Abstract
The unknown genetic basis for improved phenol production by a recombinant Pseudomonas putida S12 derivative bearing the tpl (tyrosine-phenol lyase) gene was investigated via comparative transcriptomics, nucleotide sequence analysis, and targeted gene disruption. We show upregulation of tyrosine biosynthetic genes and possibly decreased biosynthesis of tryptophan caused by a mutation in the trpE gene as the genetic basis for the enhanced phenol production. In addition, several genes in degradation routes connected to the tyrosine biosynthetic pathway were upregulated. This either may be a side effect that negatively affects phenol production or may point to intracellular accumulation of tyrosine or its intermediates. A number of genes identified by the transcriptome analysis were selected for targeted disruption in P. putida S12TPL3. Physiological and biochemical examination of P. putida S12TPL3 and these mutants led to the conclusion that the metabolic flux toward tyrosine in P. putida S12TPL3 was improved to such an extent that the heterologous tyrosine-phenol lyase enzyme had become the rate-limiting step in phenol biosynthesis.
Pseudomonas putida is a ubiquitous soil bacterium that has become increasingly important for a wide range of biotechnological applications (46). Its broad biocatalytic potential makes it highly suitable for applications such as bioremediation (8) and biocatalysis (7, 40). Of special interest are solvent-tolerant P. putida strains. These organisms have evolved several mechanisms to deal with toxic compounds, including modifications of the inner and outer membranes and active extrusion of a broad range of compounds through membrane-associated efflux pumps (18, 31, 32). Solvent-tolerant bacteria are especially useful for the biosynthesis of compounds that are toxic to other microorganisms (7, 39, 48). They have been used to produce different aromatic compounds, such as cinnamic acid (24), p-hydroxybenzoic acid (33, 43), 3-methylcatechol (35, 50), and phenol (51).
Previously, we reported the construction of a P. putida S12 strain which efficiently produces phenol from glucose as a demonstration case for the “green” production of a toxic, hydroxylated aromatic compound (51). This strain has since been converted into an efficient producer of other, value-added aromatics (43). Efficient phenol production was achieved by introduction of the tpl gene from Pantoea agglomerans, encoding the enzyme tyrosine-phenol lyase (TPL), followed by a combined approach of targeted genetic engineering, random mutagenesis, antimetabolite selection, and high-throughput screening. This approach resulted in strain P. putida S12TPL3, which was capable of converting glucose into phenol with a yield of 7% (mol/mol) (51). The optimization of phenol production was achieved mostly by random approaches. As a result, little is known about the genetic basis of the enhanced phenol production.
Transcriptome analysis has been used successfully in the past to gain comprehensive insight into complex metabolic networks (12, 27, 29) and organic solvent stress (10, 14, 34). This technique has also been successfully applied for the analysis and optimization of microbial production strains (6, 20, 25). Since the genome sequence of P. putida KT2440 has become available (23), this avenue of research is open for the study of P. putida species. Ballerstedt et al. (2) demonstrated that microarrays based on this genome sequence are suitable for the analysis of P. putida strain S12.
The goal of this work was to identify the cellular mechanisms that underlie the optimized phenol production and to identify possible bottlenecks for phenol production of strain S12TPL3. To this end, we performed comparative transcriptomics, complemented with nucleotide sequence analysis and disruption of key genes involved in phenol production.
MATERIALS AND METHODS
Strains and growth conditions.
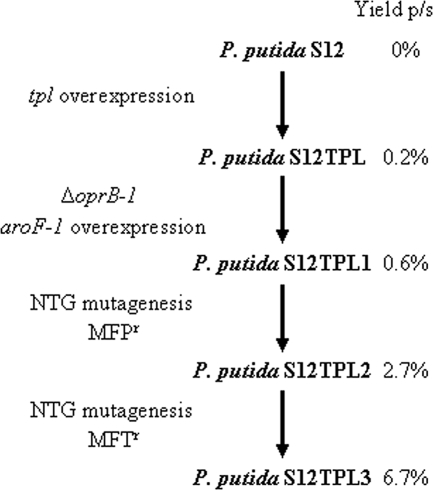
The bacterial strains and plasmids used in this study are listed in Table 1. P. putida S12 was originally isolated as a styrene-degrading bacterium (13). P. putida S12JNNmcs(t) is a negative control strain obtained by transforming P. putida S12 with the empty expression vector pJNNmcs(t) (previously known as pTn-1) (24, 51). P. putida S12TPL3 is a mutant optimized for phenol production (Fig. 1). P. putida S12TPL3c was obtained by serial cultivation of P. putida S12TPL3 in LB medium with 50 mg·liter−1 kanamycin. After five cultivations, the culture was streaked on LB agar with kanamycin. Separate colonies were inoculated on LB agar with 30 mg·liter−1 gentamicin. Colonies that had lost their Gmr phenotype were considered to be cured of the pNW1C plasmid.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference(s) |
|---|---|---|
| Strains | ||
| E. coli DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 37 |
| P. putida | ||
| S12 | Wild type | 13,47 |
| S12JNNmcs(t) | S12 transformed with empty expression vector pJNNmcs(t); negative control | 51 |
| S12TPL3 | Phenol-overproducing mutant of S12 | 51 |
| S12TPL3c | S12TPL3 without vector pNW1C | This study |
| S12TPL3r | S12TPL3c retransformed with vector pNW1C | This study |
| STΔpobA | S12TPL3 pobA::tetA | This study |
| STΔaroP1 | S12TPL3 aroP1::tetA | This study |
| STΔhpd | S12TPL3 hpd::tetA | This study |
| STΔphhA | S12TPL3 phh::tetA | This study |
| Plasmids | ||
| pNW1C | Gmr Apr; expression vector with tpl under control of the nagR/pNagAa promoter | 51 |
| pJQ200SK | Gmr; gene replacement vector | 30 |
| pTO1 | Tcr; tetA donor plasmid | 49 |
| pJQpobA::tetA | Tcr Gmr; pJQ200SK with the pobA gene interrupted by the tetA gene | 43 |
| pJQaroP1::tetA | Tcr Gmr; pJQ200SK with the aroP1 gene interrupted by the tetA gene | This study |
| pJQhdp::tetA | Tcr, Gmr; pJQ200SK with the hpd gene interrupted by the tetA gene | This study |
| pJQphhA::tetA | Tcr Gmr; pJQ200SK with the phhA gene interrupted by the tetA gene | This study |
Apr, Gmr, and Tcr, ampicillin, gentamicin, and tetracycline resistance, respectively.
FIG. 1.
Genealogy of phenol-producing mutants of P. putida S12. The left column shows how each successive mutant was obtained. The right column indicates the yield (mol phenol/mol glucose) attained in a typical shake flask culture (51). NTG, N-methyl-N′-nitro-N-nitrosoguanidine; MFPr: resistant to 100 mg m-fluoro-dl-phenylalanine·liter−1; MFTr, resistant to 100 mg m-fluoro-l-tyrosine·liter−1.
Culture conditions for shake flask cultivations, as well as medium compositions, were as described previously (51), except that 10 mM glucose was used in phenol production experiments. Chemostat cultivations were performed in 1-liter fermentors (New Brunswick Scientific) with a BioFlo 110 controller. Chemostats were inoculated with a 35-ml shake flask culture grown for approximately 8 hours in mineral salts medium (13) with 20 mM glucose supplemented with 10 mg·liter−1 gentamicin and 0.1 mM salicylate. The chemostat cultures were fed with the same medium containing 10 mM glucose as the growth-limiting nutrient. The working volume of the culture was kept at 0.7 liter by removing culture broth via a continuously working pump. The dilution rate (D) was set at 0.05 h−1 for 16 h, after which it was increased to 0.2 h−1. The pH was maintained at 7.0 by automatic addition of 4.0 M NaOH, and the temperature was kept at 30°C. Air was supplied with a flow of 1.0 liter·min−1 into the headspace. Dissolved oxygen levels were maintained at 15% air saturation by automatic adjustment of the stirring speed. Cultures were considered to be at steady state when after at least five volume changes at D = 0.2 h−1, there was no significant change in the phenol concentration in the fermentor, the optical density at 600 nm, and the stirring speed.
Sampling, mRNA isolation, and cDNA preparation for microarray analysis.
Chemostat samples were quenched in ice-cold methanol and centrifuged, and 1 ml RNAlater (Ambion) was added to the pellet. The cells were incubated for at least 1 h at 4°C, after which RNAlater was removed and the pellet flash frozen in liquid nitrogen and stored at −80°C. Frozen cell pellets were thawed by resuspension in 100 μl Tris-EDTA buffer containing 1 mg·ml−1 lysozyme. Total RNA was subsequently isolated using the Qiagen RNeasy mini purification kit according to the manufacturer's instructions. DNA was removed by on-column DNase I digestion, and total RNA was concentrated using the Qiagen RNA MinElute kit. mRNA was enriched using the Ambion MICROBExpress bacterial mRNA purification kit in combination with the specialized module for Pseudomonas according to the manufacturer's instructions. RNA concentrations were determined using an ND-1000 Nanodrop spectrophotometer (Isogen Life Sciences), and RNA quality was verified using the Bio-Rad Experion system (Bio-Rad) in combination with the Experion RNA standard- and high-sense analysis kit. Random priming cDNA synthesis, purification, fragmentation, and labeling were performed according to the microarray manufacturer's instructions (Affymetrix).
Microarray analysis.
High-density microarrays based on the genome of P. putida KT2440 were used (2). An additional 95 probe sets were added to this basic array, based on known sequences of P. putida S12 and related strains. A complete list of the features covered by these probe sets can be found in Table S1 in the supplemental material. The end-labeled cDNA fragments were hybridized to the microarray according to standard manufacturer's protocols. The chips were scanned, and the resulting .cel files were imported into Genespring GX software package version 7.2 (Agilent) using the GC RMA algorithm. After normalization, one-way analysis of variance (P > 0.05) was used to select genes that changed significantly between the conditions tested. Genes whose the expression level differed at least 1.8-fold between the two analyzed strains were used for further analysis.
Verification of transcriptome analysis by RT-qPCR.
The mRNA levels of a subset of genes were verified by real-time quantitative PCR (RT-qPCR). Specific primers (see Table S2 in the supplemental material) were designed using Primer3 software (http://frodo.wi.mit.edu/) (length maximum, 20 bases; G/C content, 50 to 60%; Tm, 55 to 60°C). PCR primer target sequences were chosen to achieve amplicon lengths of 75 to 200 bp. To predict possible amplicon secondary structures, Mfold software (http://www.bioinfo.rpi.edu/applications/mfold/) was used. RT-qPCR was performed with a spectrofluorimetric thermal cycler (iQ Cycler with optical module; Bio-Rad) using the iScript one-step RT-PCR kit with Sybr green (Bio-Rad) and equal amounts total RNA samples (100 ng) in 96-well plates according to the manufacturer's protocols (annealing temperature, 58°C). The RNase inhibitor SuperaseIn (Ambion) was added at a concentration of 0.5 U/μl to all RT-qPCR batches. Calibration curves relating the threshold cycle as a function of the log of the copy number of target gene were established using 10-fold serial dilutions (10 to 107 gene copies μl−1 template DNA) of P. putida S12 genomic DNA. All absolute quantifications were obtained using iCycler iQ real-time detection system software version 3.1 (Bio-Rad).
Nucleotide sequence analysis of key genes.
A total of 15 genes involved in the biosynthesis and degradation of tyrosine and phenol were selected for sequencing from both wild-type P. putida S12 and phenol-producing P. putida S12TPL3. PCR primer pairs were designed based on the genome sequence of P. putida KT2440 (23) and used to amplify the specific genes from the genomes of the two strains (see Table S2 in the supplemental material). PCR fragments were purified, and their nucleotide sequences were determined with the primers used for amplification. If a mutation was observed, PCR fragments were amplified and sequenced a second time to confirm the result. The tpl gene was sequenced on the plasmid isolated from P. putida S12TPL3 (primers are listed in Table S2 in the supplemental material). Nucleotide sequence analyses were performed by MWG-Biotech AG.
Targeted gene disruption.
Targeted gene disruptions were performed basically as described previously (43). Gene replacement vectors for the hpd, pobA, phhA, and aroP1 genes (Table 1) were created from pJQ200SK (30) with primers listed in Table S2 in the supplemental material. These vectors were used to mutate the selected genes in P. putida S12TPL3c by homologous recombination. Subsequently, pNW1C was transformed into the mutants by electroporation to yield P. putida STΔhpd, STΔpobA, STΔphhA, and STΔaroP1 (Table 1). All DNA manipulations were performed under permit DGM/SASIG 02-118 of the Dutch government.
TPL activity assay.
Cell extracts of P. putida S12TPL3 were produced by sonication as described previously (51) from a 100-ml shake flask culture in mineral salts medium with 20 mM glucose, 10 mg/liter gentamicin, and 0.1 mM salicylate. The conditions for the TPL activity assay were adapted from reference 19. To 1 ml of cell extract, 1.25 mM l-tyrosine and 0.1 mM pyridoxal 5′-phosphate were added. Three different concentrations of phenol (0, 0.25, and 1 mM) were added to study the effect of product inhibition. The reaction was carried out at 30°C. Samples were drawn after 0, 2, 5, 15, and 30 min, and the reaction was stopped by addition of 7.5% trichloroacetic acid. The pyruvate concentration in the samples was measured as described below. Pyruvate was measured instead of phenol because of the high background concentration of phenol already present in most samples.
Analytical methods.
Cell density, phenol, glucose, gluconic acid, 2-ketogluconic acid, and ammonium concentrations were determined as described previously (51). Pyruvate concentrations were determined by high-pressure liquid chromatography (HPLC) (Waters) using an Aminex HDP-87H column (Bio-Rad) along with a Chrompak UV detector (detection at 210 nm) with 0.016 M H2SO4 as the eluent, running at 0.6 ml/min.
Alternatively, phenol, tyrosine, phenylalanine, and 4-hydroxyphenylpyruvate concentrations were determined simultaneously by HPLC (Agilent 1100 system) using a Zorbax 3.5-μm SB C18 column (length, 5 cm; inside diameter, 4.6 mm; particle size, 3.5 μm) with acetonitrile-KH2PO4 (0.05 M, pH 2, 1% acetonitrile) as the eluent. This setup allows for simultaneous detection at multiple wavelengths and online determination of UV/visible spectra.
RESULTS
Transcriptome analysis of phenol-producing P. putida S12TPL3 and sequencing of key genes.
P. putida strain S12TPL3 was optimized for phenol production mostly by methods involving random mutagenesis. Comparative transcriptomics was applied in order to gain insight into the genetic changes in P. putida S12TPL3 connected to enhanced phenol production. To this end, both P. putida S12JNNmcs(t) and P. putida S12TPL3 (Table 1) were cultivated in chemostats. P. putida S12TPL3 produced approximately 500 μM phenol under these steady-state conditions. Phenol was added to this concentration to the feed of P. putida S12JNNmcs(t) to ensure equal growth conditions.
Transcriptome analysis yielded a total of 236 genes that showed at least a 1.8-fold change of the expression level in P. putida S12TPL3 compared to P. putida S12JNNmcs(t). Many of these genes encode hypothetical proteins and proteins with only a general function prediction. Other genes encode proteins previously shown to be related to solvent stress, such as alginate biosynthesis proteins (21); fatty acid metabolism proteins (38, 44); and antioxidants, chaperones, and heat shock proteins (10, 45). Genes involved in gluconate production and uptake (gnuK and gntP) were upregulated, likely as a result of the knockout of oprB-1 in P. putida S12TPL3. The expression levels of other genes in the peripheral pathways of glucose metabolism (9) remained unchanged. The present study focuses on a group of 38 genes that were expected to have a more apparent link to phenol production.
The selected genes were grouped into five functional categories: tyrosine biosynthesis, tryptophan biosynthesis, aromatic degradation, amino acid transport, and the tricarboxylic acid (TCA) cycle (Table 2). The transcriptomes of strains P. putida S12TPL and P. putida S12TPL2 (Fig. 1) were analyzed in a similar manner, and the same trends in expression level were observed as in P. putida S12JNNmcs(t) and P. putida S12TPL3.
TABLE 2.
Genes with an apparent link to phenol production that are differentially expressed in P. putida S12TPL3 compared to control strain P. putida S12JNNmcs(t)
| Functional group | Protein description (gene) | KT2440 locus tag | Fold changea |
|---|---|---|---|
| Tyrosine biosynthesis | DAHP synthase, class I (aroF-1) | PP2324 | 2.2 |
| Shikimate 5-dehydrogenase/quinate 5-dehydrogenase family protein | PP2406 | 2.7b | |
| 3-Dehydroquinate dehydratase, type II | PP2407 | 6.3b | |
| Shikimate dehydrogenase family protein | PP2608 | 5.1b | |
| DAHP synthase, class I (aroF-2) | PP3080 | 21.1 | |
| Quinate dehydrogenase (pyrroloquinoline quinone dependent), putative | PP3569 | 7.8 | |
| Phenylalanine-4-hydroxylase (phhA) | PP4490 | 87.6 | |
| Pterin-4-alpha-carbinolamine dehydratase (phhB) | PP4491 | 57.0 | |
| Tryptophan biosynthesis | Anthranilate synthase, component I (trpE) | PP0417 | 8.6 |
| Anthranilate synthase, component II (trpG) | PP0420 | 6.8 | |
| Anthranilate phosphoribosyltransferase (trpD) | PP0421 | 6.3 | |
| Indole-3-glycerol phosphate synthase (trpC) | PP0422 | 5.9 | |
| Aromatic degradation | 4-Hydroxybenzoate transporter (pcaK) | PP1376 | 3.4b |
| Beta-ketoadipyl coenzyme A thiolase (pcaF) | PP1377 | 7.1b | |
| 3-Oxoadipate enol-lactone hydrolase (pcaD) | PP1380 | 2.1b | |
| 4-Carboxymuconolactone decarboxylase (pcaC) | PP1381 | 1.9 | |
| BenF-like protein | PP1383 | 25.1 | |
| Tyrosine decarboxylase, putative | PP2552 | 3.7 | |
| 4-Hydroxyphenylpyruvate dioxygenase, putative | PP2554 | 3.8b | |
| Fumarylacetoacetate hydrolase family protein | PP2836 | 29.3 | |
| 4-Hydroxyphenylpyruvate dioxygenase (hpd) | PP3433 | 2.8 | |
| 4-Hydroxybenzoate hydroxylase (pobA) | PP3537 | 2.4b | |
| 3-Oxoadipate coenzyme A transferase, subunit A (pcaI) | PP3951 | 5.0b | |
| 3-Oxoadipate coenzyme A transferase, subunit B (pcaJ) | PP3952 | 3.2 | |
| Protocatechuate 3,4-dioxygenase, alpha subunit (pcaG) | PP4655 | 2.9 | |
| Protocatechuate 3,4-dioxygenase, beta subunit (pcaH) | PP4656 | 4.9b | |
| Amino acid transport | Transporter, LysE family | PP0198 | 2.1 |
| Branched-chain amino acid ABC transporter, ATP-binding protein | PP0615 | 0.6 | |
| Branched-chain amino acid ABC transporter, permease protein | PP0618 | 0.4 | |
| Branched-chain amino acid ABC transporter, periplasmic amino acid-binding protein | PP0619 | 0.5 | |
| Aromatic amino acid transporter (aroP2) | PP0927 | 10.7 | |
| Amino acid ABC transporter, permease protein | PP1070 | 0.5 | |
| Amino acid efflux protein, putative | PP3023 | 2.1 | |
| Aromatic amino acid transporter (aroP1) | PP4495 | 10.5 | |
| TCA cycle | Fumarate hydratase, class I | PP0897 | 0.5 |
| Oxidoreductase, Ldh family | PP2835 | 111.5 | |
| Isocitrate dehydrogenase, NADP dependent, prokaryotic type | PP4011 | 1.9 | |
| Malic enzyme | PP5085 | 2.2 |
Fold change in expression level in P. putida S12TPL3 compared to P. putida S12JNNmcs(t).
There was a large variation of expression levels between duplicates of P. putida S12TPL3. The average fold changes shown were confirmed by RT-qPCR.
Since N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis was used to obtain P. putida S12TPL3, point mutations that affect gene function were also expected to occur. As such mutations will not be detected with microarrays, the nucleotide sequences of several key genes related to tyrosine biosynthesis were determined in addition to the transcriptome analyses. The nucleotide sequences of three genes had changed in P. putida S12TPL3. The aroF-2 gene, encoding a 3-deoxy-d-arabino-2-heptulosonic acid 7-phosphate (DAHP) synthase (PP3080), contained a point mutation resulting in a G136E substitution. The trpE gene, encoding anthranilate synthase component I (PP0417), contained a point mutation resulting in a P290S substitution. The pykA gene, encoding pyruvate kinase II (PP1362), contained a point mutation resulting in a V219A substitution. No mutations were found in the tpl gene and genes with KT2440 genome locus tags PP1866, PP2324 (aroF-1), PP0074 (aroE-1), PP3002 (aroE-2), PP1769 (pheA), PP2170, PP1770, PP4490 (phhA), PP1972 (tyrB-1), PP3590 (tyrB-2), and PP4621 (hmgA).
Expression profiles of genes in selected functional groups. (i) Tyrosine biosynthesis.
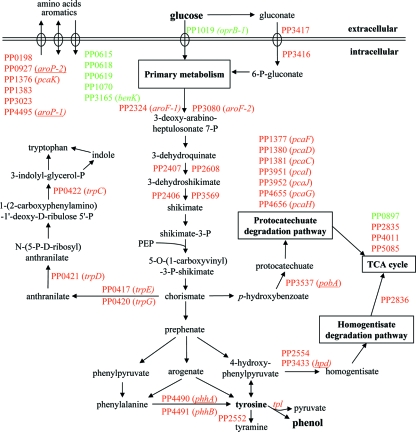
Since phenol production from glucose proceeds via tyrosine in P. putida S12TPL3, major changes in the tyrosine biosynthetic pathway were expected (Fig. 2). Indeed, eight genes encoding enzymes involved in the biosynthesis of tyrosine were upregulated in P. putida S12TPL3. Six of these genes encode (iso-)enzymes in the early shikimate pathway, which is responsible for the conversion of phosphoenolpyruvate and erythrose-4-phosphate into shikimate. Two genes (phhAB) encode enzymes responsible for the conversion of phenylalanine into tyrosine.
FIG. 2.
Schematic overview of expression profiles of genes involved in relevant pathways of phenol production, as derived from transcriptome analysis of P. putida S12TPL3. Genes are indicated by locus tags from P. putida KT2440, followed by their gene name in parentheses where applicable. Green lettering indicates downregulation, and red indicates upregulation. Genes that were selected for targeted disruption are underlined.
(ii) Tryptophan biosynthesis.
Tryptophan is produced from chorismate, which is also a precursor for tyrosine (Fig. 2) and thus for phenol in P. putida S12TPL3. The trpE gene and the trpCDG operon, required for the biosynthesis of tryptophan, are upregulated in P. putida S12TPL3. These genes are coregulated in P. putida (22).
(iii) Aromatic catabolism.
Several pathways for degradation of intermediates of the tyrosine biosynthetic pathway exist in P. putida (17). The pcaGH genes (16); the pcaCD, pcaF, and pcaIJ genes (36); and the pobA gene (3) were upregulated in P. putida S12TPL3. These genes encode eight of the nine genes necessary for the degradation of 4-hydroxybenzoate via the protocatechuate degradation pathway. This pathway can be linked to the tyrosine biosynthetic pathway through a chorismate-pyruvate lyase (PP5317) that converts chorismate into 4-hydroxybenzoate (Fig. 2). This link was confirmed by our observation that a pobA hpd double knockout mutant produced 4-hydroxybenzoate and showed, as a result of these mutations, a significantly decreased growth yield when cultured on quinate as a sole carbon source. Quinate can be metabolized only by conversion into chorismate, as was illustrated by the inability of an aroK (shikimate kinase) knockout mutant to utilize quinate as a carbon source (unpublished data).
The hpd gene and two genes with locus tags PP2554 and PP2836 were upregulated in P. putida S12TPL3. These genes encode enzymes in the homogentisate degradation pathway (1). Tyrosine can be degraded via this pathway, with 4-hydroxyphenylpyruvate as an intermediate.
(iv) Amino acid transport.
A wide range of transport systems for amino acids were found to be differentially expressed in P. putida S12TPL3. Several genes encoding proteins capable of amino acid export were upregulated, including two aroP genes for aromatic amino acid transporters and a LysE family transporter. Conversely, systems for the uptake of amino acids, such as the branched-chain amino acid ABC transporter, were downregulated.
(v) TCA cycle.
Four genes encoding enzymes in the TCA cycle were found to be differentially expressed in P. putida S12TPL3. Of these genes, three were upregulated.
Verification of leads from the transcriptome analysis.
Based on the transcriptome analysis, four genes that were upregulated in P. putida S12TPL3 were selected as targets for disruption in P. putida S12TPL3 (Fig. 2) in order to gain further insight into the enhanced phenol production characteristics of this strain. The hpd and pobA genes (locus tags PP3433 and PP3537, respectively) are responsible for degradation of shikimate pathway products. Disruption of these genes was expected to decrease the degradation of shikimate pathway intermediates, resulting in increased tyrosine production. The phhA gene (PP4490) is responsible for the conversion of phenylalanine into tyrosine. Disruption of this gene will give insight into what portion of the tyrosine is produced via phenylalanine, rather than directly via 4-hydroxyphenylpyruvate (Fig. 2). The aroP1 gene (PP4495) is responsible for transport of aromatic amino acids. Disruption of this gene could decrease export of tyrosine, increasing the intracellular tyrosine pool available to the TPL enzyme for phenol production.
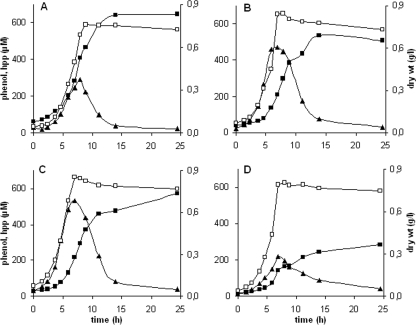
The selected genes were disrupted in P. putida S12TPL3. As a control strain, P. putida S12TPL3r (Table 1) was used. As expected, P. putida STΔphhA showed deteriorated production characteristics (Fig. 3D). The other mutants showed a decreased phenol productivity compared to P. putida S12TPL3 as well, although not as drastic as in strain STΔphhA. Unexpectedly, P. putida S12TPL3r also showed a decreased phenol productivity, comparable to that of the hpd and pobA knockout mutants (Fig. 3). This observation suggested that the curing and retransformation procedure intrinsically affected phenol production. The aroP1 knockout mutant showed impaired growth and also produced slightly less phenol than strain S12TPL3r (data not shown). A mutant with a double knockout of both isogenes aroP1 and aroP2 did not grow at all on minimal glucose medium.
FIG. 3.
Production and growth of P. putida S12TPL3 (A), P. putida S12TPL3r (B), P. putida STΔhpd (C), and P. putida STΔphhA (D). □, cell dry weight; ▪, phenol concentration; ▴, estimated 4-hydroxyphenylpyruvate (hpp) concentration. The profiles for strains STΔpobA and STΔhpd were similar; therefore, only results for STΔhpd are presented here. Cultures were performed in triplicate in mineral salts medium with 10 mM glucose as the carbon source. The variation between replicates was less than 10%.
All strains continued to produce phenol after growth had ceased (Fig. 3); however, the delay in production was more pronounced for the knockout strains and the retransformed control strain than for strain S12TPL3. The effect was most distinct in P. putida STΔhpd: as much as 62% of the phenol was produced after growth, compared to 28% in P. putida S12TPL3. Therefore, we speculated that an intermediate of the late tyrosine biosynthetic pathway accumulated, which was later converted into phenol. HPLC analyses of culture supernatant samples indeed indicated the presence of an aromatic compound with the same retention time and UV spectrum as 4-hydroxyphenylpyruvate. Since 4-hydroxyphenylpyruvate is unstable under the conditions used, exact determination of the concentration was not possible. One of the chemical degradation products overlaps with the 4-hydroxyphenylpyruvate peak. Therefore, the sum of the overlapping peak areas of 4-hydroxyphenylpyruvate and the degradation product was used to estimate the concentration of 4-hydroxyphenylpyruvate as accurately as possible (Fig. 3). Tyrosine and phenylalanine were also found, albeit in trace quantities.
Identification of bottlenecks for phenol production in P. putida S12TPL3.
The mutants that were expected to have an increased carbon flux toward tyrosine (ΔpobA and Δhpd mutants) did not show an increase in phenol production. The secretion of 4-hydroxyphenylpyruvate, a direct precursor of tyrosine, suggests that the bottleneck is either the conversion of 4-hydroxyphenylpyruvate into tyrosine or the conversion of tyrosine into phenol, causing the pathway to overflow to 4-hydroxyphenylpyruvate. Thus, we investigated whether TPL activity or tyrosine availability was the limiting factor for phenol production in P. putida S12TPL3. To study the effect of tyrosine availability, P. putida S12TPL3 was cultivated in a chemostat on mineral salts medium with 10 mM glucose as the growth-limiting nutrient. Phenol production was assessed either after addition of a tyrosine pulse or with a continuous feed of tyrosine.
For the pulse experiment, l-tyrosine was added to a steady-state culture to a final concentration of 845 μM. The phenol, tyrosine, and 4-hydroxyphenylpyruvate concentrations were monitored (Fig. 4). After 160 min, the phenol concentration in the culture had increased from 456 μM at steady state to a maximum of 560 μM. At the same time, the 4-hydroxyphenylpyruvate concentration had increased from 44 μM before the pulse to 232 μM after 160 min. The amount of biomass in the fermentor during this time remained constant at 0.59 g (dry weight) liter−1. The phenol production rate of the culture had increased 1.2-fold, from 4.8 to 5.9 μmol (g protein)−1 min−1, while the 4-hydroxyphenylpyruvate production rate increased almost fivefold from 0.5 to 2.4 μmol (g protein)−1 min−1. The average tyrosine uptake rate was 7.0 μmol (g protein)−1 min−1. The uptake of tyrosine did not result in an equivalent increase in phenol production. Most of the tyrosine was converted into 4-hydroxyphenylpyruvate instead.
FIG. 4.
Phenol and 4-hydroxyphenylpyruvate production in chemostat cultures of P. putida S12TPL3 after a tyrosine pulse. ▪, phenol concentration; ▵, tyrosine concentration; ▴, estimated 4-hydroxyphenylpyruvate (hpp) concentration. Values are the averages from duplicate experiments; variation between duplicates was less than 10%.
In order to mimic increased biosynthesis of tyrosine in the cells, l-tyrosine (1 mM) was added to the influent of the chemostat culture, and after reaching steady state, the phenol concentration was measured. With tyrosine in the influent, 611 μM phenol was produced, compared to 456 μM without tyrosine in the influent. The biomass had increased to 0.75 g (dry weight) liter−1. The specific production rate of phenol increased only marginally, from 4.9 to 5.0 μmol (g protein)−1 min−1. Thus, the increased phenol accumulation can be explained by the increased biomass density brought about by the utilization of tyrosine as an additional carbon source.
These results show that only a small amount of the additional tyrosine in the cell is converted into phenol under the conditions applied. Most tyrosine was converted into biomass, via 4-hydroxyphenylpyruvate. This suggests that TPL activity is the limiting factor for phenol production in P. putida S12TPL3, rather than tyrosine availability.
TPL activity in P. putida cell extracts and effect of exogenously added phenol on phenol production.
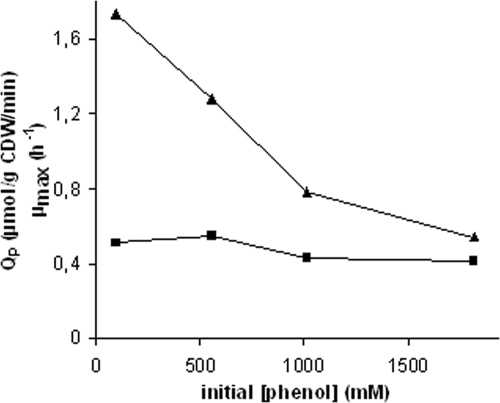
Since TPL appears to be the limiting factor for phenol production, TPL activity in cell extracts of P. putida S12TPL3 in the presence and absence of phenol was investigated. The specific TPL activity was greatly affected by the presence of phenol. In the presence of 1 mM of phenol, only 23% of the original activity remained [23.2 μmol (g protein)−1 min−1 without phenol and 5.3 μmol (g protein)−1 min−1 with 1 mM phenol]. Thus, it may be expected that the presence of phenol seriously affects its own production, through the inhibition of TPL (19, 28). This was confirmed by testing the phenol production of P. putida S12TPL3 grown in shake flask cultures in the presence of different concentrations of exogenously added phenol (Fig. 5). The effect of phenol on the μmax of the cultures was relatively small, but a drastic negative effect on phenol production was observed.
FIG. 5.
Phenol production by P. putida S12TPL3 in mineral salts medium with 20 mM glucose as the carbon source in the presence of different concentrations of phenol in the culture medium. ▪, maximum growth rate (h−1); ▴, Qp (average specific activity during the first 12 h of cultivation) [μmol (g cell dry weight)−1 min−1].
TPL activity in cell extract of P. putida STΔhpd was also compared to that in P. putida S12TPL3. Cells of both strains were harvested in logarithmic phase to ensure optimal comparability. The maximum specific activity of TPL in extracts of P. putida STΔhpd was 2.2 μmol (g protein)−1 min−1, which is about 30% of the activity in P. putida S12TPL3 [7.8 μmol (g protein)−1 min−1]. This decreased TPL activity is likely to explain the decreased phenol production and the increased 4-hydroxyphenylpyruvate production in this mutant. As the TPL expression plasmid used to transform the knockout mutants was isolated from strain S12TPL3, differences in the expression plasmid are excluded. Furthermore, no mutations in the tpl gene were observed in this plasmid (data not shown). Possibly, the lowered TPL activity may be explained by a copy number effect. The same effect may be expected for the other strains that were cured and retransformed with plasmid pNW1C, i.e., the retransformed parent strain (S12TPL3r) and the other knockout mutants.
DISCUSSION
The approach that yielded the optimized phenol producer P. putida S12TPL3 entailed several steps based on random procedures (49). In the present study, the effects on the cellular events associated with this enhanced phenol production were investigated by chemostat-based comparative transcriptomics, complemented with nucleotide sequencing of key genes. The observed altered transcription profiles in P. putida S12TPL3 could be linked to (i) cellular events effectuating improved metabolic flux toward l-tyrosine and hence toward phenol and (ii) events responding to this improved flux.
The upregulated genes of the tyrosine biosynthetic pathway fall into the first category. Almost all upregulated genes encoded enzymes in the early shikimate pathway, i.e., the first steps of aromatic amino acid biosynthesis. Of the sequenced early pathway genes, only aroF-2 contained a mutation, which was expected to have little effect, as the resulting amino acid substitution (G136E) also occurs in orthologues of Pseudomonas fluorescens strains (5). An increased metabolic flux through the early shikimate pathway, as indicated by the transcriptome data for strain S12TPL3, caused more carbon to flow toward phenylalanine in Pseudomonas aeruginosa (11). Tyrosine was produced from phenylalanine via an “overflow pathway” encoded by the phhAB genes, which are induced by phenylalanine (15). Our findings that the phhAB genes were highly upregulated in P. putida S12TPL3 and that disruption of phhA decreased phenol production by 60% were in agreement with these results. The upregulation of four genes involved in tryptophan biosynthesis (trpCDGE) suggests that the intracellular tryptophan pool was decreased in P. putida S12TPL3 (22). This decrease can be attributed to a mutation in the trpE gene, resulting in a P290S substitution adjacent to the catalytic site of the encoded anthranilate synthase component I (42). A decreased tryptophan pool contributes to the enhanced phenol production at two levels: less carbon will be directed into the tryptophan branch of the shikimate pathway, and the tryptophan-inhibited DAHP synthase could more effectively contribute to the overall carbon flux into the shikimate pathway. Thus, the transcriptome analyses indicate that the increased carbon flux toward tyrosine and, thus, phenol in strain S12TPL3 may be attributed to an upregulated early shikimate pathway, combined with a decreased carbon flux through the tryptophan branch.
The observed upregulation of genes involved in aromatic degradation pathways (1, 17), such as the homogentisate and the protocatechuate pathways, can be categorized in the second group of transcriptional events. The upregulation of these genes indicate high intracellular levels of tyrosine or its intermediates. This suggested that in P. putida S12TPL3, tyrosine is synthesized faster than it is converted into phenol. This would imply that TPL is the bottleneck in phenol synthesis. A strong indication that this is the case in P. putida S12TPL3 was provided by the tyrosine pulse and feed experiments that demonstrated that surplus tyrosine was converted mostly into 4-hydroxyphenylpyruvate (and eventually biomass) rather than into phenol. More indications that TPL limited phenol production was provided by the targeted knockout mutants of the first genes of the homogentisate and protocatechuate pathways (hpd and pobA). Also in these strains, increased 4-hydroxyphenylpyruvate levels rather than increased phenol levels were observed, indicating carbon overflow from accumulated tyrosine and showing that TPL was the limiting factor.
Other responses that indicated higher intracellular levels of tyrosine and/or its precursors were the upregulation of at least some of the putative amino acid transporters and the upregulation of three genes encoding TCA cycle enzymes. The most prominent amino acid transporters were the two aroP isogenes, each of which showed over 10-fold-higher expression levels. AroP-type transporters regulate intracellular pools of aromatic amino acids by balancing import and export (4). The aroP genes appear to be important in P. putida S12TPL3, since an aroP1 aroP2 double knockout mutant did not grow at all on minimal glucose medium (unpublished data), and an aroP1 single knockout mutant showed decreased phenol production. As the tyrosine precursor 4-hyroxyphenylpyruvate rather than tyrosine itself accumulated in strain S12TPL3, it may be speculated that AroP1 and AroP2 act as metabolic relief valves for this tyrosine precursor. The possibility of AroP1 and AroP2 as phenol transporters can be excluded, since the double knockout mutant does not grow on mineral medium in the absence of the tpl expression vector either. The induction of TCA cycle genes may be linked to the accumulation of phenol. Such a response has previously been observed in solvent-stressed pseudomonads, as a means to increase the energy supply required by the solvent tolerance response (41, 45). The upregulation of the malic enzyme may be attributed to the surplus production of pyruvate from tyrosine by TPL (26).
In conclusion, transcriptome and nucleotide sequence analyses have provided profound and novel insights into the genetic basis of the enhanced phenol production by P. putida S12TPL3, as well as in the cellular responses brought about by the enhanced metabolic flux toward tyrosine and the production of phenol. In strain S12TPL3, the carbon flow toward tyrosine was optimized to such an extent that TPL activity had become the bottleneck for phenol production. Based on these detailed insights, a rational approach for further optimization of phenol production can be pursued, with a primary focus on enhancement of TPL activity.
Supplementary Material
Acknowledgments
We thank Maaike Westerhof for her assistance with the targeted gene disruptions. We also thank Suzanne Verhoef for providing strain P. putida STΔpobA and the pobA hpd double knockout mutant and Nicole van Luijk for providing the aroP1 aroP2 double knockout mutant of P. putida S12TPL3.
Footnotes
Published ahead of print on 9 November 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Arias-Barrau, E., E. R. Olivera, J. M. Luengo, C. Fernandez, B. Galan, J. L. Garcia, E. Diaz, and B. Minambres. 2004. The homogentisate pathway: a central catabolic pathway involved in the degradation of l-phenylalanine, l-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida. J. Bacteriol. 1865062-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballerstedt, H., R. J. M. Volkers, A. E. Mars, J. E. Hallsworth, V. A. Martins dos Santos, J. Puchalka, J. Duuren, G. Eggink, K. N. Timmis, J. A. M. de Bont, and J. Wery. 2007. Genomotyping of Pseudomonas putida strains using P. putida KT2440-based high-density DNA microarrays: implications for transcriptomics studies. Appl. Microbiol. Biotechnol. 751133-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertani, I., M. Kojic, and V. Venturi. 2001. Regulation of the p-hydroxybenzoic acid hydroxylase gene (pobA) in plant-growth-promoting Pseudomonas putida WCS358. Microbiology 1471611-1620. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. D. 1971. Maintenace and exchange of the aromatic amino acid pool in Escherichia coli. J. Bacteriol. 10670-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byng, G. S., A. Berry, and R. A. Jensen. 1983. A pair of regulatory isozymes for 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase is conserved within group I pseudomonads. J. Bacteriol. 156429-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, J. H., S. J. Lee, and S. Y. Lee. 2003. Enhanced production of insulin-like growth factor I fusion protein in Escherichia coli by coexpression of the down-regulated genes identified by transcriptome profiling. Appl. Environ. Microbiol. 694737-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bont, J. A. M. 1998. Solvent-tolerant bacteria in biocatalysis. Tibtech 16493-499. [Google Scholar]
- 8.Dejonghe, W., N. Boon, D. Seghers, E. M. Top, and W. Verstraete. 2001. Bioaugmentation of soils by increasing microbial richness: missing links. Environ. Microbiol. 3649-657. [DOI] [PubMed] [Google Scholar]
- 9.del Castillo, T., J. L. Ramos, J. J. Rodriguez-Herva, T. Fuhrer, U. Sauer, and E. Duque. 2007. Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: genomic and flux analysis. J. Bacteriol. 1895142-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez-Cuevas, P., J. E. Gonzalez-Pastor, S. Marques, J. L. Ramos, and V. de Lorenzo. 2006. Transcriptional tradeoff between metabolic and stress-response programs in Pseudomonas putida KT2440 cells exposed to toluene. J. Biol. Chem. 28111981-11991. [DOI] [PubMed] [Google Scholar]
- 11.Fiske, M. J., R. J. Whitaker, and R. A. Jensen. 1983. Hidden overflow pathway to l-phenylalanine in Pseudomonas aeruginosa. J. Bacteriol. 154623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godard, P., A. Urrestarazu, S. Vissers, K. Kontos, G. Bontempi, J. van Helden, and B. Andre. 2007. Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 273065-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmans, S., J. P. Smits, M. J. van der Werf, F. Volkering, and J. A. M. de Bont. 1989. Metabolism of styrene oxide and 2-phenyl ethanol in the styrene degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 552850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi, S., R. Aono, T. Hanai, H. Mori, T. Kobayashi, and H. Honda. 2003. Analysis of organic solvent tolerance in Escherichia coli using gene expression profiles from DNA microarrays. J. Biosci. Bioeng. 95379-383. [DOI] [PubMed] [Google Scholar]
- 15.Herrera, M. C., and J. L. Ramos. 2007. Catabolism of phenylalanine by Pseudomonas putida: the NtrC-family PhhR regulator binds to two sites upstream from the phhA gene. J. Mol. Biol. 3661374-1386. [DOI] [PubMed] [Google Scholar]
- 16.Hosokawa, K. 1970. Regulation of synthesis of early enzymes of p-hydroxybenzoate pathway in Pseudomonas putida. J. Biol. Chem. 2455304-5308. [PubMed] [Google Scholar]
- 17.Jimenez, J. I., B. Minambres, J. L. Garcia, and E. Diaz. 2002. Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ. Microbiol. 4824-841. [DOI] [PubMed] [Google Scholar]
- 18.Kieboom, J., J. J. Dennis, J. A. M. de Bont, and G. J. Zylstra. 1998. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J. Biol. Chem. 27385-91. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai, H., and H. Yamada. 1970. Tyrosine phenol lyase. I. Purification, crystallization and properties. J. Biol. Chem. 2451767-1772. [PubMed] [Google Scholar]
- 20.Lee, J. H., D. E. Lee, B. U. Lee, and H. S. Kim. 2003. Global analyses of transcriptomes and proteomes of a parent strain and an l-threonine-overproducing mutant strain. J. Bacteriol. 1855442-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leone, S., A. Molinaro, F. Alfieri, V. Cafaro, R. Lanzetta, A. Di Donato, and M. Parrilli. 2006. The biofilm matrix of Pseudomonas sp. OX1 grown on phenol is mainly constituted by alginate oligosaccharides. Carbohydr. Res. 3412456-2461. [DOI] [PubMed] [Google Scholar]
- 22.Maurer, R., and I. P. Crawford. 1971. New regulatory mutation affecting some of the tryptophan genes in Pseudomonas putida. J. Bacteriol. 106331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. A. Eisen, K. N. Timmis, A. Dusterhoft, B. Tümmler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4799-808. [DOI] [PubMed] [Google Scholar]
- 24.Nijkamp, K., N. van Luijk, J. A. M. de Bont, and J. Wery. 2005. The solvent-tolerant Pseudomonas putida S12 as host for the production of cinnamic acid from glucose. Appl. Microbiol. Biotechnol. 69170-177. [DOI] [PubMed] [Google Scholar]
- 25.Park, J. H., K. H. Lee, T. Y. Kim, and S. Y. Lee. 2007. Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc. Natl. Acad. Sci. USA 1047797-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, S. J., P. A. Cotter, and R. P. Gunsalus. 1995. Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J. Bacteriol. 1776652-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, S. J., S. Y. Lee, J. Cho, T. Y. Kim, J. W. Lee, J. H. Park, and M. J. Han. 2005. Global physiological understanding and metabolic engineering of microorganisms based on omics studies. Appl. Microbiol. Biotechnol. 68567-579. [DOI] [PubMed] [Google Scholar]
- 28.Pletnev, S. V., M. N. Isupov, Z. Dauter, K. S. Wilson, N. G. Faleev, E. G. Harutyunyan, and T. V. Demidkina. 1996. Purification and crystals of tyrosine phenol-lyase from Erwinia herbicola. Biochem. Mol. Biol. Int. 3837-42. [PubMed] [Google Scholar]
- 29.Polen, T., M. Kramer, J. Bongaerts, M. Wubbolts, and V. F. Wendisch. 2005. The global gene expression response of Escherichia coli to l-phenylalanine. J. Biotechnol. 115221-237. [DOI] [PubMed] [Google Scholar]
- 30.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 12715-21. [DOI] [PubMed] [Google Scholar]
- 31.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56743-768. [DOI] [PubMed] [Google Scholar]
- 32.Ramos, J. L., E. Duque, J. J. Rodriguez-Herva, P. Godoy, A. Haidour, F. Reyes, and A. Fernandez-Barrero. 1997. Mechanisms for solvent tolerance in bacteria. J. Biol. Chem. 2723887-3890. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Gonzalez, M. I., A. Ben-Basat, M. J. Campos, and J. L. Ramos. 2003. Genetic engineering of a highly solvent-tolerant Pseudomonas putida strain for biotransformation of toluene to p-hydroxybenzoate. Appl. Environ. Microbiol. 695120-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reva, O. N., C. Weinel, M. Weinel, K. Bohm, D. Stjepandic, J. D. Hoheisel, and B. Tümmler. 2006. Functional genomics of stress response in Pseudomonas putida KT2440. J. Bacteriol. 1884079-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas, A., E. Duque, A. Schmid, A. Hurtado, J. L. Ramos, and A. Segura. 2004. Biotransformation in double-phase systems: physiological responses of Pseudomonas putida DOT-T1E to a double phase made of aliphatic alcohols and biosynthesis of substituted catechols. Appl. Environ. Microbiol. 703637-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero-Steiner, S., R. E. Parales, C. S. Harwood, and J. E. Houghton. 1994. Characterization of the pcaR regulatory gene from Pseudomonas putida, which is required for the complete degradation of p-hydroxybenzoate. J. Bacteriol. 1765771-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1982. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Santos, P. M., D. Benndorf, and I. Sa-Correia. 2004. Insights into Pseudomonas putida KT2440 response to phenol-induced stress by quantitative proteomics. Proteomics 42640-2652. [DOI] [PubMed] [Google Scholar]
- 39.Sardessai, Y. N., and S. Bhosle. 2004. Industrial potential of organic solvent tolerant bacteria. Biotechnol. Prog. 20655-660. [DOI] [PubMed] [Google Scholar]
- 40.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409258-268. [DOI] [PubMed] [Google Scholar]
- 41.Segura, A., P. Godoy, P. van Dillewijn, A. Hurtado, N. Arroyo, S. Santacruz, and J. L. Ramos. 2005. Proteomic analysis reveals the participation of energy- and stress-related proteins in the response of Pseudomonas putida DOT-T1E to toluene. J. Bacteriol. 1875937-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spraggon, G., C. Kim, X. Nguyen-Huu, M. C. Yee, C. Yanofsky, and S. E. Mills. 2001. The structures of anthranilate synthase of Serratia marcescens crystallized in the presence of (i) its substrates, chorismate and glutamine, and a product, glutamate, and (ii) its end-product inhibitor, l-tryptophan. Proc. Natl. Acad. Sci. USA 986021-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhoef, S., H. J. Ruijssenaars, J. A. M. de Bont, and J. Wery. 2007. Bioproduction of p-hydroxybenzoate from renewable feedstock by solvent- tolerant Pseudomonas putida S12. J. Biotechnol. 13249-56. [DOI] [PubMed] [Google Scholar]
- 44.Volker, U., and M. Hecker. 2005. From genomics via proteomics to cellular physiology of the Gram-positive model organism Bacillus subtilis. Cell. Microbiol. 71077-1085. [DOI] [PubMed] [Google Scholar]
- 45.Volkers, R. J. M., A. L. de Jong, A. G. Hulst, B. L. M. van Baar, J. A. M. de Bont, and J. Wery. 2006. Chemostat-based proteomic analysis of toluene-affected Pseudomonas putida S12. Environ. Microbiol. 81674-1679. [DOI] [PubMed] [Google Scholar]
- 46.Wackett, L. P. 2003. Pseudomonas putida—a versatile biocatalyst. Nat. Biotechnol. 21136-138. [DOI] [PubMed] [Google Scholar]
- 47.Weber, F. J., L. P. Ooijkaas, R. M. Schemen, S. Hartmans, and J. A. M. de Bont. 1993. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl. Environ. Microbiol. 593502-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wery, J., and J. A. M. de Bont. 2004. Solvent-tolerance of pseudomonads: a new degree of freedom in biocatalysis, p. 609-634. In J. L. Ramos (ed.), Pseudomonas, vol. 3. Kluwer Academic, Dordrecht, The Netherlands. [Google Scholar]
- 49.Wery, J., B. Hidayat, J. Kieboom, and J. A. M. de Bont. 2001. An insertion sequence prepares Pseudomonas putida S12 for severe solvent stress. J. Biol. Chem. 2765700-5706. [DOI] [PubMed] [Google Scholar]
- 50.Wery, J., D. I. Mendes da Silva, and J. A. M. de Bont. 2000. A genetically modified solvent-tolerant bacterium for optimized production of a toxic fine chemical. Appl. Microbiol. Biotechnol. 54180-185. [DOI] [PubMed] [Google Scholar]
- 51.Wierckx, N. J. P., H. Ballerstedt, J. A. M. de Bont, and J. Wery. 2005. Engineering of solvent-tolerant Pseudomonas putida S12 for bioproduction of phenol from glucose. Appl. Environ. Microbiol. 718221-8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.