Abstract
Pseudomonas aeruginosa exhibits swarming motility on 0.5 to 1% agar plates in the presence of specific carbon and nitrogen sources. We have found that PAO1 double mutants expressing neither flagella nor type IV pili (fliC pilA) display sliding motility under the same conditions. Sliding motility was inhibited when type IV pilus expression was restored; like swarming motility, it also decreased in the absence of rhamnolipid surfactant production. Transposon insertions in gacA and gacS increased sliding motility and restored tendril formation to spreading colonies, while transposon insertions in retS abolished motility. These changes in motility were not accompanied by detectable changes in rhamnolipid surfactant production or by the appearance of bacterial surface structures that might power sliding motility. We propose that P. aeruginosa requires flagella during swarming to overcome adhesive interactions mediated by type IV pili. The apparent dependence of sliding motility on environmental cues and regulatory pathways that also affect swarming motility suggests that both forms of motility are influenced by similar cohesive factors that restrict translocation, as well as by dispersive factors that facilitate spreading. Studies of sliding motility may be particularly well-suited for identifying factors other than pili and flagella that affect community behaviors of P. aeruginosa.
Pseudomonas aeruginosa is an opportunistic pathogen that can cause either acute or chronic infection in susceptible hosts. P. aeruginosa has two surface organelles responsible for motility: a single polar flagellum that promotes swimming motility in liquid environments, and polar type IV pili (TFP) responsible for twitching motility across solid surfaces (5, 25). P. aeruginosa also exhibits swarming on semisolid surfaces (0.5 to 1.0% agar) in the presence of specific nitrogen and carbon sources, such as glutamate and glucose (17, 32). Swarming motility occurs in many organisms and is often associated with changes in flagellar number and/or placement (10). Although most authors report that flagella are required for P. aeruginosa swarming motility, one of the first descriptions of P. aeruginosa swarming noted that a PAO1 fliC mutant (Flagellin−) had decreased but not abolished motility under swarming conditions (17). The role of TFP in swarming remains unclear. A recent transposon screen to identify genes required for swarming in the common laboratory strain PAO1 identified multiple pil genes involved in TFP regulation (30). This is consistent with reports that TFP are required for P. aeruginosa swarming (17). However, other authors have reported that pilA mutations in PAO1 and PA14 either have no phenotype or result in increased spreading on swarming plates (32, 38).
Swarming motility generally requires the production of surface wetting material (10). In P. aeruginosa, mono- and di-rhamnolipids, as well as their precursor 3-(3-hydroxyalkanoyloxy)alkanoic acid (HAA), are secreted during swarming (3, 7, 43). HAA and rhamnolipids are synthesized by three enzymes (RhlA, RhlB, and RhlC) that are under control of the N-butyryl homoserine lactone-activated quorum-sensing regulator RhlR. Although HAA is required for P. aeruginosa swarming under many conditions (3, 43), a recent report showed that a rhlAB mutant, which cannot synthesize HAA, still swarms on FAB plates in the presence of succinate or glutamate (38).
The identification of regulatory proteins that control swarming motility in response to environmental cues remains an area of active research. One regulator that has been implicated in swarming motility is the GacS/GacA two-component system. Mutation of the response regulator GacA results in increased swarming motility in P. aeruginosa (31). Spontaneous loss-of-function mutations that map to gacA and gacS have also been described in Pseudomonas fluorescens, where they result in hypermotile bacteria that outcompete the wild type during competitive root colonization assays (24).
GacS and GacA are also implicated in the regulation of biofilm formation, along with two hybrid sensor kinase-response regulators named RetS and LadS (9, 45). ΔretS bacteria exhibit a hyperbiofilm phenotype, while ΔladS, ΔgacS, or ΔgacA mutants are defective in biofilm formation (9, 31). It is not known how signals from these three sensor kinases are integrated during bacterial signaling; however, the current model suggests that activity of GacS/GacA and LadS leads to increased levels of the small RNAs RsmZ and RsmY, while activity of RetS results in decreased levels of these small RNAs. RsmZ and RsmY, in turn, are hypothesized to sequester the posttranscriptional regulator RsmA (12). Low levels of free RsmA lead to the expression of genes associated with biofilm formation, including the pel and psl exopolysaccharide synthesis operons.
P. aeruginosa swarming motility and biofilm formation are also regulated by pathways that use cyclic-di-GMP (c-di-GMP) as a second messenger. Two recent papers described a cyclic-di-GMP phosphodiesterase, BifA, and a diguanylate cyclase, SadC, that inversely regulate biofilm formation and swarming motility of P. aeruginosa PA14 by modulating c-di-GMP levels (18, 26). BifA and SadC likely exert some of their effects by altering exopolysaccharide synthesis; indeed, c-di-GMP binding to one of the pel-encoded enzymes, PelD, is required for exopolysaccharide synthesis (19). However, as O'Toole and colleagues demonstrated, additional c-di-GMP-regulated targets also influence swarming motility.
We have observed a novel surface behavior of P. aeruginosa PAO1 that allows bacteria to spread on semisolid surfaces in the absence of both flagella and TFP. This behavior is consistent with the description of “sliding motility” presented by Jorgen Henrichsen in his seminal 1972 review of bacterial surface translocation (11). In this work we demonstrate that sliding motility responds to many of the same regulatory proteins and environmental cues as swarming motility. These include the GacA/GacS and RetS two-component system proteins and the c-di-GMP modulators BifA and SadC.
MATERIALS AND METHODS
Strain and plasmid construction.
All bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were propagated in Luria-Broth (LB) or Vogel-Bonner minimal (VBM) medium unless otherwise noted. PPGAS medium was prepared as described elsewhere (3). Antibiotics were used as required for Escherichia coli (ampicillin, 100 μg ml−1; gentamicin, 15 μg ml−1; tetracycline, 20 μg ml−1) and P. aeruginosa (carbenicillin, 200 μg ml−1; gentamicin, 50 μg ml−1; tetracycline, 100 μg ml−1).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype or characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tcr)] | Stratagene |
| S17.1 | thi pro hsdR recA RP4-2 (Tc::Mu) (Km::Tn7) | 39 |
| P. aeruginosa strains | ||
| PAO1 | Wild type | 42 |
| PA103 | Wild type | 22 |
| PA103 pilA | PA103 ΔpilA::Gmr; Gmr | 47 |
| pilA | PAO1 ΔpilA | 6 |
| fliC | PAO1 ΔfliC::Tcr; Tcr | 6 |
| fliC pilA | PAO1 ΔfliC::Tcr ΔpilA; Tcr | 6 |
| fliC pilA | PAO1 ΔfliCΔpilA | This work |
| fliC pilA gacA | PAO1 ΔfliC::Tcr ΔpilA gacA::Tn<Gmr>; Tcr Gmr | This work |
| fliC pilA gacA | PAO1 ΔfliCΔpilA gacA::Tn<Gmr>; Gmr | This work |
| fliC pilA gacS | PAO1 ΔfliC::Tcr ΔpilA gacS::Tn<Gmr>; Tcr Gmr | This work |
| fliC pilA retS | PAO1 ΔfliC::Tcr ΔpilA retS::Tn<Gmr>; Tcr Gmr | This work |
| fliC pilA gacA attB::pilA | PAO1 ΔfliC ΔpilA gacA::Tn<Gmr> attB::pilA; Gmr | This work |
| fliC pilA rhlA | PAO1 ΔfliC::Tcr ΔpilA ΔrhlA; Tcr | This work |
| Plasmids | ||
| pX1918G | Cloning/expression vector; Gmr | 36 |
| pMOD2<MCS> | Epicentre | |
| pMOD<Gmr> | aacC1-xylE cassette cloned at MCS of pMOD2<MCS> | This work |
| mini-CTX2 | Contains attP site for integration at P. aeruginosa chromosomal attB site; Tcr | 13 |
| mini-CTX-pilA | pilA under control of its own promoter in mini-CTX2 | This work |
| pUCP-KS | Shuttle vector; Apr Cbr | 46 |
| pRhlA | rhlA under control of the Plac promoter in pUCP-SK; Apr Cbr | This work |
| pEX18 Gm | Suicide vector; Gmr; sacB | 13 |
| pEX18-ΔpilA | pilA knockout construct in pEX18 Gm; Gmr; sacB | 6 |
| pEX18-ΔfliC | fliC knockout construct in pEX18 Gm; Gmr; sacB | This work |
| pMQ30 | Suicide vector; sacB; Gmr URA3 CEN6/ARSH4 | 37 |
| pKO-rhlA | rhlA knockout construct in pMQ30; Gmr; sacB | This work |
| pMQ80 | Cloning vector; Gmr URA3 PBAD; araC | 37 |
| pBifA | His-tagged bifA under control of the PBAD promoter in pMQ80; Gmr URA3 | 18 |
| pSadC | C-terminal HA-tagged sadC under control of the PBAD promoter in pMQ80; Gmr URA3 | 26 |
Abbreviations: Ap, ampicillin; Cb, carbenicillin; Gm, gentamicin; Tc, tetracycline; Km, kanamycin.
Standard molecular biology techniques were employed for restriction digests, ligations, transformations, and selections (35). PCR primers were designed based on sequence information in the PAO1 genome database (http://v2.pseudomonas.com) and are listed in Table 2 (42). PCR amplifications were carried out using recombinant Pfu Turbo polymerase (Stratagene, La Jolla, CA) or Taq polymerase (New England BioLabs, Beverly, MA). E. coli strain XL-1 Blue was used for cloning and plasmid propagation; vectors were transformed into E. coli S17.1 for mobilization by mating into P. aeruginosa. All P. aeruginosa mutants were constructed in the PAO1 background. The ΔpilA strain was constructed by allelic exchange. Upstream (PCR amplified with pilA N1 and pilA N2) and downstream (PCR amplified with pilA C1 and pilA C2) (Table 2) regions flanking the pilA gene were cloned in tandem in pEX18 Gmr to make pEX18-ΔpilA; this plasmid was then mobilized into PAO1 by mating. Exconjugants were initially selected on VBM-gentamicin and then plated to VBM-sucrose (5%) to select for loss of vector backbone sequences, as previously described (6, 36). Candidates were screened by PCR. The ΔfliC::Tcr and ΔfliC::Tcr ΔpilA mutants were made as previously described (6). An unmarked ΔfliC ΔpilA strain was constructed by mating ΔfliC::Tcr ΔpilA with E. coli S17.1 carrying pEX18-ΔfliC and selecting for the loss of Tcr. Again, all strains were screened for gene deletion by PCR; in each case, the correct genotype was confirmed by Southern blot analysis. For complementation of ΔpilA, PCR primers pilA N1 and pilA C2 were used to amplify the full-length pilA gene from PAO1 along with its promoter. This product was cloned into the mini-CTX2 plasmid and integrated into the chromosomal attB site (1).
TABLE 2.
Primers
| Primer | Sequencea |
|---|---|
| pilA N1 | CTCGAGCTCGGTGCTGAACTGGACATCATTG |
| pilA N2 | GAATTCCATGAATCTCTCCGTT |
| pilA C1 | GAATTCGATAACTAAGGTGATCGAAGGTG |
| pilA C2 | CTGCAGCCGCGAGTGCTGGTG |
| rhlA expN | CGAGCTCAAGAGCACCTACGCGCGTTG |
| rhlA exp C | CGGTACCGGTCTTCGCAGGTCAAGG |
| rhlA N1 | CAGACCGCTTCTGCGTTCTGATTTAATCTGTATCAGGCGAACTCCTTCGCCCTGGC |
| rhlA N2 | CCGGCCAGGCCGGGTCTTCGCAGGTCAAGGGTTTCAGGCGTAGCCGCGCCGCATTTCACAC |
| rhlA C1 | GCCTGTTCGAAAATTTTTGGAGGTGTGAAATGCGGCGCGGCTACGCCTGAACCCTTGACCTGC |
| rhlA C2 | GTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCAGCGTTGCAGTTCGTCGTC |
| gacA expN | CCGAATTCAATGCGCGACGAGGTGCAG |
| gacA expC | GGGCGGCCGCGATTGCTACAGGTAGCGAGG |
| Tn for 1 | ATGCCTGCAGGTCGACTCTAGAGGATC |
| Tn for 2 | CAAGCTTGCCAACGACTACGCAC |
| Tn rev 1 | CGAGAACACCCGAGAAAATTCATC |
| Tn rev 2 | CATATTGGCTCGAATTCCGAT |
Underlined portions of sequences represent restriction sites incorporated into primers to facilitate cloning.
The rhlA deletion strains were constructed with pMQ30, which allows for plasmid construction by gap repair in yeast (37). PCR primers rhlA N1 and rhlA N2 and rhlA C1 and rhlA C2 were used to amplify DNA flanking rhlA (Table 2). These PCR products as well as SmaI-linearized pMQ30 were transformed into Saccharomyces cerevisiae EGY40 (MATα trp1 his3 ura3 leu2:0LexAop-LEU2), and transformants were selected on Ura- dropout medium (Q-Biogene, Irvine, CA) as previously described (37). The pMQ30-ΔrlhA plasmid was purified from yeast, transformed into electrocompetent E. coli XL-1 Blue, and verified by analytic restriction digests. The pMQ30-ΔrlhA vector was subsequently mobilized into PAO1 by mating, and candidate ΔrhlA mutants were selected, screened, and confirmed as described above.
Full-length rhlA and gacA genes were amplified with a 1:1 ratio of Pfu Turbo and Taq polymerases using primer pairs rhlA expN with rhlA expC and gacA expN with gacA expC, respectively (Table 2). The PCR products were cloned into pUCP-KS, placing them under control of the constitutively active lac promoter in P. aeruginosa. All constructs were verified by sequencing to confirm that no errors were introduced during PCR amplification and then transformed into electrocompetent PAO1 (4).
Motility assays.
Swarming motility was assayed as previously described on 0.5% M8 plates supplemented with 0.2% glucose and 0.05% glutamate (28) or 0.5% plates with FAB medium supplemented with 12 mM of either glutamate, succinate, or glucose (38). Motility was also measured on 0.5% agar PPGAS plates prepared as described above. Single colonies were plated overnight at 30°C and then placed at room temperature for an additional 24 h. Twitching motility was determined by subsurface stab assays through 1.5% LB agar plates. The twitching zone size at the plastic-agar interface was visualized by Coomassie blue staining after overnight incubation at 37°C. Swimming motility was assayed by point inoculation of 0.3% LB agar plates; zone sizes were measured after overnight incubation at 30°C. Motility assays were carried out on three to five replicates and repeated at least three times. The images presented are representative with regard to both the extent of spreading and morphology of motile colonies.
Construction of pMOD-2-<Gmr> and transposon mutagenesis.
A 2.3-kb fragment containing the xylE aacC1 cassette was generated by EcoRI digestion of pX1918G and subcloned into the unique EcoRI site of pMOD-2-<MCS> (Epicentre Biotechnologies, Madison, WI) to generate pMOD-2-<Gmr>. The EZ-Tn5<Gmr> transposon was prepared by digesting pMOD-2-<Gmr> with PshA. A 200-ng aliquot of transposon DNA was incubated with EZ-Tn5 transposase (Epicentre Biotechnologies) in the presence of glycerol to form transposon complexes according to the manufacturer's protocols. Electrocompetent ΔfliC::Tcr ΔpilA was prepared and electroporated with one-eighth of the transposon mixture and then incubated for 2 h at 37°C in LB and plated onto LB-gentamicin plates overnight (4). The next day single colonies were picked to 150- by 15-mm 0.5% M8 swarming plates. The parent strain was included on each plate as a positive control. Colonies that displayed altered dispersion were then plated to LB-gentamicin plates for further evaluation.
Inverse PCR was done to map the location of the transposons found to alter bacterial spreading. Chromosomal DNA was prepared using the Wizard kit (Promega, Madison, WI). A 1.5-μg aliquot of chromosomal DNA was digested with either MspI or HinpI for 4 h at 37°C. The enzymes were heat inactivated at 65°C for 25 min. Ten μl of digested chromosomal DNA was ligated with 1 μl T4 ligase (New England BioLabs, Beverly, MA) overnight in a total volume of 400 μl at 4°C. The DNA was precipitated with sodium acetate and ethanol, washed with 70% ethanol, and air dried, and the pellet was resuspended in 100 μl Tris-EDTA buffer. Five μl of this material was used as template in PCRs (total volume, 25 μl). Sequences flanking the transposon ends were amplified with Taq polymerase (94°C for 5 min, 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 45 s, and then 72°C for 10 min), and PCR products were sequenced using primers Tn for1, Tn for2, Tn rev1, and Tn rev 2 (Table 2).
Rhamnolipid and drop collapse assays.
Rhamnolipid was measured via the orcinol method as previously described (20). Filtered supernatants prepared from M8 plus casein or PPGAS cultures grown with aeration at 30°C for 36 to 48 h were used for this assay (n = 3). To confirm that the supernatants contained biologically active surfactant, drop collapse assays were also performed on PPGAS filtered supernatants (above) or on filtered supernatants prepared from LB overnight cultures for fliC pilA rhlA and fliC pilA rhlA pRhlA. Filtered supernatant was serially diluted with sterile water, and 30-μl drops were placed on the lid of a Corning 96-well dish, allowing drop collapse activity to be visualized as described elsewhere (3). Each assay was repeated a minimum of three times.
RESULTS
P. aeruginosa exhibits sliding motility in the absence of flagella and TFP.
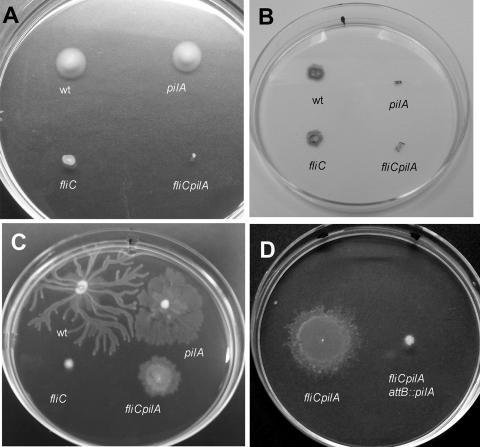
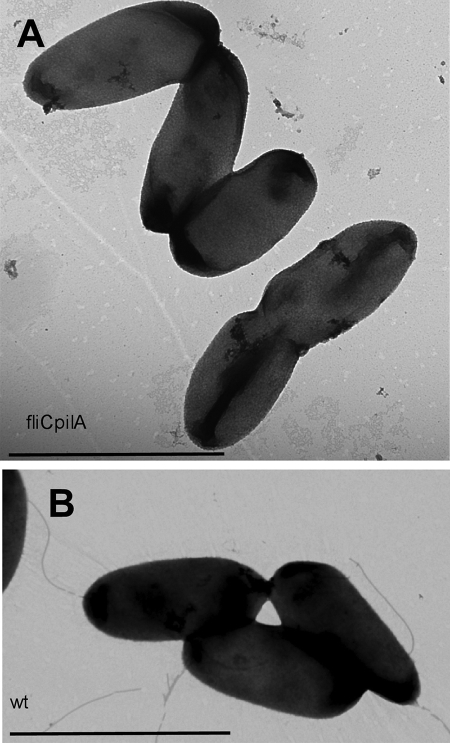
We are interested in understanding the factors that influence P. aeruginosa motility. The standard laboratory strain, PAO1, reproducibly displays swarming motility on 0.5% M8 plates supplemented with 0.2% glucose and 0.05% glutamate. We constructed a double mutant lacking both pili and flagella, fliC pilA, to serve as a negative control in motility assays. Like its isogenic parent fliC, the double mutant did not swim in liquid broth (data not shown) or on 0.3% agar swimming plates (Fig. 1A) (6). Subsurface stab assays demonstrated that the double mutant was also defective for twitching motility (Fig. 1B). We were surprised, however, to observe that the fliC pilA mutant still showed surface spreading on swarming motility plates (Fig. 1C). fliC showed no swarming motility, as expected; however, an isogenic pilA mutant showed increased spreading compared to wild-type PAO1 (Fig. 1C). To confirm that the double mutant lacked both TFP and flagella, we harvested bacteria from swarming motility plates and examined them by transmission electron microscopy (TEM). As seen in Fig. 2A, both flagella and TFP were absent from the surface of the double mutant, though these could clearly be visualized on the surfaces of wild-type PAO1 harvested from swarming plates (Fig. 2B) (6).
FIG. 1.
PAO1 motility in strains lacking type IV pili and/or flagella. A) Flagellum-dependent swimming motility on 0.3% LB agar plates. B) TFP-dependent twitching motility at the plastic-1.5% LB agar interface. C) Motility on 0.5% M8 agar plates supplemented with 0.05% glutamate and 0.2% glucose. D) Restoration of pilin expression inhibits motility of fliC pilA bacteria. wt, wild type.
FIG. 2.
Motility organelles are not present on sliding fliC pilA bacteria. TEM of fliC pilA bacteria (A) and isogenic wild-type (wt) bacteria (B) harvested directly from swarming plates and stained with 1.5% uranyl acetate. Bar, 2 μm.
The surface spreading of fliC pilA on semisolid agar is consistent with “sliding motility,” i.e., “a kind of surface translocation produced by the expansive forces in a growing culture in combination with special surface properties of the cells resulting in reduced friction between cell and substrate” (11). Unlike swarming, sliding motility does not require flagella (8, 11, 23). Complementation of fliC pilA and pilA with a copy of the wild-type pilA gene integrated into the chromosomal attB site restored twitching motility in both strains and abolished surface spreading in the double mutant (Fig. 1D and data not shown), confirming that the observed differences in motility on semisolid agar between these two strains were indeed due to the loss of pilin expression. This suggests that type IV pili inhibit sliding motility, possibly by increasing interactions between the cell and substrate.
Rhamnolipids facilitate motility in the absence of TFP and flagella.
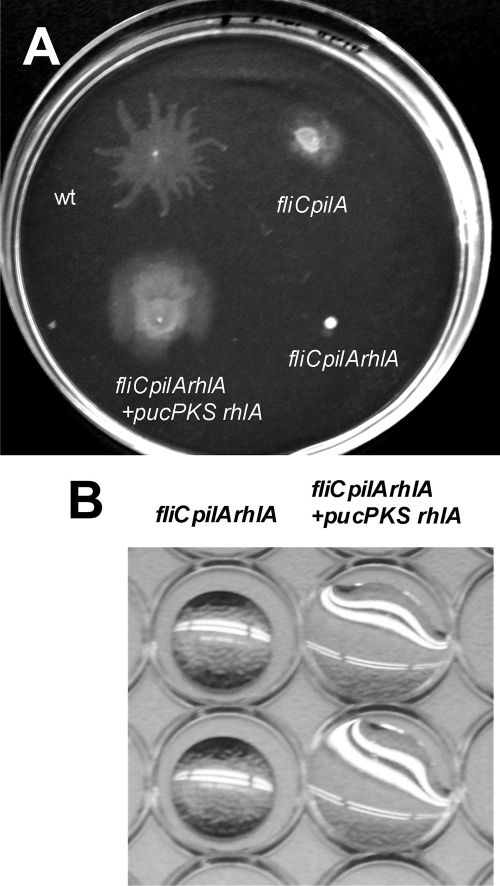
Motility on swarming plates usually appears to be dependent on the production of rhamnolipid surface wetting agents. We tested whether motility of fliC pilA on swarming plates also required rhamnolipid production. An unmarked in-frame deletion of rhlA, which encodes the protein required for synthesis of the rhamnolipid precursor HAA, was constructed in the fliC pilA background and tested for motility and surfactant production. As seen in Fig. 3B, the triple mutant showed no drop collapse activity; this could be complemented by expressing RhlA from a plasmid. The fliC pilA rhlA strain showed decreased surface spreading compared to its isogenic parent, fliC pilA; motility could be restored, however, by plasmid-encoded RhlA (Fig. 3A and Table 3). These data demonstrate that motility of fliC pilA bacteria on swarming plates is enhanced by HAA and/or rhamnolipid production. This enhancement of flagellum-independent spreading by increased expression of surface wetting agents (i.e., rhamnolipids) is characteristic of sliding motility (11).
FIG. 3.
rhlA positively influences sliding motility. (A) Dispersion of fliC pilA and fliC pilA rhlA bacteria was compared on 0.5% agar M8 plates after overnight incubation at 30°C. The extent of spread is dependent upon RhlA expression. (B) Undiluted supernatants prepared from overnight LB cultures of fliC pilA rhlA and the complemented strain fliC pilA rhlA pRhlA were assayed for drop collapse activity.
TABLE 3.
Colony sizes on swarming plates
| Strain | Colony diama (mm) |
|---|---|
| PAO1 | 42.5 ± 5.2 |
| fliC pilA | 19.5 ± 2.6 |
| fliC pilA gacA | 34.3 ± 5.0 |
| fliC pilA retS | 2.25 ± 0.50 |
| fliC pilA rhlA | 9.25 ± 1.30 |
| fliC pilA rhlA pRhlA | 19.5 ± 4.0 |
The largest diameter of the colony was measured after overnight incubation at 30°C on 0.5% M8 swarming plates supplemented with 0.2% glucose and 0.05% glutamate. Data are means ± standard deviations (n = 5).
Sliding motility is regulated by gacS/gacA and retS.
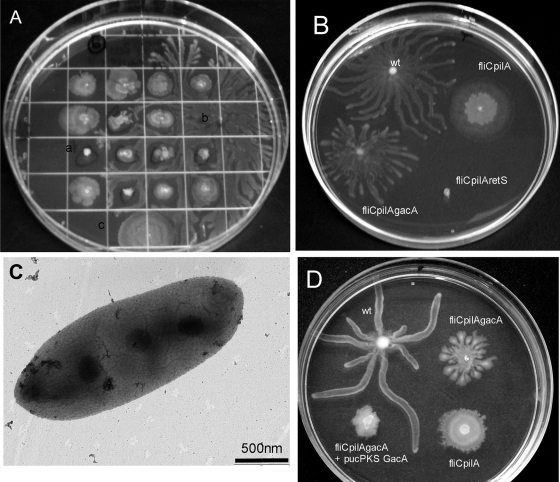
To identify other gene products that influence P. aeruginosa sliding motility, we performed random transposon mutagenesis of fliC pilA using miniTn5<Gmr>. A group of ca. 1,000 Gmr insertion mutants were screened for increased or decreased spreading on swarming motility plates. Figure 4A shows examples of mutants that demonstrated either decreased (type a) or increased (type b) motility compared to the fliC pilA parent (type c). We were particularly interested in b type mutants, which showed tendril formation reminiscent of that observed during wild-type PAO1 swarming. Two transposon insertions that resulted in this phenotype were mapped by inverse PCR to gacA and gacS (Fig. 4B and data not shown). Of interest, one of the transposon insertions that abolished motility of fliC pilA mapped to the retS gene (Fig. 4B). These sensor kinase response regulator proteins have been shown to inversely affect swarming behavior of wild-type (i.e., Fla+ Pil+) P. aeruginosa and P. fluorescens (9, 12).
FIG. 4.
GacA and RetS influence sliding motility. (A) Transposon insertion mutants of fliC pilA were screened on 0.5% M8 swarming plates for changes in motility. Mutants exhibiting both diminished (a) and increased (b) spreading were obtained; the parental fliC pilA strain was included on each plate as a control (c). Note the appearance of tendril formation in the strain marked b. (B) Sliding motility of fliC pilA gacA and fliC pilA retS mutants was assayed on 0.5% M8 plates after overnight incubation at 30°C. (C) fliC pilA gacA bacteria grown on swarming plates were harvested from the end of a tendril and visualized by TEM. No bacteria had recognizable surface structures; a representative image is shown. (D) Expression of GacA from a plasmid complements the fliC pilA gacA phenotype on 0.5% M8 plates supplemented with carbenicillin (200 μg/ml). Note that tendril formation is eliminated when GacA is reintroduced. The isogenic control strains all carry the empty vector pUCP-KS. wt, wild type.
fliC pilA gacA and fliC pilA retS bacteria exhibit no swimming or twitching motility (data not shown). As sliding motility is affected by bacterial growth rate (11), we confirmed that growth curves of fliC pilA, fliC pilA gacA, and fliC pilA retS bacteria in M8-glucose-glutamate-0.5% Casamino Acids liquid medium were identical (data not shown). We also harvested fliC pilA gacA mutants from swarming plates and examined them by TEM to confirm the absence of pili, flagella, cup fimbriae (44), or other surface structures that might be associated with increased motility; no such structures could be visualized (Fig. 4C). Complementation of fliC pilA gacA with a plasmid expressing wild-type GacA (pGacA) resulted in decreased motility relative to the parental fliC pilA strain (Fig. 4D). We also restored pilin expression to the fliC pilA gacA triple mutant by integrating a wild-type copy of the pilA gene under its own promoter at the chromosomal attB site. The resulting strain, fliC pilA gacA attB::pilA, showed restored twitching motility (data not shown) but failed to spread on swarming motility plates (see Fig. S1 in the supplemental material). This underscores the ability of pili to inhibit sliding motility in nonflagellated bacteria and is consistent with the hypothesis that the gacA mutation alters expression of genes and/or proteins other than those that target assembly of pili and flagella to result in a hypermotility phenotype.
fliC pilA retS PAO1 produces rhamnolipid and exhibits drop-collapse activity.
As demonstrated above, HAA and/or rhamnolipid production enhances sliding motility of fliC pilA. GacA and GacS activities positively regulate rhamnolipid production (12); however, the effect of retS mutations on rhamnolipid production has not been described. We tested whether gacA or retS mutants showed differences in rhamnolipid production in any of several media, including PPGAS and M8 media supplemented with casein. Extraction and quantification of rhamnolipids using the orincol method demonstrated that all strains showed equivalent rhamnolipid production (data not shown).
The orcinol assay measures rhamnose and does not detect the presence of the rhamnolipid precursor HAA, which also affects swarming motility. HAA was therefore measured indirectly by carrying out drop collapse assays on the same bacterial culture supernatants. Equal amounts of surfactant were present in the supernatants of all strains tested (data not shown), arguing that changes in total HAA and/or rhamnolipid production are unlikely to account for differences in sliding motility observed between fliC pilA, fliC pilA gacA, and fliC pilA retS.
Sliding motility of fliC pilA gacA responds to changes in available carbon and nitrogen sources.
P. aeruginosa swarming motility varies with the carbon and glucose sources provided in the swarming plate (17, 38). To determine whether sliding motilities of fliC pilA and fliC pilA gacA showed a similar nutritional dependence, we examined the extent and pattern of dispersion on 0.5% PPGAS plates and on 0.5% FAB plates supplemented with either 12 mM succinate or glutamate. The plates were incubated overnight at 30°C and then placed at room temperature and examined 48 to 72 h later. As seen in Fig. S2 of the supplemental material, both wild-type PAO1 and fliC pilA showed different extents of swarming and sliding, respectively, depending on available carbon and nitrogen sources. fliC pilA gacA spread to a similar extent under all conditions but exhibited distinct morphological patterns on each medium (see Fig. S2 in the supplemental material). We could not induce motility of fliC pilA retS on any swarming medium that we tested; likewise, fliC pilA rhlA bacteria failed to spread on 0.5% FAB plates with glutamate or succinate (see Fig. S2 in the supplemental material).
Overexpression of SadC and BifA alters dispersal of fliC pilA on swarming plates.
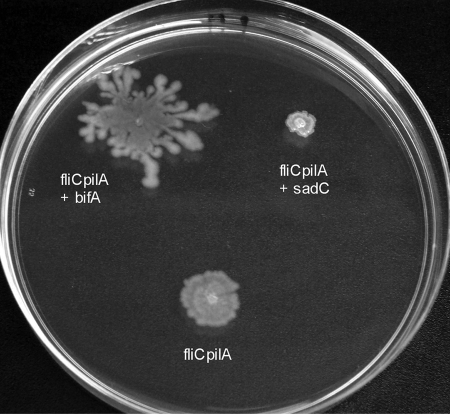
The diguanylate cyclase SadC and the c-di-GMP phosphodiesterase BifA have recently been shown to regulate swarming motility and biofilm formation in PA14. Overexpression of SadC represses swarming motility (26), while overexpression of BifA increases swarming zone size (18). We tested whether overexpression of these proteins in the fliC pilA background would similarly affect sliding motility. As seen in Fig. 5, SadC expression inhibited sliding, while overexpression of BifA resulted in both increased sliding and the appearance of tendril formation. These results argue that sliding motility, like swarming, is regulated by c-di-GMP levels.
FIG. 5.
BifA and SadC reciprocally regulate sliding motility. Dispersion of fliC pilA bacteria expressing BifA or SadC under control of the inducible PBAD promoter was assayed on 0.5% M8 agar plates supplemented with 0.2% glucose and 0.05% glutamate plates that contained gentamicin and arabinose (0.5%). Plates were incubated overnight at 30°C and then left at room temperature for an additional 48 h. The control strain carries the empty vector pMQ80.
DISCUSSION
Bacteria can move through their environment in many different ways. One relatively complex form of motility exhibited on semisolid surfaces by several genera of bacteria, including pseudomonads, is swarming motility. This form of motility appears to be “powered” by flagella but is also influenced by elaboration of surface structures (such as TFP), production of surfactants, and the presence of specific environmental signals. In this work we present evidence that P. aeruginosa can spread on semisolid surfaces in the absence of flagella or pili, a behavior that matches the description of sliding motility originally presented by Henrichsen (11). We also demonstrate that sliding motility is regulated by many of the factors that are known to influence classic swarming motility. Rhamnolipid production promoted sliding motility, specific carbon/nitrogen sources altered its extent, and known regulators of swarming, such as GacA/GacS, RetS, BifA, and SadC, inhibited or promoted sliding as well as swarming motility. Therefore, sliding motility may allow P. aeruginosa to colonize surfaces under conditions where flagellar expression is downregulated, e.g., within the human airway (14, 40).
Although we were initially quite surprised to observe surface spread by fliC pilA bacteria on swarming plates, the behavior of the single and double mutants is consistent with the notion that bacterial translocation of any sort is restricted by cohesive forces and facilitated by both propulsive forces and those that reduce friction between bacterium and substrate. In wild-type PAO1, TFP mediate interactions between bacteria that appear to antagonize the flagellum-dependent movement of bacteria away from each other. Thus, the disappearance in a pilA mutant of the tight tendrils that characterize a wild-type swarming colony is a manifestation of a net decrease in cell-cell interactions. Likewise, the increased motility of a double fliC pilA mutant compared to its fliC counterpart is facilitated by a decrease in the cell-cell interactions that restrict spreading of the piliated fliC strain. The importance of pilin-mediated cell-cell interactions is underscored by comparing the phenotypes of fliC pilA gacA with the same strain now complemented for pilin expression: tendril formation and spreading are strongly repressed in the piliated strain.
The absence of TFP and flagella is not sufficient for sliding motility. A pilA mutant constructed in the aflagellate PA103 background does not spread on swarming plates (data not shown), suggesting that sliding motility requires more than the absence of surface pili. This work provides evidence that rhamnolipids, which are not produced by PA103 (data not shown), are one of the factors that positively regulate sliding motility of fliC pilA bacteria. Nonetheless, a quantitative defect in total rhamnolipid production does not account for the failure of the fliC pilA retS cells to spread. Our assays, however, cannot rule out that changes in the relative amounts of HAA and mono- and di-rhamnolipids produced by the retS and gacA transposon insertion mutants underlie the different patterns and degrees of motility that they exhibit.
Many of the regulators that influence swarming motility also appear to affect production of exopolysaccharide (EPS), at either the transcriptional or posttranscriptional level (9, 18, 26). In agreement with published results, mutation of retS and overexpression of SadC in the fliC pilA background increased Congo Red staining of colonies, consistent with increased EPS production (data not shown). Thus, the increased cohesive forces provided by increased EPS production may inhibit both swarming and sliding motility. However, it is not clear that diminished or absent EPS production alone is sufficient to promote either swarming or sliding motility. Thus, while mutations in the EPS synthetic locus, pel, result in increased swarming of wild-type PA14, the same mutation introduced into a nonswarming ΔbifA mutant does not rescue swarming motility (18). These observations are consistent with the hypothesis that additional factors that promote swarming or sliding are also under the control of these regulators.
The surface translocation of P. aeruginosa in the absence of recognized motility organelles likely reflects the spread of dividing organisms in an environment where dispersive factors, such as surfactant production, outweigh the cohesive force usually provided by pili. This “spreading by expansion,” termed sliding motility by Henrichsen (11), has been recently described in mycobacteria (23) and nonflagellated Bacillus subtilis (8). Acetylated glycopeptidolipids function as surface-active compounds required for spreading of mycobacteria (33, 34), while mutations in a surfactin synthetic locus abolish sliding motility of B. subtilis (15, 16). Nonetheless, we cannot rule out the existence of an as-yet-unrecognized form of motility independent of pili and flagella, as has been described for other organisms, such as Myxococcus xanthus (27, 41), Flavobacterium johnsoniae (2, 21), and Mycoplasma mobile (29). Our ongoing screen of transposon mutants that abolish sliding motility has not yet identified genes encoding novel surface structures or homologs of motility genes found in these other organisms. Continuing identification of transposon insertions that either abolish or enhance sliding motility will provide additional information regarding regulators not only of this unusual surface behavior but also of swarming motility.
Supplementary Material
Acknowledgments
We thank Maria Lebron, Xiao Bai, and Isaac Elias for assistance with plasmid and strain construction, Amit Kunte for optimizing inverse PCR conditions, and Marc Pypaert for assistance with TEM. We thank George O'Toole for generously providing plasmids.
This work was supported by the Winchester Fund (Yale New Haven Hospital) and NIH training grant T32 AI07210 postdoctoral fellowship to T.S.M. and grants from the NIH (R01 AI054920), the Catherine and Patrick Weldon Donaghue Medical Research Foundation, and the Burroughs Wellcome Fund to B.K.
Footnotes
Published ahead of print on 7 December 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single copy chromosomal lacZ and lux gene fusions. BioTechniques 29948-952. [DOI] [PubMed] [Google Scholar]
- 2.Braun, T. F., M. K. Khubbar, D. A. Saffarini, and M. J. McBride. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J. Bacteriol. 1876943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caiazza, N. C., R. M. Q. Shanks, and A. G. O'Toole. 2005. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J. Bacteriol. 1877351-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, K. H., A. Kumar, and H. P. Schweizer. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64391-397. [DOI] [PubMed] [Google Scholar]
- 5.Dasgupta, N., M. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50809-824. [DOI] [PubMed] [Google Scholar]
- 6.de Kerchove, A. J., and M. Elimelech. 2007. Impact of alginate conditioning film on deposition kinetics of motile and non-motile Pseudomonas aeruginosa strains. Appl. Environ. Microbiol. 735227-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deziel, E., F. Lepine, S. Milot, and R. Villemur. 2003. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 1492005-2013. [DOI] [PubMed] [Google Scholar]
- 8.Fall, R., D. B. Kearns, and T. Nguyen. 2006. A defined medium to investigate sliding motility in a Bacillus subtilis flagella-less mutant. BMC Microbiol. 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman, A. L., B. Kulasekara, A. Rietsch, D. Boyd, R. S. Smith, and S. Lory. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7745-754. [DOI] [PubMed] [Google Scholar]
- 10.Harshey, R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57249-273. [DOI] [PubMed] [Google Scholar]
- 11.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heurlier, K., F. Williams, S. Heeb, C. Dormond, G. Pessi, D. Singer, M. Camara, P. Williams, and D. Haas. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 1862936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 21277-86. [DOI] [PubMed] [Google Scholar]
- 14.Jyot, J., A. Sonawane, W. Wu, and R. Ramphal. 2007. Genetic mechanisms involved in the repression of flagellar assembly by Pseudomonas aeruginosa in human mucus. Mol. Microbiol. 631026-1038. [DOI] [PubMed] [Google Scholar]
- 15.Kinsinger, R. F., D. B. Kearns, M. Hale, and R. Fall. 2005. Genetic requirements for potassium ion-dependent colony spreading in Bacillus subtilis. J. Bacteriol. 1878462-8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsinger, R. F., M. C. Shirk, and R. Fall. 2003. Rapid surface motility in Bacillus subtilis is dependent on extracellular surfactin and potassium ion. J. Bacteriol. 1855627-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechere. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 1825990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuchma, S. L., K. M. Brothers, J. H. Merritt, N. Liberati, F. M. Ausubel, and G. A. O'Toole. 2007. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 1898165-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, V. T., J. M. Matewish, J. L. Kessler, M. Hyodo, Y. Hayakawa, and S. Lory. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 651474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leech, A. J., and J. S. Mattick. 2006. Effect of site-specific mutations in different phosphotransfer domains of the chemosensory protein ChpA on Pseudomonas aeruginosa motility. J. Bacteriol. 1888479-8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, J., M. J. McBride, and S. Subramaniam. 2007. Cell surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J. Bacteriol. 1897503-7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis: identity of the lethal toxins produced in vitro and in vivo. J. Infect. Dis. 116481-489. [DOI] [PubMed] [Google Scholar]
- 23.Martinez, A., S. Torello, and R. Kolter. 1999. Sliding motility in mycobacteria. J. Bacteriol. 1817331-7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Granero, F., R. Rivilla, and M. Martin. 2006. Rhizosphere selection of highly motile phenotypic variants of Pseudomonas fluorescens with enhanced competitive colonization ability. Appl. Environ. Microbiol. 723429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56289-314. [DOI] [PubMed] [Google Scholar]
- 26.Merritt, J. H., K. M. Brothers, S. L. Kuchma, and G. A. O'Toole. 2007. SadC reciprocally influence biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J. Bacteriol. 1898154-8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mignot, T., J. W. Shaevitz, P. L. Hartzell, and D. Zusman. 2007. Evidence that focal adhesion complexes power bacterial gliding motility. Science 315853-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, T. S., and B. Kazmierczak. 2006. FlhF is required for swimming and swarming in Pseudomonas aeruginosa. J. Bacteriol. 1886995-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai, R., and M. Miyata. 2006. Gliding motility of Mycoplasma mobile can occur by repeated binding to N-acetylneuraminyllactose (sialyllactose) fixed on solid surfaces. J. Bacteriol. 1886469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overhage, J., S. Lewenza, A. K. Marr, and R. E. Hancock. 2007. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J. Bacteriol. 1892164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkins, M. D., H. Ceri, and D. G. Storey. 2001. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 401215-1226. [DOI] [PubMed] [Google Scholar]
- 32.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 974885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recht, J., and R. Kolter. 2001. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J. Bacteriol. 1835718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recht, J., A. Martinez, S. Torello, and R. Kolter. 2000. Genetic analysis of sliding motility in Mycobacterium smegmatis. J. Bacteriol. 1824348-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 15815-22. [DOI] [PubMed] [Google Scholar]
- 37.Shanks, R. M., N. C. Caiazza, S. M. Hinsa, C. M. Toutain, and G. A. O'Toole. 2006. Saccharamyces cerevisiae-based molecular tool kit for manipulation of genes of gram-negative bacteria. Appl. Environ. Microbiol. 725027-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrout, J. D., D. L. Chopp, C. L. Just, M. Hentzer, M. Givskov, and M. R. Parsek. 2006. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 621264-1277. [DOI] [PubMed] [Google Scholar]
- 39.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1784-791. [Google Scholar]
- 40.Sonawane, A., J. Jyot, R. During, and R. Ramphal. 2006. Neutrophil elastase an innate immunity effector molecule represses flagellin transcription in Pseudomonas aeruginosa. Infect. Immun. 746682-6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spormann, A. M. 1999. Gliding motility in bacteria: insights from studies of Myxococcus xanthus. Microbiol. Mol. Biol. Rev. 63621-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stover, C., X. Pham, A. Erwin, S. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, Jr., R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406959-964. [DOI] [PubMed] [Google Scholar]
- 43.Tremblay, J., A.-P. Richardson, F. Lepine, and E. Deziel. 2007. Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behavior. Environ. Microbiol. 92622-2630. [DOI] [PubMed] [Google Scholar]
- 44.Vallet, I., J. W. Olson, S. Lory, A. Lazdunski, and A. Filloux. 2001. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 986911-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ventre, I., A. L. Goodman, I. Vallet-Gely, P. Vasseur, C. Soscia, S. Molin, S. Bleves, A. Lazdunski, S. Lory, and A. Filloux. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA 103171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson, A. A., R. A. Alm, and J. S. Mattick. 1996. Construction of improved vectors for protein production in Pseudomonas aeruginosa. Gene 172163-164. [DOI] [PubMed] [Google Scholar]
- 47.Whitchurch, C. B., S. A. Beatson, J. C. Comolli, T. Jakobsen, J. L. Sargent, J. J. Bertrand, J. West, M. Klausen, L. L. Waite, P. J. Kang, T. Tolker-Nielsen, J. S. Mattick, and J. N. Engel. 2005. Pseudomonas aeruginosa fimL regulates multiple virulence functions by intersecting with Vfr-modulated pathways. Mol. Microbiol. 551357-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.