intervals for 60 min at room temperature. Reactions were started by addition of ATP to a final concentration of 0.5 mM and His6-PilB to a concentration of 5 μM in a total volume of 100 μl. Phosphate release was determined by comparing the A360 to a standard curve prepared with KH2PO4 as described previously (48). The blank reaction was a reaction mixture including protein but without ATP. The blank value was subtracted from determinations of A360, the release of free phosphate estimated from the standard curve, and specific activity calculated.
Determination of ATPase activity of His6-PilT.
Reaction mixtures (25 μl) containing 15 μM of His6-PilT in NLT buffer supplemented with 50 mM KCl and 5 mM MgCl2 were prepared. Reactions were started by addition of radioactively labeled [α-32P]ATP (GE Heathcare) giving a specific activity of 3.2 MBq per ml and a final ATP concentration of 0.2 mM. After 30 min of incubation at room temperature, protein was removed by ultrafiltration using a Microcon filter with a 30-kDa molecular-mass cutoff (Millipore). Labeled adenosine phosphates were separated by thin-layer chromatography (TLC) as described previously (39). Briefly, aliquots of 1.0 μl of the eluate were applied to a poly(ethyleneimine)-cellulose FTLC plate (Merck) with 2.4 M formic acid as the solvent system. The labeled nucleotides on the TLC plates were visualized and quantified by phosphorimaging and analyzed using the ImageQuant software. [α-32P]ATP and [α-32P]ADP were located on the TLC plates using the products of ATP hydrolysis by the apyrase ATPase (Sigma-Aldrich) as markers.
Antibody generation.
To generate rabbit polyclonal anti-PilT and anti-PilB antibodies, His6-PilT and His6-PilB were overexpressed from pSL4 and pSL5, respectively, and purified under denaturing conditions as described for His6-PilT and used to immunize rabbits by standard procedures (42).
Transmission electron microscopy of T4P.
Transmission electron microscopy was used to visualize T4P essentially as described previously (54). Briefly, 50 μl of exponentially growing M. xanthus culture was placed on Parafilm. A small piece of carbon-coated mica was dipped into the drop for 30 s, allowing cells to adsorb to the surface of the carbon film. The carbon film was picked with a copper grid, excess liquid was soaked off, the film was placed briefly on a drop of distilled water, excess liquid was soaked off again, and the film was transferred on a drop of 2% uranyl acetate (wt/vol) for 2 seconds and blotted dry. Transmission electron microscopy was performed on a Philips EM 301 electron microscope at calibrated magnifications.
RESULTS
PilB and PilT of M. xanthus are ATPases.
PilB and PilT of M. xanthus conform to the general description of secretion ATPases and contain all four conserved sequence motifs in the C-terminal domain (Fig. 1). These analyses, taken together with the observation that PilB and PilT of M. xanthus are required for T4P formation and retraction (58, 59), respectively, made these two proteins a good pair for systematically analyzing the requirements for ATP binding and hydrolysis by PilB and PilT in T4P function.
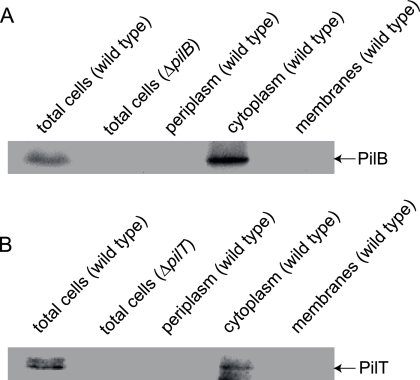
It has previously been suggested that PilT is localized to the periplasm (19). A periplasmic localization of PilT would argue against ATPase activity being important for function. To determine the localization of PilB and PilT, we carried out fractionation experiments in which total cell extract of strain DK1622GFP was separated into fractions enriched for cytoplasmic, periplasmic, and membrane proteins and subsequently probed with antibodies against either full-length His6-PilB or full-length His6-PilT. In immunoblot analyses of total cell extracts from DK1622GFP and DK10416, which carries an in-frame deletion of pilB, the anti-PilB antibodies recognized a protein with a size of 63 kDa in DK1622GFP, which was not present in DK10416 (Fig. 2A). The size of this protein is in good agreement with the calculated molecular mass of PilB of 63.2 kDa, thus confirming that the antibodies are specific. Likewise, in immunoblot analyses of total cell extracts from DK1622GFP and DK10409, which carries an in-frame deletion of pilT, the anti-PilT antibodies recognized a protein with a size of 39 kDa in DK1622GFP, which was not present in DK10409 (Fig. 2B). The size of this protein is in good agreement with the calculated molecular mass of PilT of 40.7 kDa, thus confirming that the antibodies are specific. In the cell fractionation experiments, PilB and PilT fractionated with the cytoplasmic fraction (Fig. 2A and B). Control experiments in which the fractions were tested with antibodies against the cytoplasmic GFP, full-length CsgA protein in the outer membrane (23, 25), and the inner membrane protein PilC (V. Jakovljevic and L. Søgaard-Andersen, unpublished) demonstrated that the fractionation functioned properly (data not shown). Thus, we conclude that PilB and PilT are cytoplasmic proteins.
FIG. 2.
Subcellular localization of PilB and PilT in M. xanthus. (A) PilB is a cytoplasmic protein. Cells of the wild-type DK1622GFP were separated into fractions enriched for periplasmic, cytoplasmic, and membrane proteins. Following separation by SDS-PAGE, the fractions were subjected to immunoblot analysis with anti-PilB antibodies. As controls, total cell lysates of DK1622GFP and ΔpilB cells (DK10416) were included. Protein from 5 × 106 cells was loaded per lane. (B) PilT is a cytoplasmic protein. The experiment was done as described for panel A except that anti-PilT antibodies were used and the control included ΔpilT cells (DK10409).
To test whether PilB of M. xanthus has ATPase activity, the pilB gene was cloned and PilB overexpressed in E. coli as an N-terminal hexahistidine fusion protein. His6-PilB was purified under native conditions by Ni2+ affinity chromatography. The apparent molecular mass of purified His6-PilB was 62 kDa as calculated from SDS-PAGE and was in good agreement with the calculated molecular mass of 63.8 kDa for His6-PilB (Fig. 3A). The specific ATPase activity of purified His6-PilB was measured using a coupled enzyme assay, which detects release of phosphate from ATP. As shown in Fig. 3C, we observed increased phosphate release with time when His6-PilB was incubated with ATP, demonstrating that His6-PilB possesses ATPase activity in vitro. We calculated the specific ATPase activity of His6-PilB to be 2.9 ± 0.6 nmol of phosphate min−1 mg−1 (mean ± standard deviation). As a positive control for ATPase activity, we used the enzyme apyrase, which was found to have a specific activity of 5.3 ± 0.2 μmol of phosphate min−1 mg−1 (data not shown), similar to that previously observed (48).
FIG. 3.
PilB and PilT of M. xanthus are ATPases. (A) Purification of wild-type and mutant PilB proteins. E. coli JM109/pMS421 containing pSL5 was grown in the absence of IPTG (uninduced) and in the presence of 0.1 mM IPTG (induced). Soluble His6-PilB was purified by Ni2+ affinity chromatography as described in Materials and Methods. His6-PilB(K327A) and His6-PilB(E391A) proteins were purified using a similar procedure. After SDS-PAGE, proteins were stained with Coomassie brilliant blue. Molecular size markers, with sizes in kDa, are included on the left. (B) PilT purification. E. coli JM109/pMS421 containing pSL4 was grown in the absence of IPTG (uninduced) and in the presence of 0.1 mM IPTG (induced). His6-PilT was isolated from inclusion bodies and purified by Ni2+ affinity chromatography as described in Materials and Methods. His6-PilT(K137A) and His6-PilT(E205A) proteins were purified using a similar procedure. After SDS-PAGE, proteins were stained with Coomassie brilliant blue. Molecular size markers, with sizes in kDa, are included on the left. (C) Time dependence of the ATPase activities of purified His6-PilB, His6-PilB(K327A), and His6-PilB(E391A) proteins. His6-PilB proteins were incubated as described in Materials and Methods, and the A360 was followed over time and converted into Pi released. Diamonds, wild type His6-PilB; squares, His6-PilB(K327A); triangles, His6-PilB(E391A); crosses, incubation buffer. (D) ATPase activities of His6-PilT, His6-PilT(K137A), and His6-PilT(E205A) proteins, showing an autoradiogram of labeled adenosine phosphates after incubation of [α-32P]ATP with the indicated His6-PilT proteins or apyrase followed by TLC. The positions of [α-32P]ATP and [α-32P]ADP are indicated.
We also sought to test whether His6-PilB forms a homo-oligomer in vitro using gel filtration chromatography. Under all conditions tested, i.e., in the presence or absence of ATP, ADP, the nonhydrolyzable ATP homolog ATP-γ-S, and Mg2+, His6-PilB eluted as a sharp peak with an estimated molecular mass of ∼60 kDa, corresponding to the size of the His6-PilB monomer (data not shown). Thus, we could not demonstrate that His6-PilB forms an oligomer.
PilT has been previously purified from A. aeolicus as a soluble C-terminal hexahistidine fusion (13) and as soluble N-terminal hexahistidine fusion proteins in the case of two cyanobacterial species (32, 33). We cloned pilT of M. xanthus and attempted to overexpress the protein either with an N-terminal hexahistidine tag or with a C-terminal hexahistidine tag. We obtained expression of only the N-terminal hexahistidine fusion protein (Fig. 3B). Under all expression conditions tested, His6-PilT was found in inclusion bodies (data not shown). Consequently, we purified His6-PilT under denaturing conditions and renatured it as described in Materials and Methods. The apparent molecular mass of purified His6-PilT was 39 kDa as calculated from SDS-PAGE, in agreement with the calculated molecular mass of 41.3 kDa for His6-PilT (Fig. 3B). In order to test His6-PilT for ATPase activity, we initially used the coupled enzyme assay described above for His6-PilB. However, under all conditions tested His6-PilT formed precipitates in this assay, thus precluding the determination of enzyme activity. Instead we used an enzyme assay in which the release of [α-32P]ADP from [α-32P]ATP was measured. His6-PilT remained soluble under the conditions used in this assay. Importantly, we observed a low but significant increase in accumulation of [α-32P]ADP when His6-PilT was incubated with α-32P-ATP (Fig. 3D). However, the specific ATPase activity of His6-PilT could not be determined because ATP hydrolysis reached saturation within 5 to 10 min of incubation (data not shown). As a positive control for ATPase activity in this assay we used apyrase, which was found to have a specific activity in this assay similar to that found under the conditions used to test His6-PilB (data not shown).
We also sought to determine whether His6-PilT forms a homo-oligomer in vitro using gel filtration chromatography. The A. aeolicus PilT protein was reported to form an oligomer in the absence of ATP and Mg2+ (13). The His6-PilT protein of M. xanthus eluted as a sharp peak with an estimated molecular mass of ∼ 40 kDa, corresponding to the size of a His6-PilT monomer, under all conditions tested, i.e., in the presence or absence of ATP, ADP, the nonhydrolyzable ATP homolog ATP-γ-S, and Mg2+ (data not shown). Thus, also for His6-PilT we could not demonstrate that it forms an oligomer.
Substitutions in the Walker A and Walker B boxes of PilB and PilT abolish ATPase activity.
Based on the recently solved structures of several secretion ATPases, the conserved lysine residue in the Walker A box is important for ATP binding and the conserved glutamate residue in the Walker B box is important for ATP hydrolysis (12, 40, 44, 46, 47, 60, 61).To investigate the roles of the Walker A and B boxes in PilB and PilT ATPase activity in vitro, the conserved lysine residue in the Walker A box and the conserved glutamate residue in the Walker B box were replaced individually with alanine in both proteins (Fig. 1).
The two mutant PilB proteins, PilB(K327A) and PilB(E391A), were purified as N-terminal hexahistidine tagged proteins as soluble proteins as described for the wild type protein (Fig. 3A). The two mutant PilT proteins, PilT(K137A) and PilT(E205A), were purified as N-terminal hexahistidine tagged proteins under denaturing conditions and then renatured as described for wild type His6-PilT (Fig. 3B).
The His6-PilB(K327A) and His6-PilB(E391A) proteins were strongly reduced in ATPase activity (Fig. 3C). Likewise, we were unable to detect ATP hydrolysis in the presence of the His6-PilT(K137A) and His6-PilT(E205A) proteins (Fig. 3D). These observations confirm that the ATPase activities measured for the wild type proteins were due to His6-PilB and His6-PilT rather than contaminating proteins. Moreover, these observations verify the importance of the conserved lysine residue in the Walker A box and the conserved glutamate residue in the Walker B box for PilB and PilT ATPase activity in vitro. Based on the structure of secretion ATPases and the analyses of mutant afGspE proteins (60), we suggest that the Walker A box substitutions (in His6-PilB(K327A) and His6-PilT(K137A)) abolish ATPase activity indirectly by interfering with ATP binding and that the Walker B box substitutions (in His6-PilB(E391A) and His6-PilT(E205A)) abolish ATPase activity by directly interfering with ATP hydrolysis.
PilB and PilT ATPase activity is essential for T4P-dependent motility.
In-frame deletion mutants of pilB and pilT of M. xanthus abolish T4P-dependent motility (58, 59). To determine genetically whether ATP binding and hydrolysis by PilB affect T4P-dependent motility in M. xanthus, we introduced the wild type pilB allele and the two mutant pilB alleles, pilB(K327A) and pilB(E391A), into the strain DK10416, which carries an in-frame deletion of pilB. In the three plasmids containing the pilB alleles, the pilB genes were expressed from the constitutively active pilA promoter. The three plasmids were integrated by site-specific recombination at the phage Mx8 attB site on the chromosome giving rise to SA2021 (ΔpilB/pilB+), SA2414 (ΔpilB/pilB(K327A)) and SA2415 (ΔpilB/pilB(E391A)). To determine whether ATP binding and hydrolysis by PilT affect T4P-dependent motility, we used a similar strategy and introduced plasmids containing the wild type pilT allele and the two mutant pilT alleles, pilT(K137A) and pilT(E205A), into the strain DK10409, which carries an in-frame deletion of pilT, giving rise to SA2020 (ΔpilT/pilT+), SA2412 (ΔpilT/pilT (K137A)) and SA2413 (ΔpilT/pilT(E205A)).
We analyzed the six strains for T4P-dependent motility defects on 0.5% agar, which favors motility by means of T4P using the wild type strain DK1622 as a positive control, and the strains DK10416 (ΔpilB), DK10409 (ΔpilT) and DK10410, which carries an in-frame deletion of the pilA gene that codes for the T4P subunit, as negative controls. As shown in Fig. 4A, the wild type DK1622 formed colonies with large rafts of cells at the edge typical of T4P-dependent motility whereas DK10416 (ΔpilB), DK10409 (ΔpilT) and DK10410 (ΔpilA) did not form rafts of cells at the edge, thus, confirming that pilB, pilT and pilA are required for T4P-dependent motility. Moreover, SA2021 (ΔpilB/pilB+) and SA2020 (ΔpilT/pilT+) displayed a motility phenotype similar to that of the wild type DK1622. Thus, pilB+ and pilT+ expressed from the pilA promoter fully complemented the defects in T4P-dependent motility in the respective in-frame deletion mutants. Importantly, SA2414 (ΔpilB/pilB(K327A)) and SA2415 (ΔpilB/pilB(E391A)) displayed motility defects similar to those observed in DK10416 (ΔpilB). Thus, pilB(K327A) and pilB(E391A) were unable to complement the defect in T4P-dependent motility caused by the ΔpilB mutation. Also, SA2412 (ΔpilT/pilT(K137A)) and SA2413 (ΔpilT/pilT(E205A)) displayed motility defects similar to those observed in DK10409 (ΔpilT). Thus, pilT(K137A) and pilT(E205A) were unable to complement the defect in T4P-dependent motility caused by the ΔpilT mutation.
FIG. 4.
T4P-dependent motility of M. xanthus strains containing mutant pilB and pilT alleles. (A) T4P-dependent motility of the indicated strains on 0.5% agar plates. Cells were incubated for 24 h on 0.5% agar supplemented with 0.5% CTT. Strain names and relevant genotypes are indicated above each panel. Bar, 1 mm. (B) Accumulation of PilB and PilT wild-type and mutant proteins. Cells from exponentially growing cultures of the indicated strains were harvested, and total protein was separated by SDS-PAGE and analyzed by immunoblotting using anti-PilB (left panel) or anti-PilT (right panel) antibodies. Protein from 5 × 107 cells was loaded per lane.
Immunoblot analysis with anti-PilB and anti-PilT antibodies confirmed that the PilB+, PilB(K327A), and PilB(E391A) proteins in SA2021, SA2414, and SA2415, respectively, accumulated at levels similar to that observed for PilB+ in the wild-type DK1622 and that the PilT+, PilT(K137A), and PilT(E205A) proteins in SA2020, SA2412, and SA2413, respectively, accumulated at levels similar to that observed for PilT+ in DK1622 (Fig. 4B). From these analyses we conclude that ATPase activity of PilB as well as of PilT is essential for T4P-dependent motility in M. xanthus. Moreover, our data suggest that not only ATP hydrolysis [likely directly abolished in PilB(E391A) and PilT(E205A)] but also ATP binding by PilB and PilT [likely directly abolished in PilB(K327A) and PilT(K137A)] is essential for T4P-dependent motility in M. xanthus.
PilB ATPase activity is essential for T4P extension, and PilT ATPase activity is essential for T4P retraction.
We used transmission electron microscopy to determine whether the lack of T4P-dependent motility in the Walker A and Walker B box mutants of PilB and PilT was due to the lack of T4P or due to nonfunctionality of T4P. Approximately 20 cells of each strain were analyzed for the presence of T4P (Fig. 5 and Table 2). As previously reported (20), the wild-type strain DK1622 assembled T4P in a unipolar pattern. On average nine T4P per cell assembled per pole. As previously reported (58, 59), DK10416 (ΔpilB) and DK10410 (ΔpilA) did not assemble T4P, whereas DK10409 (ΔpilT) assembled T4P in a unipolar pattern. T4P formation was restored to wild-type levels in SA2021 (ΔpilB/pilB+), whereas SA2414 [ΔpilB/pilB(K327A)] and SA2415 [ΔpilB/pilB(E391A)] did not assemble T4P. SA2020 (ΔpilT/pilT+), SA2412 [ΔpilT/pilT(K137A)], and SA2413 [ΔpilT/pilT(E205A)] all formed T4P at wild-type levels, and in all cells analyzed the T4P were localized to one pole. These data, taken together with the defects in T4P-dependent motility in the various mutants, suggest that ATP binding and ATP hydrolysis by PilB are important for T4P extension and that ATP binding and ATP hydrolysis by PilT are not essential for T4P formation but are essential for T4P retraction.
FIG. 5.
Electron micrographs of M. xanthus strains. Cells from exponentially growing cultures of the indicated strains were directly transferred to a grid, stained with 2% (wt/vol) uranyl acetate, and visualized using transmission electron microscopy. Bar, 0.1 μm.
TABLE 2.
T4P formation in M. xanthus mutants carrying substitutions in the Walker A and Walker B boxes of PilB and PilT
| Strain | Genotype | No. of cells
|
No. of pili per cell | |||
|---|---|---|---|---|---|---|
| Total scored | Nonpiliated | Piliated at one pole | Piliated at both poles | |||
| DK1622 | Wild type | 21 | 6 | 15 | 0 | 9 |
| DK10410 | ΔpilA | 21 | 21 | 0 | 0 | 0 |
| DK10416 | ΔpilB | 20 | 20 | 0 | 0 | 0 |
| SA2021 | ΔpilB/pilB+ | 20 | 2 | 18 | 0 | 7 |
| SA2414 | ΔpilB/pilB(K328A) | 20 | 20 | 0 | 0 | 0 |
| SA2415 | ΔpilB/pilB(E392A) | 20 | 20 | 0 | 0 | 0 |
| DK10409 | ΔpilT | 21 | 2 | 19 | 0 | 7 |
| SA2020 | ΔpilT/pilT+ | 21 | 2 | 19 | 0 | 10 |
| SA2412 | ΔpilT/pilT(K138A) | 21 | 3 | 18 | 0 | 7 |
| SA2413 | ΔpilT/pilT(E206A) | 21 | 5 | 16 | 0 | 9 |
DISCUSSION
Here, we present the first report of a study in which the function of a pair of PilB and PilT proteins acting in the same T4P system has been analyzed systematically in vitro and in vivo. We showed directly in vitro that both PilB and PilT of M. xanthus have ATPase activity. PilB and PilT of M. xanthus both contain the conserved Walker A and Walker B boxes. The structures of five secretion ATPases (12, 40, 44, 46, 47, 60, 61) have revealed that the conserved lysine residue in the Walker A box is important for binding of ATP by making contacts to the β-phosphate and that the conserved glutamate residue in the Walker B box is pointing toward the γ-phosphate of ATP and appropriately positioned to activate a water molecule during hydrolysis. The five structures of secretion ATPases allowed us to use a structure-guided approach to replace amino acid residues in PilB and PilT that are predicted to be involved in ATP binding or ATP hydrolysis, respectively. To determine the importance of ATP binding for PilB and PilT activity, we replaced the conserved lysine residues in the Walker B box with alanine. To determine the importance of ATP hydrolysis for PilB and PilT activity, we replaced the conserved glutamate residue in the Walker B box with alanine. As expected, all four mutant proteins displayed a loss of ATPase activity in vitro, confirming the importance of the replaced residues for ATPase activity as suggested by the structural analyses. Importantly, when tested in vivo, the Walker A and Walker B box mutants of PilB were unable to complement the defect in T4P-dependent motility in a ΔpilB mutant. Moreover, a ΔpilB mutant containing the mutant pilB alleles did not assemble T4P. These data strongly suggest that ATP binding as well as ATP hydrolysis by PilB is essential for T4P assembly. For the two mutant PilT proteins we observed that the Walker A and Walker B box mutants were unable to complement the defect in T4P-dependent motility in a ΔpilT mutant. However, the ΔpilT mutant containing the mutant PilT proteins still assembled T4P. These data strongly suggest that ATP binding as well as ATP hydrolysis by PilT is essential for T4P retraction. The distinct phenotypes of insertion mutations in pilB and pilT previously led to the suggestion by Whitchurch et al. (56) that PilB and PilT act at distinct steps in the T4P extension/retraction cycle and function antagonistically in T4P extension/retraction, with PilB promoting the addition of pilin subunits to the growing pilus and PilT promoting their removal. Our data support this model, with the addition that ATP binding and hydrolysis by PilB and PilT are essential for their antagonistic functions.
For His6-PilB a specific ATPase activity of 2.9 nmol min−1 mg−1 was measured. This specific activity is similar to that previously reported for the PilB ortholog PilQ of the thin conjugative pilus system in E. coli (1 nmol min−1 mg−1) (41) is but significantly lower than that for the PilB ortholog BfpD of the bundle-forming pili in E. coli (62.2 nmol min−1 mg−1) (8). It has previously been reported that PilT proteins of mesophilic bacteria tend to be poorly soluble (1). Consistently, we observed that His6-PilT of M. xanthus under all conditions tested formed inclusion bodies. His6-PilT purified from the inclusion bodies, denatured, and renatured formed precipitates under the assay conditions used to monitor ATPase activity of His6-PilB. However, using an assay in which we monitored the conversion of [α-32P]ATP to [α-32P]ADP, we measured a low but significant ATPase activity of His6-PilT. However, because His6-PilT did not follow standard reaction kinetics, the specific ATPase activity could not be determined. The specific activities of two PilT proteins have been reported; i.e., PilT of A. aeolicus has a specific activity of 15.7 nmol min−1 mg−1 (13), and PilT of M. aeruginosa has a specific activity of 37.5 nmol min−1 mg−1 (32). In vivo several factors may contribute to increasing the ATPase activity of secretion ATPases; i.e., for BfpD it was reported that ATPase activity is stimulated 1,200-fold by the interaction with cytoplasmic domains of the inner membrane proteins BfpC and BfpE (8), for EpsE of the TT2S in V. cholerae acidic phospholipids were reported to stimulate activity in combination with a cytoplasmic domain of the inner membrane protein EpsL (2), and for XpsE of the T2SS in X. campestris ATPase activity is stimulated by interaction with the cytoplasmic domain of the inner membrane protein XpsL (50). Structural analyses of traffic ATPases strongly suggest that the active forms of these proteins are hexameric and that intersubunit interactions in the hexamers would be important for ATPase activity (12, 40, 44, 47, 60, 61). We observed hexamer formation neither by His6-PilB nor by His6-PilT of M. xanthus. We speculate that the active form of PilB and PilT in vivo is hexameric and that the absence of hexamer formation in vitro may contribute to the low ATPase activities observed for PilB and PilT of M. xanthus.
The molecular mechanisms by which PilB and PilT function in the extension/retraction cycle of T4P remain unknown. In principle, PilB and PilT could act as molecular motors that facilitate the incorporation of and removal of pilin subunits, respectively (28). According to this model, ATP binding by PilB followed by hydrolysis would provide the energy for the insertion of pilin subunits from a reservoir in the inner membrane into the growing pilus, and ATP binding by PilT followed by ATP hydrolysis would provide the energy for the removal of pilin subunits from the shrinking pilus into the inner membrane. A model for direct assembly of pilin subunits stimulated by PilB has been proposed in which ATP hydrolysis by PilB provides force to an unknown inner membrane protein, which in turn acts like a mechanical piston to push pilin subunits into a growing pilus (7). In the “power stroke” model, PilT was proposed to retract T4P by direct removal of pilin subunits from the base of the pilus (19). However, the power stroke model is based on the assumption that PilT is localized to the periplasm, which is in disagreement with our observations of the cytoplasmic localization of PilT.
However, other models are also possible for the function of PilB and PilT. Some biological fibers assemble and disassemble spontaneously (26). Merz and Forest (28) noticed that formally only one step in T4P function requires energy, with the second process occurring spontaneously. Thus, if T4P assembly is energetically favorable and with pilin subunits melting spontaneously from the inner membrane into the growing T4P, then PilT could act as a molecular motor generating the energy required to retract T4P. In this model, PilB ATP binding and hydrolysis would have a regulatory function to stimulate the switch from retraction to assembly. Alternatively, assembly of T4P could be energy dependent and with PilB acting as a molecular motor providing the energy required for the incorporation of pilin subunits into the growing pilus. In this model, PilT ATP binding and hydrolysis could have a regulatory function, which would serve to stimulate the switch from assembly to retraction. Interestingly, it was recently observed in M. xanthus that a 2-fold decrease in the level of accumulation of the pilin subunit PilA resulted in a 10-fold decrease in the level of assembled T4P (17). These observations seem to support a spontaneous T4P assembly hypothesis. Clearly, our data do not allow us to distinguish between these models. However, our data show that irrespective of the function of PilB (molecular motor or regulator) and PilT (molecular motor or regulator), ATP hydrolysis is required by both proteins.
Acknowledgments
We thank Dale Kaiser for providing strains and Bernd Bukau for helpful discussions.
The graduate program “Intra- and intercellular transport and communication” and the Max Planck Society supported this work.
Footnotes
Published ahead of print on 25 January 2008.
REFERENCES
- 1.Aukema, K. G., E. M. Kron, T. J. Herdendorf, and K. T. Forest. 2005. Functional dissection of a conserved motif within the pilus retraction protein PilT. J. Bacteriol. 187611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camberg, J. L., T. L. Johnson, M. Patrick, J. Abendroth, W. G. Hol, and M. Sandkvist. 2007. Synergistic stimulation of EpsE ATP hydrolysis by EpsL and acidic phospholipids. EMBO J. 2619-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camberg, J. L., and M. Sandkvist. 2005. Molecular analysis of the Vibrio cholerae type II secretion ATPase EpsE. J. Bacteriol. 187249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonnelle, E., S. Helaine, L. Prouvensier, X. Nassif, and V. Pelicic. 2005. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fiber stability and function. Mol. Microbiol. 5554-64. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., S.-J. Shiue, C.-W. Huang, J.-L. Chang, Y.-L. Chien, N.-T. Hu, and N.-L. Chan. 2005. Structure and function of the N-terminal domain, an essential component of the Xanthomonas campestris type II secretion system. J. Biol. Chem. 28042356-42363. [DOI] [PubMed] [Google Scholar]
- 6.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenecity. Nat. Rev. Microbiol. 2363-378. [DOI] [PubMed] [Google Scholar]
- 7.Craig, L., N. Volkmann, A. S. Arvai, M. E. Pique, M. Yeager, E. H. Egelman, and J. A. Tainer. 2006. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23651-662. [DOI] [PubMed] [Google Scholar]
- 8.Crowther, L. J., A. Yamagata, L. Craig, J. A. Tainer, and M. S. Donnenberg. 2005. The ATPase activity of BfpD is greatly enhanced by zinc and allosteric interactions with other Bfp proteins. J. Biol. Chem. 28024839-24848. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Dubnau, D. 1999. DNA uptake in bacteria. Annu. Rev. Microbiol. 53217-244. [DOI] [PubMed] [Google Scholar]
- 10.Durand, E., A. Bernadac, G. Ball, A. Lazdunski, J. N. Sturgis, and A. Filloux. 2003. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J. Bacteriol. 1852749-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grana, D., T. Gardella, and M. M. Susskind. 1988. The effects of mutations in the ant promoter of phage P22 depend on context. Genetics 120319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hare, S., R. Bayliss, C. Baron, and G. Waksman. 2006. A large domain swap inthe VirB11 ATPase of Brucella suis leaves the hexameric assembly intact. J. Mol. Biol. 36056-66. [DOI] [PubMed] [Google Scholar]
- 13.Herdendorf, T. J., D. R. McCaslin, and K. T. Forest. 2002. Aquifex aeolicus PilT, homologue of a surface motility protein, is a thermostable oligomeric NTPase. J. Bacteriol. 1846465-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgkin, J., and D. Kaiser. 1977. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 742938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgkin, J., and D. Kaiser. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol. Gen. Genet. 171177-191. [Google Scholar]
- 16.Hu, N. T., W. M. Leu, M. S. Lee, A. Che, S. C. Cheng, Y. L. Song, and L. Y. Chen. 2002. XpsG, the major pseudopilin in Xanthomonas campestris pv campestris, forms a pilis-like structure between cytoplasmic and outer membrane. Biochem. J. 365205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelsbak, L., and D. Kaiser. 2005. Regulating pilin expression reveals a threshold for S motility in Myxococcus xanthus. J. Bacteriol. 1872105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelsbak, L., and L. Søgaard-Andersen. 2002. Pattern formation by a cell surface-associated morphogen in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 992032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser, D. 2000. Bacterial motility: how do pili pull? Curr. Biol. 10R777-780. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 765952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashefi, K., and P. L. Hartzell. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF defect. Mol. Microbiol. 15483-494. [DOI] [PubMed] [Google Scholar]
- 22.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 5061-68. [DOI] [PubMed] [Google Scholar]
- 23.Kruse, T., S. Lobedanz, N. M. S. Berthelsen, and L. Søgaard-Andersen. 2001. C-signal: a cell surface-associated morphogen that induces and coordinates multicellular fruiting body morphogenesis and sporulation in M. xanthus. Mol. Microbiol. 40156-168. [DOI] [PubMed] [Google Scholar]
- 24.Lanzer, M., and H. Bujard. 1988. Promoters largely determine the efficiency of repressor action. Proc. Natl. Acad. Sci. USA 858973-8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobedanz, S., and L. Søgaard-Andersen. 2003. Identification of the C-signal, a contact-dependent morphogen coordinating multiple developmental responses in Myxococcus xanthus. Genes Dev. 172151-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahadevan, L., and P. Matsudaira. 2000. Motility powered by supramolecular springs and ratchets. Science 28895-100. [DOI] [PubMed] [Google Scholar]
- 27.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56289-314. [DOI] [PubMed] [Google Scholar]
- 28.Merz, A. J., and K. T. Forest. 2002. Bacterial surface motility: slime trails, grappling hooks and nozzles. Curr. Biol. 12R297-R303. [DOI] [PubMed] [Google Scholar]
- 29.Merz, A. J., M. So, and M. P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature 40798-102. [DOI] [PubMed] [Google Scholar]
- 30.Mignot, T., J. P. Merlie, Jr., and D. R. Zusman. 2005. Regulated pole-to-pole oscillations of a bacterial gliding motility protein. Science 310855-857. [DOI] [PubMed] [Google Scholar]
- 31.Morand, P. C., E. Bille, S. Morelle, E. Eugène, J. L. Beretti, M. Wolfgang, T. F. Meyer, M. Koomey, and X. Nassif. 2004. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 232009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakasugi, K., R. Alexova, C. J. Svenson, and B. A. Neilan. 2007. Functional analysis of PilT from the toxic cyanobacterium Microcystis aeruginosa PCC 7806. J. Bacteriol. 1891689-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto, S., and M. Ohmori. 2002. The cyanobacterial PilT protein responsible for cell motility and transformation hydrolyzes ATP. Plant Cell Physiol. 431127-1136. [DOI] [PubMed] [Google Scholar]
- 34.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30295-304. [DOI] [PubMed] [Google Scholar]
- 35.Peabody, C. R., Y. J. Chung, M.-R. Yen, D. Vidal-Ingigliardi, A. P. Pugsley, and M. H. Saier, Jr. 2003. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 1493051-3072. [DOI] [PubMed] [Google Scholar]
- 36.Planet, P. J., S. C. Kachlany, R. DeSalle, and D. H. Figurski. 2001. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc. Natl. Acad. Sci. USA 982503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Possot, O., and A. P. Pugsley. 1994. Molecular characterization of PulE, a protein required for pullulanase secretion. Mol. Microbiol. 12287-299. [DOI] [PubMed] [Google Scholar]
- 38.Py, B., L. Loiseau, and F. Barras. 1999. Assembly of the type II secretion machinery of Erwinia chrysanthemi: direct interaction and associated conformational change between OutE, the putative ATP-binding component and the membrane component OutL. J. Mol. Biol. 289659-670. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen, A. A., S. Wegener-Feldbrügge, S. L. Porter, J. P. Armitage, and L. Søgaard-Andersen. 2006. Four signalling domains in the hybrid histidine protein kinase RodK of Myxococcus xanthus are required for activity. Mol. Microbiol. 60525-534. [DOI] [PubMed] [Google Scholar]
- 40.Robien, M. A., B. E. Krumm, M. Sandkvist, and W. G. Hol. 2003. Crystal structure of the extracellular protein secretion NTPase EpsE of Vibrio cholerae. J. Mol. Biol. 333657-674. [DOI] [PubMed] [Google Scholar]
- 41.Sakai, D., T. Horiuchi, and T. Komano. 2001. ATPase activity and multimer formation of PilQ protein are required for thin pilus biogenesis in plasmid R64. J. Biol. Chem. 27617968-17975. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Sandkvist, M., M. Bagdasarian, S. P. Howard, and V. J. DiRita. 1995. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 141664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satyshur, K. A., G. A. Worzalla, L. S. Meyer, E. K. Heiniger, K. G. Aukema, A. M. Misic, and K. T. Forest. 2007. Crystal structures of the pilus retraction motor PilT suggest large domain movements and subunit cooperation drive motility. Structure 15363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauvonnet, N., G. Vignon, A. P. Pugsley, and P. Gounon. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 192221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savvides, S. N. 2007. Secretion superfamily ATPases swing big. Structure 15255-257. [DOI] [PubMed] [Google Scholar]
- 47.Savvides, S. N., H. J. Yeo, M. R. Beck, F. Blaesing, R. Lurz, E. Lanka, R. Buhrdorf, W. Fischer, R. Haas, and G. Waksman. 2003. VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. EMBO J. 221969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sexton, J. A., J. S. Pinkner, R. Roth, J. E. Heuser, S. J. Hultgren, and J. P. Vogel. 2004. The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J. Bacteriol. 1861658-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, W., and D. R. Zusman. 1993. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc. Natl. Acad. Sci. USA 903378-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiue, S.-J., K.-M. Kao, W.-M. Leu, L.-Y. Chen, N.-L. Chan, and N.-T. Hu. 2006. XpsE oligomerization triggered by ATP binding, not hydrolysis, leads to its association with XpsL. EMBO J. 251426-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skerker, J. M., and H. C. Berg. 2001. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA 986901-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, H., D. R. Zusman, and W. Shi. 2000. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 101143-1146. [DOI] [PubMed] [Google Scholar]
- 53.Turner, L. R., J. C. Lara, D. N. Nunn, and S. Lory. 1993. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 1754962-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valentine, R. C., B. M. Shapiro, and E. R. Stadtman. 1968. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry 72143-2152. [DOI] [PubMed] [Google Scholar]
- 55.Wall, D., and D. Kaiser. 1999. Type IV pili and cell motility. Mol. Microbiol. 321-10. [DOI] [PubMed] [Google Scholar]
- 56.Whitchurch, C. B., M. Hobbs, S. P. Livingston, V. Krishnapillai, and J. S. Mattick. 1991. Characterisation of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system widespread in eubacteria. Gene 10133-44. [DOI] [PubMed] [Google Scholar]
- 57.White, D. J., R. Merod, B. Thomasson, and P. L. Hartzell. 2001. GidA is an FAD-binding protein involved in development of Myxococcus xanthus. Mol. Microbiol. 42503-517. [DOI] [PubMed] [Google Scholar]
- 58.Wu, S. S., and D. Kaiser. 1995. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol. Microbiol. 18547-558. [DOI] [PubMed] [Google Scholar]
- 59.Wu, S. S., J. Wu, and D. Kaiser. 1997. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 23109-121. [DOI] [PubMed] [Google Scholar]
- 60.Yamagata, A., and J. A. Tainer. 2007. Hexameric structures of the archaeal secretion ATPase GspE and implications for a universal secretion mechanism. EMBO J. 26878-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeo, H. J., S. N. Savvides, A. B. Herr, E. Lanka, and G. Waksman. 2000. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 61461-1472. [DOI] [PubMed] [Google Scholar]