Abstract
Vacuolar myelopathy (VM) is a frequent central nervous system complication of human immunodeficiency virus type 1 (HIV-1) infection. We report here that transgenic (Tg) mice expressing even low levels of Nef in oligodendrocytes under the regulation of the myelin basic protein (MBP) promoter (MBP/HIVNef) developed VM similar to the human disease in its appearance and topography. The spinal cords of these Tg mice showed lower levels of the myelin proteins MAG and CNPase and of the 21-kDa isoform of MBP prior to the development of vacuoles. In addition, Tg oligodendrocytes in primary in vitro cultures appeared morphologically more mature but, paradoxically, exhibited a less mature phenotype based on O4, O1, CNPase, and MBP staining. In particular, mature CNPase+ MBP+ Tg oligodendrocytes were less numerous than non-Tg oligodendrocytes. Therefore, Nef appears to affect the proper differentiation of oligodendrocytes. These data suggest that even low levels of Nef expression in human oligodendrocytes may be responsible for the development of VM in HIV-1-infected individuals.
Vacuolar myelopathy (VM) develops in more than one-fourth of human immunodeficiency virus type 1 (HIV-1)-infected individuals (12, 14, 36, 45). The disease is characterized by the presence of interlamellar vacuoles in white matter tracts of the spinal cord, mainly at the cervical and thoracic level, often associated with infiltrating macrophages around vacuoles. The pathogenesis of this HIV-1-associated disease is not well understood. Infection of oligodendrocytes by HIV-1 has been reported to be undetectable (8, 16, 25, 26, 34, 44, 53, 54) or infrequent (15, 19, 40, 50) in the central nervous systems (CNS) of AIDS patients. However, the more sensitive in situ reverse transcription-PCR technique has demonstrated that the oligodendrocytes of AIDS patients are targets for HIV-1 infection (2, 4). Furthermore, primary cultures of HIV-1-infected microglial cells, the principal resident CNS targets for HIV-1 infection, are able to transmit virus to primary human oligodendrocyte cultures (1). Thus, oligodendrocytes appear to be a potential CNS cell target population for infection by HIV-1 and as such may be directly susceptible to the detrimental effects of HIV-1 proteins.
We previously characterized a transgenic (Tg) mouse model of HIV-1-associated VM, where the entire genome of HIV-1 (strain pNL4-3) was expressed in oligodendrocytes under the control of the myelin basic protein (MBP) promoter (MBP/HIVwt) (17). These Tg mice developed VM closely resembling that described in human AIDS, with lesions in cervical and thoracic spinal cord associated with spastic paralysis in aged mice.
To identify the viral gene(s) responsible for inducing vacuole formation, we began a mutational analysis of the HIV-1 genome. We first generated Tg mice harboring the MBP/HIVNef transgene in which all of the coding sequences of HIV-1 except nef were mutated. The rationale for this choice came from two observations in our laboratory: that expression of HIV-1 Nef in the immune systems of Tg mice resulted in the development of a severe AIDS-like disease (20, 39) and that Nef was the predominant protein detected in the CNS of MBP/HIVwt Tg mice (17). Moreover, the finding that Nef is also expressed in another glial cell type (astrocytes) of HIV-1-infected individuals (41, 43, 52) influenced our choice.
We report here that MBP/HIVNef Tg mice develop VM which is histologically indistinguishable from that observed in MBP/HIVwt Tg mice. We found that even low levels of Nef expression were sufficient to elicit disease. In addition, studies of primary enriched oligodendrocytes in culture revealed an abnormal differentiation of Tg oligodendrocytes. These results suggest that, like T cells expressing Nef (20), oligodendrocyte signal transduction pathway(s) may be altered by the presence of Nef.
MATERIALS AND METHODS
Transgene construction and generation of Tg mice.
MBP promoter sequences (51) were ligated to the G mutant of HIV-1 pNL4-3 (20) to generate the MBP/HIV-1Nef transgene (Fig. 1). Tg founders and their offspring were bred as heterozygotes with C3H/He mice (Charles River Canada). The identification of MBP/HIV-1Nef Tg mice was done on tail DNA by the Southern procedure by using a 32P-labeled 1.4-kbp HindIII-SacI HIV-1 pNL4-3 DNA fragment as a hybridizing probe as previously described (17) or by the PCR procedure with the HIV-1-related sense (5′-CATGGAGCAATCACAAGTAG) and antisense (5′-GGTACTAGCTTGAAGCACCA) oligonucleotides. Tg and non-Tg littermates were housed in the same room.
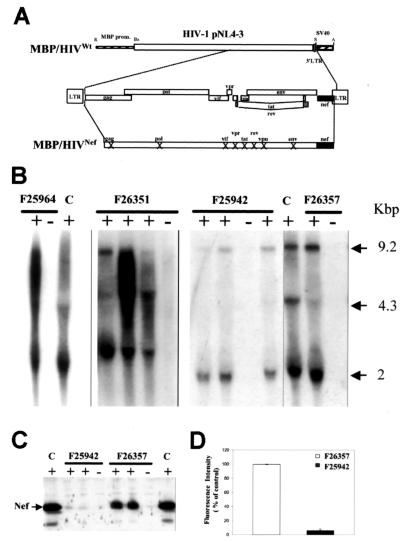
FIG. 1.
Structure and expression of the MBP/HIVNef transgene. (A) Diagram of the structure of the MBP/HIVNef transgene. Thin hatched box, MBP gene promoter sequences; open box, HIV-1 pNL4-3 DNA fragment; thick hatched box, simian virus 40 polyadenylation sequences. A, AatII; Bs, BsshII; R, EcoRI; S, SacI, LTR, long terminal repeat. The Xs in the lowest bar indicate HIV-1 open reading frames that have been mutated. (B) Northern blotting was done with total RNA (10 μg) extracted from CNS tissues with the 32P-labeled HIV-1-specific probe. RNA from the brain of an MBP/HIVwt Tg mouse was the control. C, control. (C) Western blot of HIV-1 Nef protein. Protein extracts (70 μg) from the thoracic spinal cord were evaluated with anti-Nef Ab (1/1,000). Protein extracts from CD4C/HIVMutG Tg thymus were the controls. (D) Quantitation of Nef protein in two founders. Expression in F26357 mice was adjusted to 100%.
RNA purification and Northern blotting.
Total RNAs from brains and spinal cords were prepared and hybridized as previously described (17).
In situ hybridization (ISH).
Tissue sections were hybridized with α-35S-UTP-labeled antisense and sense HIV-1 or CNPase (2′, 3′-cyclic nucleotide 3′-phosphodiesterase)-specific riboprobes as previously described (17). The CNPase riboprobe was synthesized from full-length rat cDNA (2.3 kbp) (18) subcloned into the EcoRI site of pBluescript SK.
Immunocytochemistry (IHC).
Detection of Nef was carried out on cryostat sections of spinal cord with rabbit anti-Nef antisera (21) (1/100) and with a VECTASTAIN anti-rabbit peroxidase kit (Vector Laboratories). Cells of the oligodendrocyte lineage were identified by labeling with antigalactocerebroside (anti-[GalC]) O1 or antisulfatide O4 monoclonal antibodies (MAb) (5) and with anti-CNPase and anti-MBP (Sternberger Monoclonals Inc.) antibodies (Abs). For O4 and O1 labeling, cells were fixed in 4% paraformaldehyde and incubated with the Ab for 1 h at room temperature. For CNPase and MBP immunostaining, cells were permeabilized with 0.3% Triton X-100 after fixation with 4% paraformaldehyde and then incubated overnight at 4°C with the antisera. An anti-mouse horseradish peroxidase-conjugated secondary Ab (Sigma) was used.
Combined ISH and IHC.
Combined ISH and IHC was performed essentially as described previously (17). Briefly, IHC was first carried out to detect either Nef or CNPase protein by using anti-Nef Ab or anti-CNPase MAb. Following IHC, sections underwent ISH as previously described (17) to detect CNPase RNA (for anti-Nef IHC) or Tg HIV-1 RNA (for anti-CNPase IHC).
Detection of proteins by Western blotting.
Western blotting was carried out as previously described (17). Briefly, the spinal cords were homogenized in RIPA lysis buffer (1% Nonidet P-40, 10 mM Tris-Hcl [pH 8], 150 mM NaCl, 1% sodium desoxycholate, 0.1% sodium dodecyl sulfate) with protease inhibitors (2 μg of leupeptin/ml, 2 μg of aprotinin/ml, 1 μg of pepstatin/ml, and 100 μg of phenylmethylsulfonyl fluoride/ml). The homogenates were then incubated on ice for 30 min and centrifuged at 15,000 × g for 30 min at 4°C. Equal amounts of lysates (70 μg of protein) were electrophoresed on sodium dodecyl sulfate-polyacrylamide gels (10% for CNPase and myelin-associated glycoprotein (MAG) and 12% for MBP and Nef) and transferred to Millipore Immobilon P membranes. The membranes were probed with Abs against MBP, CNPase (Sternberger Monoclonals Inc.), MAG (Santa Cruz), and Nef (21). Anti-mouse or anti-rabbit Alexa 680 (Molecular Probes)-conjugated Abs were used as secondary Abs. Quantification of protein fluorescence intensity was done with the Li-Cor Odyssey Infrared Imaging system. All values were normalized to those obtained with Abs against actin (no. 2066; Sigma). The anti-Nef Ab was also used with anti-rabbit horseradish peroxidase (no. A0545; Sigma).
Microscopic analysis.
Mice were perfused with 4% paraformaldehyde buffered with phosphate-buffered saline (PBS), and the brains and spinal cords were dissected and embedded in paraffin. For each animal, the cervical and thoracic spinal cord was sectioned transversely to allow evaluation of four to five different levels. The sections were stained with hematoxylin and eosin as previously described (17). Electron microscopic evaluation of the cervical spinal cord transverse sections was performed as described previously (17).
Cell cultures.
Oligodendrocyte progenitor primary cultures were prepared from 5-day-old neonatal mouse brains, essentially as described previously (31). In brief, forebrains containing half of the brainstem were isolated and kept in cold PBS containing penicillin (1000 U/ml) and streptomycin (0.1 mg/ml), while the spleen DNA was extracted and PCR amplified to type for the presence or lack of presence of the transgene. Brains from non-Tg and Tg animals (three to four animals for each group) were dissociated mechanically in PBS containing antibiotics and trypsinized for 30 min at 37°C, and cells were collected by centrifugation at 300 × g for 5 min. The cell suspension was then passed through a 143-μm-pore-size nylon mesh and centrifuged, and the pellet was resuspended and passed again through a 60-μm-pore-size nylon mesh. After centrifugation, the cells were suspended, counted, and plated in 80-cm2 tissue culture flasks precoated with poly-l-lysine (6 × 106 to 12 × 106 cells per flask) in Dulbecco modified Eagle medium containing 10% complement-inactivated fetal bovine serum. At day 5, the medium was changed for the first time, and then it was changed every 3 days until day 11, when the preparation was shaken overnight at 210 rpm (New Brunswick Orbital Shaker). The cell suspension was passed through a 30-μm-pore-size nylon mesh and centrifuged, and the pellet was resuspended and plated on a bacterium-grade culture petri dish for 45 min at 37°C. This step enriches for oligodendrocyte precursors, since the contaminating microglial cells adhere to the plastic of bacterium-grade petri dishes. After this incubation period, the dishes were swirled gently and the medium containing oligodendrocyte precursor cells was centrifuged and the cells were counted and plated again in poly-l-lysine-coated 35-mm tissue culture dishes. About 1.7 × 104 cells were then incubated at 37°C in one drop of medium (0.08 ml) and allowed to adhere for 1 h before 1 ml of medium was added. The medium was changed the next day, and the cells were fixed the day after. This population is designated a 2-day-old oligodendrocyte-enriched culture. All the in vitro culture studies were performed with such 2-day-old oligodendrocyte-enriched cultures from non-Tg and Tg mice.
Morphological assessment of cells in vitro.
Quantitation of oligodendrocyte proximal processes and their secondary branchings was performed on cells labeled with the O4 MAb.
RESULTS
Construction of Tg mice.
The MBP/HIVNef transgene was constructed by ligating the MBP promoter to the full-length HIV-1MutG fragment described previously (20) (Fig. 1A). This HIV-1 genome harbors mutations in all of the known coding regions of HIV-1 except nef, thus expressing only one gene product, Nef. Four independent founder lines (F25942, F26351, F26357, and F25964) were established and routinely examined for signs of disease.
Expression of the transgene.
Expression of the transgene was first assessed by Northern blot analysis. All four founder lines were found to express the transgene in the CNS at different levels, some lines expressing at relatively high levels (F26351, F25964, and F26357) while one line expressed at low levels (F25942) in comparison to the reference MBP/HIVwt Tg line (Fig. 1B). The three forms of the HIV-1 transcripts could be detected in all of the founder lines. Interestingly, the 2-kb multispliced RNA appeared to be more abundant than the other species.
Nef expression, assessed by Western blot analysis, was readily detected in cervical and thoracic spinal cord protein extracts of the high-expresser F26357 line, consistent with our previous results for MBP/HIVwt Tg mice (17), but was barely detectable in the low-expresser F25942 Tg line (Fig. 1C). Quantitation of Western blot data revealed a 20-fold difference in expression between these two Tg lines (Fig. 1D).
Although the cell type specificity of MBP/HIV transgene expression was previously documented (17), this analysis was confirmed with CNS tissues from the high-expresser (F26357) MBP/HIVNef line. Brain tissue and cervical and thoracic spinal cord specimens were evaluated by ISH and IHC. Robust Tg RNA expression was observed in both brain (data not shown) and spinal cord, principally in white matter tracts in cells exhibiting an oligodendrocyte-like morphology (Fig. 2P and Q) consistent with what had previously been documented for MBP/HIV-1wt Tg mice (17). However, in the low-expresser F25942 line, Tg RNA was undetectable in paraformaldehyde-perfusion-fixed, paraffin-embedded CNS tissue sections and was only weakly detected in white matter of fresh-frozen CNS tissue sections (data not shown). Nef immunoreactivity was detected in brain (Fig. 2E) and spinal cord (Fig. 2A and C) sections, principally in the white matter, again in cells with an oligodendrocyte-like morphology (Fig. 2C). Double labeling by ISH and IHC (anti-Nef) demonstrated expression of transgene HIV-1 RNA and Nef protein in the same oligodendrocyte-like cells, as expected (data not shown), and was located principally in the white matter tracts. Finally, double labeling IHC to detect Nef protein and CNPase, an oligodendrocyte-specific marker, showed a high degree of coincident labeling in cell processes (Fig. 2K and N) and coincident labeling of some cell bodies (Fig. 2N). Taken together, these results strongly suggest that the MBP promoter is faithfully directing Nef expression in oligodendrocytes, both in the brains and in the spinal cords of the MBP/HIVNef Tg mice, as reported for other surrogate genes (22, 24).
FIG. 2.
Detection of MBP/HIV-1Nef Tg expression in the spinal cord by ISH and IHC. (A through H) IHC of anti-Nef immunoreactivity. Shown are spinal cord sections of MBP/HIV-1Nef Tg mice (F26357) at low (A) and high (C) power and Tg corpus callosum (E) and non-Tg spinal cord (G) at high power reacted with anti-Nef Ab. Normal rabbit serum (NRS) was used for the control (B, D, F, andH). The bright field was stained with hematoxylin. (I through O) Codistribution of Tg protein and CNPase in thoracic spinal cord transsection. Tg tissue (F26357) was reacted with anti-Nef and anti-CNPase antisera and with secondary antisera conjugated to Alexa 633 (red, Nef) and Alexa 488 (green, CNPase). Confocal images of Nef (I and L) and CNPase (J and M) and merged images (K and N) at low and high power are shown. A merged image of anti-mouse isotype and control NRS is shown in panel O. (P and Q) Discordance of localization of CNPase and HIV-1 RNA. Spinal cord sections were processed for combined ISH and IHC by using HIV-1-specific ribobrobe for ISH and anti-CNPase for IHC. Note that not all cells expressing the HIV-1 transgene are positive for CNPase. (R) The control section was reacted with HIV-1-specific sense riboprobe and with the secondary Ab alone. Magnification for panels P through R, ×285.
To further evaluate the partial overlap of transgene expression in oligodendrocyte cell bodies, spinal cord sections from MBP/HIVNef Tg mice were subjected to double labeling, involving the detection of Tg RNA by ISH and of that of CNPase by IHC with anti-CNPase Abs (Fig. 2P and Q). The reverse experiment (detecting Tg protein by IHC and CNPase expression by ISH) was also performed (data not shown). With both experiments, a minority (<5%) of cells (assessed at the cell body) expressing HIV-1 RNA or Nef protein were seen to coexpress CNPase RNA or protein.
Further studies have documented downregulation of CNPase in oligodendrocytes of these Tg mice, a phenomenon most likely explaining this lack of colocalization (see below).
Pathological assessment of the MBP/HIV-1Nef Tg mice.
Clinically, most of the MBP/HIV-1Nef Tg mice observed (n = 47) appeared normal for up to 14 months of age. However, in the Tg lines most extensively bred (F26357 and F25942), decreased fertility, especially of Tg male mice from the high-expresser line (F26357), was noticed. This line is now maintained by breeding female Tg mice. This phenotype suggests that motor and/or autonomic nervous functions may be affected in these Tg animals.
Histological examination of the spinal cords of the animals revealed a much higher incidence of pathology, with vacuolar changes evident in the anterior and lateral, but rarely in the dorsal, funiculi (Fig. 3A, C, and D), in more than 70% of the Tg animals from four distinct Tg lines assessed (Table 1). Despite robust Tg expression throughout the brain (F26357), vacuolation in brain white matter tracts was infrequently observed. Surprisingly, vacuolation and other signs of disease (see below) were even apparent in mice from the low-expresser Tg line F25942. Electron microscopy examination also revealed myelin splitting (Fig. 3F), as was previously documented in MBP/HIVwt Tg mice (17). The vacuolar changes observed in these MBP/HIV-1Nef Tg mice were indistinguishable from those observed previously in the MBP/HIV-1wt Tg mice (17).
FIG. 3.
Spinal cord pathology in MBP/HIV-1Nef Tg mice. (A through D) Light microscopy comparing thoracic spinal cord transsections from MBP/HIV-1Nef Tg mice (A, C, and D) and from an age-matched control animal (B). Note the appearance of vacuoles, which sometimes form clusters, in the anterior and lateral funiculi of MBP/HIV-1Nef Tg mice (A, C, and D). Vacuolation of the dorsal funiculi is uncommon. An inflammatory lesion in the lateral funiculus of an MBP/HIV-1Nef mouse exhibiting significant vacuolar changes can be seen in panel D. The bright field was stained with hematoxylin and eosin. (E and F) Electron microscopy of non-Tg (E) and Tg (F) cervical spinal cords in transsection. Note the splitting of the myelin sheaths in the Tg tissue (F). Magnification for panels A and B, ×77; for panel C, ×174; for panel D, ×145; for panels E and F, ×5,820.
TABLE 1.
Incidence of spinal cord pathology in MBP/HIVNef Tg mice
Nef-expressing oligodendrocytes from MBP/HIVNef Tg mice exhibit lower levels of MAG, CNPase, and MBP proteins.
The myelin defects observed in MBP/HIVNef Tg mice led us to evaluate MAG, CNPase, and MBP protein levels in the cervical and thoracic spinal cord of 5-month-old mice, using quantitative Western blotting. MAG and CNPase protein levels were decreased by ∼50% in mice (n = 5 or 6) of the high-expresser founder line F26357 compared to those in the controls (Fig. 4A through C). Such a decrease was not consistently observed in Tg mice from the low-expresser line (F25942). Interestingly, a similar (∼50%) decrease in the level of the 21-kDa species of the MBP protein but not of the 17- to 18-kDa species was documented in mice from both the high (n = 5)- and the low (n = 6)-expresser lines (Fig. 4A and D). These decreases were statistically significant for MAG and MBP but just failed to reach statistical significance for CNPase. These results suggest that Nef may modulate the levels of major myelin proteins.
FIG. 4.
Expression levels of myelin proteins (MAG, MBP, and CNPase) in the spinal cord of MBP/HIVNef Tg mice. Spinal cord lysates (70 μg of proteins) from 5-month-old MBP/HIVNef Tg and non-Tg control mice were processed for Western blot analysis with Abs against MAG, MBP, and CNPase (A). After stripping, the membranes were reacted with antiactin. The intensity of fluorescence of each band was quantified by using the Odyssey Infrared Imaging system. The amount of each protein species was normalized to the amount of actin (B through D). The highest protein level observed in a mouse from the non-Tg control group was arbitrarily assigned a value of 100%. All the other values from the remaining non-Tg and Tg mice were expressed relative to this 100% set value. Significance was assessed by analysis of variance with Dunnett's multiple comparison test. One asterisk (*) indicates a P value of <0.05; two asterisks (**) indicate a P value of <0.01).
Oligodendrocytes from MBP/HIVNef Tg mice appear morphologically more mature.
To further study the impact of Nef expression on oligodendrocyte structure and differentiation, oligodendrocyte-enriched primary cultures were prepared from young (5-day-old) MBP/HIVNef Tg mice and their non-Tg littermates. We used the MAb O4 against sulfatide to detect cells of the oligodendrocyte lineage. O4 expression begins early following lineage commitment and continues throughout oligodendrocyte differentiation (37).
O4-positive oligodendrocytes from MBP/HIVNef Tg mice were found to develop more complex networks of cellular extensions than those from non-Tg mice. The number of proximal cell processes and the number of secondary branchings arising from these proximal processes were quantitated (Fig. 5A). The extent of secondary branching did not appear to be affected by Nef expression (Fig. 5C). In contrast, this analysis showed that Nef expression induced a significant increase in the proportion of O4-stained oligodendrocytes with more (5 to 8) proximal processes and a decrease in the proportion of those with fewer (2 to 4) proximal processes (Fig. 5B). Similar results were obtained with mice from the high (F26357)- and the low (F24942)-expresser lines. These results suggested that these Tg oligodendrocytes were morphologically more differentiated than the control non-Tg oligodendrocytes.
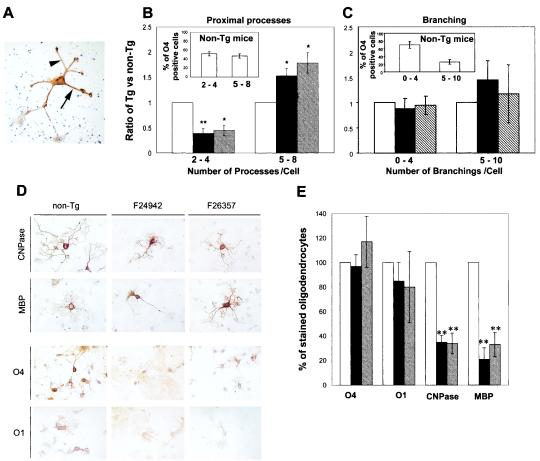
FIG. 5.
Cultured oligodendrocytes from MBP/HIVNef Tg mice exhibit altered morphology and differentiation in marker expression. (A) Oligodendrocyte-enriched cultures derived from telencephalons of MBP/HIVNef Tg mice from the low (F24942)- and high (F26357)-expresser lines as well as from non-Tg littermates were fixed and stained with O4 antiserum. Arrow, proximal extensions; arrowhead, distal extensions. (B and C) Quantitation of the number of proximal and distal extensions of O4-positive cells. The proportion of O4-positive cells observed is shown in the inset of each panel. The numbers obtained for non-Tg mice were arbitrarily assigned a value of 1.0. The total numbers of cells counted were 101, 45, and 58 for non-Tg, F24942 Tg, and F26357 Tg mice, respectively. Each value represents the mean ± the standard error of the mean of the results from three independent experiments. (D) Immunophenotype. After fixation, the cells were stained with antiserum against CNPase, MBP, O1, or O4. Note that the intensity of the O4 and O1 immunostaining shows a dramatic decrease in Tg oligodendrocytes compared to that in non-Tg oligodendrocytes. (E) Quantitation of oligodendrocytes. Between 1,700 and 3,000 hematoxylin-stained cells (corresponding to 12 to 14 fields) were first counted for each animal. Then the numbers of O4-, O1-, CNPase- or MBP-positive cells were also counted for each mouse. The data are expressed as percentages of Ab-positive cells adjusted to 100% for non-Tg cells. Each value represents the mean ± the standard error of the mean of the results from three or four independent experiments. Open bar, non-Tg mice; black and hatched bars, Tg mice from founder lines F25942 and F26357, respectively. One asterisk (*) indicates a P value of <0.05, and two asterisks (**) indicate a P value of <0.01 as determined by the two-tailed Student's t test.
Oligodendrocytes from MBP/HIVNef Tg mice appear less mature.
The stages of maturation of oligodendrocytes have been relatively well defined with various oligodendrocyte-specific Abs (28, 37). Immature oligodendrocytes express sulfatide recognized by the O4 MAb. As they mature, GalC is expressed and can be recognized by the O1 MAb. O1-positive cells also start expressing CNPase. More mature oligodendrocytes (O4+, O1+, and CNPase+) also express MBP. Based on these four differentiation markers, the immunophenotype observed for oligodendrocytes from normal mice was consistent with that previously published (5, 6). Expression of all of the phenotypic markers was observed, with immunostaining being higher in cell bodies than in processes (Fig. 5D). Moreover, the proportion of cells positive for each of these markers in six independent experiments (35 ± 3%, O4; 20 ± 6%, O1; 5.4 ± 1.8%, CNPase; and 4.3 ± 1%, MBP [means ± standard errors of the means {SEM}]) was consistent with published data (6).
We next studied the maturation profile of Nef-expressing Tg oligodendrocytes. The percentages of O4- and O1-positive cells from MBP/HIVNef Tg mice were not statistically different from those from their non-Tg littermates (Fig. 5E). However, a dramatic diminution of the intensity of the lipid immunostaining was observed with these Abs in both low- and high-expresser lines (Fig. 5D). In contrast, a significant decrease in the percentages of CNPase- and MBP-positive cells was observed for both low- and high-expresser mice compared to those for non-Tg mice (Fig. 5E). A similar decrease in the percentage of CNPase-immunostained cells was also observed in cultured oligodendrocytes from MBP/HIVwt Tg mice, as previously described (17) (data not shown). However, the intensity of CNPase/MBP immunostaining in Tg oligodendrocytes did not differ from that of the controls (Fig. 5D).
Together, these data indicate that the recovery of oligodendrocytes (O4+ and O1+) in 2-day-old enriched cultures is comparable between Tg and non-Tg mice and that Tg oligodendrocytes exhibit a deficit in galactolipid accumulation. These results further show that the number of mature oligodendrocytes (CNPase+ and MPB+) is lower in Tg than in non-Tg cultures, suggesting that Nef prevents maturation of these oligodendrocytes, as defined by these markers. This contrasts with the more mature phenotype of Tg oligodendrocytes observed morphologically.
DISCUSSION
HIV-1 Nef harbors a major determinant of CNS white matter vacuolation.
In a previous study, we demonstrated that expression of the complete HIV-1 genome in oligodendrocytes of Tg mice (MBP/HIVwt) induces VM (17). In the present work, we found that the expression of a single HIV-1 gene, nef, in oligodendrocytes (MBP/HIVNef) was sufficient to induce VM. Although it is unlikely, we cannot exclude the possibility that Tg RNA itself or an unknown HIV-1 peptide encoded by a yet unrecognized open reading frame results in the observed pathology. The development of the same disease in four independent founder lines rules out the possibility that the observed phenotype was due to an integration event. The histopathological CNS lesions induced by expression of the whole HIV-1 coding sequence (HIV-1wt) (17) or of Nef alone (HIVNef) (Fig. 3) were indistinguishable. They represent interlamellar vacuoles which are concentrated in the anterior and lateral white matter tracts of the cervical and thoracic spinal cord. This limited distribution cannot be accounted for by the different levels of Nef expression, which was found to be as high in brain as in cervical and thoracic oligodendrocytes. Such a restricted topography of the myelin deficit has also been reported in Fyn-deficient mice (49). The cellular basis for the preferential development of vacuoles in the cervical and thoracic spinal cord of MBP/HIVNef Tg mice remains unknown. It may be related to the existence of separate lineages of oligodendrocytes (47, 48).
In other cell types, Nef has been found to significantly affect cell proliferation and survival as well as to deregulate gene expression (3, 30, 42, 46). This finding was highlighted in our in vivo study showing that expression of Nef in cells of the immune systems of Tg mice (CD4C/HIV) leads to the development of an AIDS-like syndrome with several independent phenotypes (20, 23, 39).
We have performed similar work with MBP/HIVNef Tg mice to study some of the mechanisms by which Nef-expressing oligodendrocytes may lead to the formation of white matter vacuoles. Nef was found to have a profound effect on the expression of genes associated with proper oligodendrocyte function and/or differentiation both in vivo and in vitro. In vivo, Tg oligodendrocytes harbor reduced levels of CNPase, 21-kDa MBP, and MAG proteins. MAG plays a role in the interaction between oligodendrocyte processes and axons (32), and its lower levels in Tg mice may contribute to early myelin splitting. MPBs are a major class of myelin proteins produced in multiple isoforms (9). The selective reduction of the 21-kDa MBP species in MBP/HIVNef Tg mice is intriguing and may participate in vacuole formation. In vitro, we also documented that Tg oligodendrocytes from both the high- and low-expresser founder lines exhibited an increased number of proximal processes, a characteristic of more mature oligodendrocytes, and at the same time exhibited a lower level of galactolipid content and that those expressing CNPase and MBP were less numerous, all features of less mature oligodendrocytes. This discordant phenotype suggests a profound perturbation of the differentiation of these Nef-expressing Tg oligodendrocytes. It is possible that these changes reflect a reprogramming of Tg oligodendrocytes which is responsible for the initial breakdown of the cohesion of the myelin lamellae, leading to splitting of these lamellae and eventually to formation of vacuoles.
A similar, but inverse, discordance has been reported in oligodendrocyte cultures where the src-related kinase Fyn was inhibited, either pharmacologically or with a dominant-negative Fyn mutant: these oligodendrocytes had no or few processes, but expressed MBP and MAG (35). Fyn is expressed at high levels in cultured oligodendrocytes (35) and was shown to have a crucial function in oligodendrocyte differentiation (35, 49). It is therefore tempting to suggest that Fyn is activated by Nef in Tg oligodendrocytes. Nef has been reported to associate with src-related protein kinases, including Fyn, and to activate them (42). Consistent with these observations, we have found that disease progression in CD4C/HIV Tg mice was delayed when these Tg mice were bred on an hck-deficient background (21). Thus, the molecular mechanism underlying the oligodendrocyte process outgrowth may involve the activation of the Fyn signaling pathway.
In contrast, another signaling pathway appears to be inhibited by Nef in Tg oligodendrocytes: the galactolipid biosynthetic pathway responsible for the synthesis of GalC and its derived sulfatide. Our results show that the intensity of the immunostaining of both lipids was significantly reduced in the presence of Nef (Fig. 5D), suggesting that Nef may inhibit the synthesis and/or the activity of the enzyme UDP-galactose ceramide galactosyltransferase (33). Consistent with this hypothesis, ceramide galactosyltransferase-deficient mice, which lack expression of both GalC and sulfatide, share some characteristics with the MBP/HIVNef Tg model, in particular the progressive development of vacuoles in the ventral region of the spinal cord associated with myelin splitting (10, 11). Together, these findings suggest that Nef may have an inhibitory effect on this galactolipid biosynthetic pathway.
Perhaps the most intriguing finding emerging from our studies with these MBP/HIVNef Tg mice is that both in vivo and in vitro phenotypes were comparable in mice from the high- and low-expresser Tg lines. These results suggest that the threshold level of Nef required to elicit these changes is rapidly saturated. This low-threshold response to Nef may also be present in human oligodendrocytes and may significantly impact our understanding of the human disease (see below).
The fact that obvious myelin changes were rarely apparent in various brain areas outside the cervical and thoracic spinal cord does not necessarily indicate absence of CNS lesions. A recent report on the CNPase-encoding gene (cnp1) indeed shows that in Cnp-1-deficient mice, axonal loss and neurodegeneration occur throughout the brain as a consequence of the dysfunction of oligodendrocytes but in the absence of apparent myelin changes (27, 38). Further studies will be required to determine whether Nef-expressing oligodendrocytes also affect the function and integrity of brain neurons.
Is Nef involved in the development of HIV-1-associated human VM?
Several indications suggest that the CNS disease developing in MBP/HIVNef Tg mice may be a relevant model for the human HIV-1-associated VM. First, in both MBP/HIVNef Tg mice and HIV-1-infected individuals, the disease is progressive. Second, the topography of the vacuoles is similar in both diseases, being largely restricted to the white matter tracts of the cervical and thoracic spinal cord. Third, in both diseases the vacuoles arise between the myelin lamellae. Such interlamellar vacuolation is a relatively rare phenotype in human CNS diseases, being particularly associated with rare genetic Canavan's disease (13). Fourth, multispliced RNA is the predominant HIV-1 RNA species being produced both in infected human astroglial cells (7, 29) and to some extent in oligodendroglial cells of MBP/HIVwt/Nef Tg mice. Consistent with the preferential accumulation of these Nef-coding multispliced RNA species in vitro, in cultured cells Nef protein has been found to be efficiently produced in vivo, in glial cells both in murine oligodendrocytes (17) (Fig. 1C) and in astrocytes of HIV-1-infected children (43, 52) and adults (41).
However, despite these strong resemblances, an apparent paradox resides in the fact that the documentation of HIV-1 expression in oligodendrocytes of HIV-1-infected individuals is controversial, with expression being reported as either present, but rare, (4, 15, 19, 40, 50) or absent (8, 16, 25, 26, 34, 44, 53, 54). The present work may help to clarify this issue. In this model, virtually all of the in vivo and in vitro phenotypes were observed not only in Tg mice from the high-expresser line F26357 but also in Tg mice from the very-low-expresser line F25942. We therefore hypothesize that expression of low levels of Nef in oligodendrocytes is sufficient to induce the development of human VM. Such a low level of HIV-1 expression by oligodendrocytes has previously been proposed to account for the reactive hyperplasia of oligodendrocytes observed in AIDS patients exhibiting mild myelin damage (15). In Tg mice, such low levels of expression could not be detected by the in situ techniques used in perfusion-fixed frozen or paraffin-embedded tissues (IHC with anti-Nef Ab and ISH with HIV-1-specific riboprobes). Such low levels of Nef RNA or proteins are also unlikely to be detectable in human tissues prepared with the same or less stringent (postmortem) techniques. Moreover, we also found that Nef expression leads to the downregulation of two markers present on mature oligodendrocytes (CNPase and MBP) which are frequently used to stain and identify oligodendrocytes. Therefore, even if HIV-1 expression in oligodendrocytes is high in some HIV-1 individuals with VM, these cells may be very difficult, if not impossible, to identify with oligodendrocyte markers, as we found in the MBP/HIVNef Tg mice, and are likely to be scored as nonoligodendrocytes.
Our results suggest that low levels of expression coupled with downregulation of oligodendrocyte markers may largely explain the difficulty of detecting HIV-1 infection of oligodendrocytes in human patients with VM. If this hypothesis is correct, the MBP/HIVNef Tg model will have proven to be useful in understanding an important aspect of HIV-1-associated VM.
Acknowledgments
This work was supported by grants to P.J. from the CIHR (grant MA-7926) and NIAID (grant AI-38490-03).
We thank Karina Lamarre, Jean-René Sylvestre, Benoît Laganière, Lin Jia, and Chunyan Hu for excellent technical assistance. We thank Miguel Chagnon, Université de Montréal, and Peter Braun, McGill University, for helpful discussions and the latter for providing the O1 and O4 MAb and the CNPase cDNA clone. We are grateful to Aleks Spurmanis (Concordia University) for facilitating access to the Odyssey Infrared Imaging System.
REFERENCES
- 1.Albright, A. V., J. Strizki, J. M. Harouse, E. Lavi, M. O'Connor, and F. Gonzalez-Scarano. 1996. HIV-1 infection of cultured human adult oligodendrocytes. Virology 217:211-219. [DOI] [PubMed] [Google Scholar]
- 2.An, S. F., M. Groves, B. Giometto, A. A. Beckett, and F. Scaravilli. 1999. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. 98:481-487. [DOI] [PubMed] [Google Scholar]
- 3.Arendt, C. W., and D. R. Littman. 2001. HIV: master of the host cell. Genome Biol. 2:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. [DOI] [PubMed] [Google Scholar]
- 5.Bansal, R., A. E. Warrington, A. L. Gard, B. Ranscht, and S. E. Pfeiffer. 1989. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J. Neurosci. Res. 24:548-557. [DOI] [PubMed] [Google Scholar]
- 6.Bansal, R., S. Winkler, and S. Bheddah. 1999. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J. Neurosci. 19:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack-Werner, R., A. Kleinschmidt, A. Ludvigsen, W. Mellert, M. Neumann, R. Herrmann, M. C. Khim, A. Burny, N. Muller-Lantzsch, and D. Stavrou. 1992. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS 6:273-285. [PubMed] [Google Scholar]
- 8.Budka, H., and H. Lassmann. 1988. Human immunodeficiency virus in glial cells? J. Infect. Dis. 157:203-205. [DOI] [PubMed] [Google Scholar]
- 9.Campagnoni, A. T. 1988. Molecular biology of myelin proteins from the central nervous system. J. Neurochem. 51:1-14. [DOI] [PubMed] [Google Scholar]
- 10.Coetzee, T., N. Fujita, J. Dupree, R. Shi, A. Blight, K. Suzuki, and B. Popko. 1996. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell 86:209-219. [DOI] [PubMed] [Google Scholar]
- 11.Coetzee, T., K. Suzuki, and B. Popko. 1998. New perspectives on the function of myelin galactolipids. Trends Neurosci. 21:126-130. [DOI] [PubMed] [Google Scholar]
- 12.Dal Pan, G. J., J. D. Glass, and J. C. McArthur. 1994. Clinicopathologic correlations of HIV-1-associated vacuolar myelopathy: an autopsy-based case-control study. Neurology 44:2159-2164. [DOI] [PubMed] [Google Scholar]
- 13.Davies, K. 1993. The cause of Canavan's disease. Nature 365:590. [Google Scholar]
- 14.Eilbott, D. J., N. Peress, H. Burger, D. LaNeve, J. Orenstein, H. E. S. R. Gendelman, and B. Weiser. 1989. Human immunodeficiency virus type 1 in spinal cords of acquired immunodeficiency syndrome patients with myelopathy: expression and replication in macrophages. Proc. Natl. Acad. Sci. USA 86:3337-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esiri, M., C. S. Morris, and P. R. Millard. 1991. Fate of oligodendrocytes in HIV-1 infection. AIDS 5:1081-1088. [DOI] [PubMed] [Google Scholar]
- 16.Gosztonyi, G., J. Artigas, L. Lamperth, and H. D. Webster. 1994. Human immunodeficiency virus (HIV) distribution in HIV encephalitis: study of 19 cases with combined use of in situ hybridization and immunocytochemistry. J. Neuropathol. Exp. Neurol. 53:521-534. [DOI] [PubMed] [Google Scholar]
- 17.Goudreau, G., S. Carpenter, N. Beaulieu, and P. Jolicoeur. 1996. Vacuolar myelopathy in transgenic mice expressing human immunodeficiency virus type 1 proteins under the regulation of the myelin basic protein gene promoter. Nat. Med. 2:655-661. [DOI] [PubMed] [Google Scholar]
- 18.Gravel, M., D. DeAngelis, and P. E. Braun. 1994. Molecular cloning and characterization of rat brain 2′,3′-cyclic nucleotide 3′-phosphodiesterase isoform 2. J. Neurosci. Res. 38:243-247. [DOI] [PubMed] [Google Scholar]
- 19.Gyorkey, F., J. L. Melnick, and P. Gyorkey. 1987. Human immunodeficiency virus in brain biopsies of patients with AIDS and progressive encephalopathy. J. Infect. Dis. 155:870-876. [DOI] [PubMed] [Google Scholar]
- 20.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 21.Hanna, Z., X. Weng, D. G. Kay, J. Poudrier, C. Lowell, and P. Jolicoeur. 2001. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J. Virol. 75:9378-9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsuki, M., M. Sato, M. Kimura, M. Yokoyama, K. Kobayashi, and T. Nomura. 1988. Conversion of normal behavior to shiverer by myelin basic protein antisense cDNA in transgenic mice. Science 241:593-595. [DOI] [PubMed] [Google Scholar]
- 23.Kay, D. G., P. Yue, Z. Hanna, S. Jothy, E. Tremblay, and P. Jolicoeur. 2002. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 Nef in cells of the immune system. Am. J. Pathol. 161:321-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura, M., M. Sato, A. Akatsuka, S. Nozawa-Kimura, R. Takahashi, M. Yokoyama, T. Nomura, and M. Katsuki. 1989. Restoration of myelin formation by a single type of myelin basic protein in transgenic shiverer mice. Proc. Natl. Acad. Sci. USA 86:5661-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenig, S., H. E. Gendelman, J. M. Orenstein, M. C. Dal Canto, G. H. Y. M. Pezeshkpour, F. Janotta, A. Aksamit, M. A. Martin, and A. S. Fauci. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089-1093. [DOI] [PubMed] [Google Scholar]
- 26.Korber, B. T., K. J. Kunstman, B. K. Patterson, M. Furtado, M. McEvilly, and R. Levy, and S. M. Wolinsky. 1994. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J. Virol. 68:7467-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lappe-Siefke, C., S. Goebbels, M. Gravel, E. Nicksch, J. Lee, P. E. Braun, I. R. Griffiths, and K. A. Nave. 2003. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Gen. 33:366-374. [DOI] [PubMed] [Google Scholar]
- 28.Levine, J. M., R. Reynolds, and J. W. Fawcett. 2001. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 24:39-47. [DOI] [PubMed] [Google Scholar]
- 29.Li, J., Y. Liu, I. W. Park, and J. J. He. 2002. Expression of exogenous Sam68, the 68-kilodalton SRC-associated protein in mitosis, is able to alleviate impaired Rev function in astrocytes. J. Virol. 76:4526-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangasarian, A., and D. Trono. 1997. The multifaceted role of HIV Nef. Res. Virol. 148:30-33. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy, K. D., and J. de Vellis. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85:890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montag, D., K. P. Giese, U. Bartsch, R. Martini, Y. Lang, H. Bluthmann, J. Karthigasan, D. A. Kirschner, E. S. Wintergerst, and K. A. Nave. 1994. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron 13:229-246. [DOI] [PubMed] [Google Scholar]
- 33.Morell, P., and N. S. Radin. 1969. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry 8:506-512. [DOI] [PubMed] [Google Scholar]
- 34.Nuovo, G. J., F. Gallery, P. MacConnell, and A. Braun. 1994. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am. J. Pathol. 144:659-666. [PMC free article] [PubMed] [Google Scholar]
- 35.Osterhout, D. J., A. Wolven, R. M. Wolf, M. D. Resh, and M. V. Chao. 1999. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J. Cell Biol. 145:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petito, C. K., B. A. Navia, E. S. Cho, B. D. Jordan, D. C. George, and R. W. Price. 1985. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 312:874-879. [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer, S. E., A. E. Warrington, and R. Bansal. 1993. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3:193-197. [DOI] [PubMed] [Google Scholar]
- 38.Popko, B. 2003. Myelin: not just a conduit for conduction. Nat. Gen. 33:327-328. [DOI] [PubMed] [Google Scholar]
- 39.Poudrier, J., X. Weng, D. G. Kay, G. Paré, E. L. Calvo, Z. Hanna, M. H. Kosco-Vilbois, and P. Jolicoeur. 2001. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-γ and IL-6. Immunity 15:173-185. [DOI] [PubMed] [Google Scholar]
- 40.Pumarola-Sune, T., B. A. Navia, C. Cordon-Cardo, E. S. Cho, and R. W. Price. 1987. HIV antigen in the brains of patients with the AIDS dementia complex. Ann. Neurol. 21:490-496. [DOI] [PubMed] [Google Scholar]
- 41.Ranki, A., M. Nyberg, V. Ovod, M. Haltia, I. Elovaara, R. Raininko, H. Haapasalo, and K. Krohn. 1995. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9:1001-1008. [DOI] [PubMed] [Google Scholar]
- 42.Renkema, G. H., and K. Saksela. 2000. Interactions of HIV-1 Nef with cellular signal transducing proteins. Front. Biosci. 5:D268-D283. [DOI] [PubMed] [Google Scholar]
- 43.Saito, Y., L. R. Sharer, L. G. Epstein, J. Michaels, M. Mintz, M. G. K. Louder, T. A. Cvetkovich, and B. M. Blumberg. 1994. Overexpression of Nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology 44:474-481. [DOI] [PubMed] [Google Scholar]
- 44.Sharer, L., and J. W. Prineas. 1988. Human immunodeficiency virus in glial cells? J. Infect. Dis. 157:204. [DOI] [PubMed] [Google Scholar]
- 45.Sharer, L. R. 1992. Pathology of HIV-1 infection of the central nervous system. A review. J. Neuropathol. Exp. Neurol. 51:3-11. [DOI] [PubMed] [Google Scholar]
- 46.Skowronski, J., M. E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A. J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harbor Symp. Quant. Biol. 64:453-463. [DOI] [PubMed] [Google Scholar]
- 47.Spassky, N., C. Goujet-Zalc, E. Parmantier, C. Olivier, S. Martinez, A. Ivanova, K. Ikenaka, W. Macklin, I. Cerruti, B. Zalc, and J. L. Thomas. 1998. Multiple restricted origin of oligodendrocytes. J. Neurosci. 18:8331-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spassky, N., C. Olivier, E. Perez-Villegas, C. Goujet-Zalc, S. Martinez, J. Thomas, and B. Zalc. 2000. Single or multiple oligodendroglial lineages: a controversy. Glia 29:143-148. [PubMed] [Google Scholar]
- 49.Sperber, B. R., E. A. Boyle-Walsh, M. J. Engleka, P. Gadue, A. C. Peterson, P. L. Stein, S. S. Scherer, and F. A. McMorris. 2001. A unique role for Fyn in CNS myelination. J. Neurosci. 21:2039-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoler, M. H., T. A. Eskin, S. Benn, R. C. Angerer, and L. M. Angerer. 1986. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA 256:2360-2364. [PubMed] [Google Scholar]
- 51.Takahashi, N., A. Roach, D. B. Teplow, S. B. Prusiner, and L. Hood. 1985. Cloning and characterization of the myelin basic protein gene from mouse: one gene can encode both 14 kd and 18.5 kd MBPs by alternate use of exons. Cell 42:139-148. [DOI] [PubMed] [Google Scholar]
- 52.Tornatore, C., R. Chandra, J. R. Berger, and E. O. Major. 1994. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481-487. [DOI] [PubMed] [Google Scholar]
- 53.Vazeux, R., N. Brousse, A. Jarry, D. Henin, C. Marche, C. Vedrenne, J. W. M. Mikol, C. Michon, W. Rozenbaum, et al. 1987. AIDS subacute encephalitis. Identification of HIV-infected cells. Am. J. Pathol. 126:403-410. [PMC free article] [PubMed] [Google Scholar]
- 54.Wiley, C. A., R. D. Schrier, J. A. Nelson, P. W. Lampert, and M. B. Oldstone. 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA 83:7089-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]