Abstract
Background:
The dopamine precursor levodopa has shown some, albeit relatively weak, promise in treating cocaine dependence. This study sought to identify the most appropriate behavioral therapy platform for levodopa pharmacotherapy by evaluating its effect when administered in combination with behavioral platforms of varying intensities.
Method:
A total of 161 treatment-seeking cocaine dependent subjects received sustained release levodopa/carbidopa (400/100 mg bid, Sinemet) or placebo delivered in combination with Clinical Management (ClinMan); ClinMan+Cognitive Behavioral Therapy (CBT); or ClinMan+CBT+Voucher-Based Reinforcement Therapy (VBRT) in a 12-week randomized, placebo-controlled, double-blind (for medication condition) trial. Medication compliance was monitored with riboflavin (100 mg/capsule) and the Medication Event Monitoring System. Protocol compliance was addressed in weekly, 10-minute nurse-delivered ClinMan sessions. Weekly, 1-hour CBT sessions focused on coping skills training. VBRT (with escalating reinforcer value) provided cash-valued vouchers contingent on cocaine-negative urine toxicology results. Urine benzoylecgonine assays collected thrice-weekly were analyzed by intention-to-treat criteria using generalized linear mixed models.
Results:
Levodopa main effects were found on all outcome measures of cocaine use. Contrasts testing the levodopa-placebo difference within each behavioral platform found reliable effects, favoring levodopa, only in the VBRT platform. Levodopa treatment with vouchers produced higher proportions of cocaine-negative urines and longer periods of consecutive abstinence compared to other treatment combinations.
Conclusion:
This is the first study to find a significant treatment effect for levodopa and, in doing so, to demonstrate that the magnitude of this effect is dependent upon conditions of the behavioral therapy platform. The data support use of levodopa with abstinence-based reinforcement therapy as one efficacious combination in cocaine dependence disorder treatment.
Keywords: levodopa-carbidopa, cocaine treatment, contingency management, Voucher-Based Reinforcement Therapy, VBRT
1. Introduction
Cocaine abuse and dependence continue to represent a significant public-health concern and treatment priority. Approximately 1.5 million people can be classified as dependent or abusing cocaine, and rates of cocaine initiation have steadily increased (SAMHSA, 2004). In 2004, cocaine abuse accounted for 14 percent of admissions to substance abuse treatment services (Substance Abuse and Mental Health Services Administration (SAMHSA), 2006). Treatment development efforts have advanced in two main directions. Pharmacologically, the search for an effective medication treatment continues, with some degree of success. A number of candidate drugs have been eliminated (e.g., risperidone, olanzapine) or retained (e.g., modafinil, disulfiram) as potential medications for cocaine dependence. Behavioral interventions, notably contingency management (CM) procedures, have been effective in treating substance dependence. Since Higgins' seminal study showing a greater than twofold increase in rates of continuous cocaine abstinence under contingent versus non-contingent voucher delivery (Higgins et al., 1994), CM studies across a range of substances of abuse have continued to provide strong empirical support for this approach (e.g., Budney et al., 2000; Higgins et al., 2004; Lussier et al., 2006; Petry and Martin, 2002; Silverman et al., 1999).
Recently it has proven useful to test potential cocaine medications in the context of specific behavior therapy platforms. We (Grabowski et al., 1995) and others (Covi et al., 1995) reported fluoxetine combined with cognitive psychotherapy to be ineffective but later demonstrated efficacy when evaluating fluoxetine in combination with abstinence contingent incentives (Schmitz et al., 1998). Kosten and colleagues (Kosten et al., 2003) reported an additive effect of the antidepressant desipramine and CM in the treatment of cocaine use in buprenorphine-maintained patients. In that study, the combination treatment had almost twofold greater efficacy in improving cocaine-negative urines than either treatment alone. Poling and colleagues (Poling et al., 2006) reported a synergistic effect of CM and bupropion for the treatment of cocaine dependence in methadone-maintained patients. Cocaine use was lower in the group receiving CM treatment with 300 mg/d of bupropion than in the group receiving CM with placebo, or in the two voucher control conditions with or without bupropion. Thus, the behavioral therapy context may be a particularly important determinant of effectiveness for some medications.
Levodopa, the dopamine precursor considered uniquely efficacious in treatment of Parkinson's disease, has particular relevance as a candidate medication in the treatment of stimulant abuse since dopamine plays a critical role in mediating the acute reinforcing effects of cocaine (Koob, 1992; Volkow et al., 2004), and the depletion of dopamine associated with chronic cocaine use is thought to underlie cocaine craving and relapse following periods of abstinence (Dackis & Gold, 1985; Shalev et al., 2002). Early evidence that chronic cocaine administration might result in dopamine depletion came from reports of hyperprolactinemia in abstinent patients following chronic cocaine abuse (Dackis et al., 1984; Dackis & Gold, 1985; Teoh et al., 1990). The clinical observation that dopamine-regulated prolactin levels vary inversely with available dopamine in the synapse provided the rationale for studying dopamine agonist drugs like bromocriptine which presumably worked by reversing dopamine supersensitivity caused by dopamine depletion (Dackis & Gold, 1985; Giannini et al., 1987; Giannini & Billett, 1987). Neurochemical studies in animals have shown changes in brain dopamine function consistent with the dopamine depletion hypothesis (e.g., Pradhan et al., 1978; Mateo et al., 2005). Imaging studies of the human brain have linked cocaine-induced decreases in dopamine concentrations with disruptions of prefrontal regions involved with reward, motivation, memory, and cognitive control (reviewed in Volkow et al., 2003). Thus, the collective findings support the hypothesis that chronic cocaine exposure produces dopaminergic dysfunction and that successful pharmacological interventions might be those having a restorative effect on this neurotransmitter system.
Levodopa, converting to dopamine in the brain, should increase CNS levels thus ameliorating deficits and enhancing function following cocaine discontinuation. Cocores and colleagues (Cocores et al., 1989) were the first to report reduced cocaine withdrawal symptomatology when levodopa or bromocriptine was administered in addition to standard outpatient treatment. Wolfsohn et al. (Wolfsohn and Angrist, 1990) reported similar encouraging results in an open trial, but these findings were not replicated in a subsequent placebo-controlled double-blind trial (Wolfsohn et al., 1993). More recently in a NIDA sponsored Clinical Rapid Efficacy Screening Trial (Shoptaw et al., 2005), levodopa/carbidopa (300/75 mg/d) was not superior to placebo on urine benzoylecgonine (BE) or self-reported measures of cocaine use. Our exploratory pilot and subsequent dose-ranging studies (placebo vs. levodopa/carbidopa 400/100 mg/d or 800/200 mg/d) indicated safety and tolerability but no reduction in cocaine use (Mooney et al., 2007). Thus, a study combining levodopa with a more potent behavioral therapy was predicated on work by us and others indicating instances of CM enhanced medication effectiveness (e.g., Kosten et al., 2003; Poling et al., 2006; Schmitz et al., 1998).
A growing number of empirically supported, manual-guided behavioral therapies are now available as “platforms” for pharmacotherapy efficacy research. Whereas abstinence or reduced substance use is the target for all these platforms, specific therapies differ in intensity and potency (Carroll et al., 2004b). More intense therapies have been recommended for medications having weak or partial effects, so that continued heavy drug use does not override potential pharmacotherapy effects (Carroll et al., 2004b). Still, in the conduct of clinical trials, Carroll and colleagues describe the need to strike a balance between reducing substance use with behavioral treatments while avoiding ceiling effects that might obscure or prohibit detection of medication effects. To pursue this objective, we designed a clinical trial to compare levodopa versus placebo when delivered in combination with behavioral platforms of differing intensities. All cocaine dependent subjects received brief clinical management (ClinMan), to which was added cognitive-behavioral therapy (CBT), or CBT plus Voucher-Based Reinforcement Therapy (VBRT). We posited that: (1) levodopa would be superior to placebo in reducing cocaine use; and (2) this difference would be significant only in the most intense behavioral therapy platform (i.e., VBRT).
2. Methods
2.1. Subjects
All participants were enrolled at the outpatient treatment research clinic located at the Substance Abuse Research Center in Houston, Texas (described in Grabowski et al., 1997). Inclusion required meeting Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (American Psychiatric Association, 1994) criteria for current cocaine dependence and self-reported recent use of cocaine (confirmed by qualitative urine benzoylecgonine testing during a 1-week screening period). Exclusion criteria included: (1) dependence on drugs other than cannabis or nicotine; (2) current non-substance induced Axis I psychotic, depressive, or anxiety disorder; (3) presence of significant suicidal or homicidal ideation; (4) having a major medical illness or condition (e.g., severe pulmonary or cardiovascular disease, renal function impairment); (5) concomitant medications interacting with levodopa/carbidopa (e.g., MAO inhibitors, anticonvulsants); (6) pregnancy or nursing; and (7) inability to read, write, or speak English.
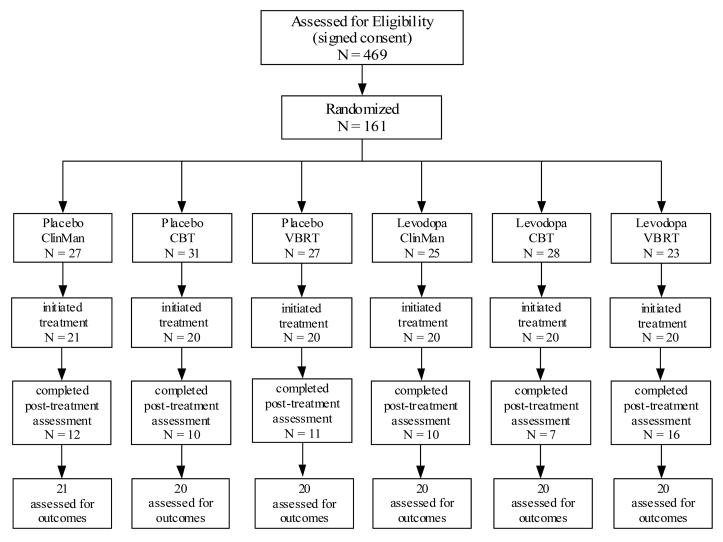
Enrollment took place between March 2000 and June 2005, during which time we screened 469 individuals for eligibility. Of those screened in person, 169 did not meet inclusion criteria, 7 chose not to participate, and 132 failed to complete the intake evaluation. The most common exclusion reasons were medical (28%), psychiatric (23%), and non-recent use of cocaine (9%). The intent-to-treat sample consisted of 161 subjects who were randomized to one of the six treatment conditions (see Figure 1, Study Flow Chart). All subjects provided written informed consent. The research, consent, and all materials were reviewed and approved by the Committee for the Protection of Human Subjects (CPHS) of the University of Texas Medical School, Houston.
Figure 1.
Study participation flow figure. Note. ClinMan = Clinical management. CBT = Cognitive behavioral therapy. VBRT = Voucher-based reinforcement therapy.
2.2. Study Design and Procedures
This randomized, placebo-controlled, double-blind (for medication condition) trial used a 2 × 3 factorial design to compare levodopa effects across three behavioral platforms. Following a 2-week intake evaluation phase, subjects were assigned to one of the six treatment conditions using urn randomization procedures to ensure even distribution of treatment groups with respect to gender, employment status, severity of cocaine addiction, motivation to change, and concurrent alcohol use (Stout et al., 1994). Treatment conditions combined medication (levodopa, placebo) with ClinMan only, ClinMan with CBT (referred to hereafter as CBT), or ClinMan with CBT plus VBRT (referred to hereafter as VBRT). During the 12-week treatment phase, participants obtained medication and provided urine samples at thrice-weekly (M, W, F) visits. Participants were dismissed for missing three consecutive or nine total clinic visits. At the end of 12 weeks all participants were contacted to complete post-treatment assessments.
2.3. Medications
For participants assigned to the levodopa condition, therapy began with a one-week dose escalation schedule (Days 1-2, one 50/12.5 levodopa/carbidopa sustained-release tablet, Sinemet® CR, BID; Days 3-4, one 100/25 tablet BID; Days 5-6, one 200/50 tablet BID; Day 7, one 400/100 tablet BID), followed by maintenance for 11 weeks, and a 7-day dose reduction at week 12. Medication and placebo were packed in identical capsules with riboflavin (100 mg) and dispensed in Medication Event Monitoring Systems (MEMS). MEMS caps electronically record bottle openings (i.e., presumptive doses). All investigators and staff, except the pharmacist, were blind to medication assignment.
2.4. Behavioral Platforms
Clinical Management
The NIMH Collaborative Study on the Treatment of Depression (Fawcett et al., 1987) guidelines provided the framework for clinical management (ClinMan). ClinMan seeks to develop a supportive relationship with the patient that: (1) promotes medication and study compliance; and (2) reinforces continued progress and participation using common or non-specific therapeutic elements. Three study nurses were trained to conduct manual-based ClinMan sessions that included systematic assessment of concomitant medications and study medication side effects along with supportive discussion regarding clinical progress. Weekly ClinMan sessions lasted 10-15 minutes.
Cognitive Behavioral Therapy
Cognitive-behavioral therapy (CBT), according to the model of Marlatt and Gordon (Marlatt and Gordon, 1985), emphasizes coping skill development to achieve abstinence and prevent relapse. Support for CBT in the treatment of cocaine dependence has been demonstrated (Carroll et al., 1991, 1994; Maude-Griffin et al., 1998; McKay et al., 1997; Rohsenow et al., 2000; Schmitz et al., 1997), with recent evidence suggesting additive effects when combining CBT with pharmacotherapy (Carroll et al., 2004a; Carroll et al., 1998; Schmitz et al., 2001). CBT is considered to be more powerful than ClinMan, but not sufficiently potent to override medication effects (Carroll et al., 2004b).
CBT therapists teach patients to recognize and cope with high-risk situations through: (1) self-monitoring and functional analysis of situational craving or drug use stimuli/factors; (2) coping skills training (i.e., didactic presentations, role-playing, specific home practice exercises); and (3) general lifestyle modification (e.g., increasing pleasant drug-free events, anger management, interpersonal skills, general problem-solving). Masters' level therapists underwent training to establish their competence and adherence in delivery of the manual driven CBT (evaluated previously in Schmitz et al., 1997; Schmitz et al., 2001). For ongoing supervision, therapists met weekly with a senior therapist who reviewed manual adherence and clinical case materials.
Voucher-Based Reinforcement Therapy
Subjects assigned to receive VBRT earned monetary rewards for each cocaine-negative urine sample. The reinforcement schedule followed standard recommendations (Budney and Higgins, 1998), with voucher values starting at $2.50 and increasing by $1.25 for each consecutive cocaine-negative urine sample. A $10 bonus voucher was awarded for providing three consecutive cocaine-negative urine samples. Missed or refused samples were considered positive and reset the voucher value to $2.50. Five consecutive negative urines after submission of a positive urine sample could return the voucher value to its previous level. Subjects received a weekly written and verbal statement indicating their previous week's urine test results. Vouchers earned could be exchanged at any time for gift certificates (e.g., local restaurants, movie theatre) or redeemed as direct cash payments.
2.5. Assessments
Psychiatric diagnostic and addiction severity information were collected at intake using the Structured Clinical Interview for DSM-IV (First et al., 1995) and the Addiction Severity Index (McLellan et al., 1992). Safety assessments at intake included a medical history and physical examination, laboratory evaluation of liver and thyroid function, and cardiac functioning (i.e., 12-lead electrocardiogram). Vital signs (including heart rate, blood pressure, and weight) were obtained weekly during treatment. In addition, tests were conducted for pregnancy (urine), drug toxicology (e.g., urine, for over 90 illicit and prescription drugs), tuberculosis, and HIV. Each week participants completed a 33-item side effects checklist consisting of side effects common in many substance using patients (i.e., nausea, vomiting, anxiety/nervousness, depression, dizziness, and problems concentrating), including some characteristic of levodopa. Adverse events were evaluated by the study nurse and physician that included a standardized reporting system when appropriate. Pill taking was observed on clinic visit days and tracked by riboflavin fluorescence testing (Del Boca et al., 1996; Mooney et al., 2004) and MEMS. Cocaine craving ratings were obtained at each visit using the Brief Substance Craving Scale (BSCS: Mezinskis et al., 1998; Mezinskis et al., 2001).
The primary outcome measure, cocaine use, determined by benzoylecgonine (BE) urine sample levels as a dichotomous outcome (> 300ng/ml), was obtained thrice-weekly (M, W, F). Onsite analysis (Analytical Neurochemistry Division) was conducted with the Syva EMIT and Varian Thin Layer chromatography Toxi-Lab systems. Indices of treatment efficacy based on cocaine use included mean proportion of cocaine-positive urines and maximum number of consecutive cocaine-negative urines. Indices of performance in therapy for those receiving CBT and VBRT were examined based on session attendance and cumulative earnings, respectively. Secondary outcomes included cocaine craving, medication compliance, and side effects.
2.6. Data Analysis
All analyses were performed on the intent-to-treat population using the Statistical Analysis System, Version 9.13 (SAS, 2006) with statistical significance designated as p < .05. Baseline differences in treatment groups were evaluated using ANOVA and chi-square tests. Proportional hazards Cox regression analyses were used to test for differences in time to dropout from treatment. Multilevel mixed models were used for repeated measures analyses with each including the between-subjects factors of medication and therapy platform (except for side effects, vital signs, and medication compliance analyses in which therapy was removed since it was not hypothesized to influence these outcomes) and the within-subjects factor of time. The first model tested main effect terms. The second set of models, constructed as statistical contrasts, tested the a priori hypothesis that the levodopa-placebo difference would be significant in the VBRT platform only. For these contrasts, the medication effect was tested within each of the three platform conditions. To further examine the superiority of levodopa+VBRT on cocaine use outcomes, this treatment condition was coded as the reference group and compared to each of the other five treatment combinations in a set of pairwise statistical contrasts. Longest continuous abstinence was analyzed using Poisson regression. Post-hoc follow-up tests for all pair-wise comparisons employed Tukey's adjustment for type I error rates while other multiple comparisons use Holm's (Holm, 1979) Bonferroni-Stepdown Method.
3. Results
3.1. Sample Characteristics
The demographic and substance use characteristics of participants at randomization are presented in Table 1. Subjects across groups did not differ on any of these baseline variables. Recent cocaine use was reported to be 14.0 (SD = 8.7) days in the past 30, with lifetime cocaine use reported to be 11.8 (SD = 5.8) years. The majority of subjects (64%) reported previous drug abuse treatment. Cocaine was detected in 86% of the samples submitted on the first intake visit. During the two-week intake evaluation period prior to treatment, the percentage of cocaine-positive urines was 76%, with comparable rates across groups.
Table 1.
Demographic and drug use characteristics of participants at baseline by randomization status.
| Variable | Placebo | Levodopa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ClinMana n =27 |
CBTb n =31 |
VBRT c n = 27 |
ClinMana n =25 |
CBTb n =28 |
VBRT c n = 23 |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Female | 3 | 11.1 | 6 | 19.4 | 3 | 11.1 | 3 | 12.0 | 9 | 32.1 | 3 | 13.0 |
| Race | ||||||||||||
| White | 7 | 25.9 | 6 | 19.4 | 8 | 29.6 | 6 | 24.0 | 7 | 25.0 | 5 | 21.7 |
| Black | 17 | 63.0 | 24 | 77.4 | 16 | 59.3 | 14 | 56.0 | 20 | 71.4 | 18 | 78.3 |
| Hispanic | 3 | 11.1 | 1 | 3.3 | 3 | 11.1 | 4 | 16.0 | 1 | 3.6 | 0 | 0.0 |
| Employed | 16 | 59.3 | 12 | 38.7 | 11 | 40.7 | 13 | 52.0 | 14 | 50.0 | 12 | 52.2 |
| Previous drug treatment | 20 | 74.1 | 20 | 64.5 | 18 | 66.7 | 13 | 52.0 | 20 | 71.4 | 12 | 52.2 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 41.3 | 6.5 | 41.3 | 7.7 | 41.1 | 6.9 | 39.1 | 7.6 | 42.1 | 5.8 | 43.3 | 7.0 |
| Education (Years) | 12.5 | 2.2 | 12.6 | 1.4 | 12.8 | 2.1 | 12.7 | 1.6 | 12.8 | 2.0 | 13.1 | 1.5 |
| Cocaine use (past 30 days) | 14.9 | 8.4 | 14.4 | 9.6 | 12.6 | 10.0 | 14.1 | 8.2 | 16.0 | 8.4 | 12.3 | 7.6 |
| Lifetime cocaine (years) | 13.6 | 6.9 | 11.6 | 5.7 | 11.3 | 5.9 | 10.2 | 5.4 | 12.7 | 5.5 | 10.8 | 4.6 |
| Alcohol use (past 30 days) | 8.4 | 9.6 | 9.8 | 10.9 | 6.6 | 7.9 | 9.9 | 9.9 | 10.7 | 9.3 | 10.1 | 10.6 |
| Lifetime alcohol (years) | 16.9 | 9.5 | 17 | 10.6 | 19.8 | 8.8 | 15.9 | 9.2 | 20.2 | 7.7 | 16.5 | 11.3 |
Note.
Clinical management.
Cognitive behavioral therapy.
Voucher-based reinforcement therapy.
3.2. Treatment Retention
One-hundred sixty one subjects were randomized and 121 initiated treatment (i.e., competed at least 1 week of treatment after randomization). Survival analysis indicated no differences in dropout rates by medication, χ2 (1) = 3.12, p = 0.0770, therapy, χ2 (2) = .6172, p = 0.7345, or their interaction, χ2 (2) = 1.0164, p = 0.6016 (see Figure 2, top panel). The average number of visits attended during treatment was 16.3 (SD = 12.0) of a possible 36, with a trend suggesting higher attendance in the levodopa compared to the placebo group (M = 17.9, SD = 1.6; M = 14.7, SD = 1.4, respectively).
Figure 2.
Proportion of participants remaining over the 12-week treatment period (top panel). Observed rates of cocaine use by medication and behavioral therapy platform (bottom panel). Note. ClinMan = Clinical management. CBT = Cognitive behavioral therapy. VBRT = Voucher-based reinforcement therapy.
3.3. Cocaine Use Outcomes
Proportion of Cocaine-Positive Urines
Figure 2 (bottom panel) illustrates the rates of cocaine-positive urines over time for each treatment group. Mixed model analyses showed a significant medication effect, F (1, 2100) = 5.15, p ≤ 0.0233, with participants receiving levodopa having lower rates of cocaine positive urines during treatment (levodopa, M = 61.6%; placebo, M = 79.1%). A reliable main effect of therapy also emerged, F (1, 2100) = 3.75, p ≤ 0.0236, with the difference driven by lower rates of cocaine use in the VBRT versus CBT groups, t (2092) = 2.76, p = 0.0162 (VBRT, M = 59%; CBT, M = 84%). No effect of time or the interaction terms with time were found. Planned contrasts testing the simple effect of medication in each therapy platform condition indicated a significant difference in the VBRT condition, t (817) = −3.48, p = 0.0015, but not in any of the other therapy conditions. Within the VBRT condition, the levodopa group demonstrated a cocaine use rate of 40% while the placebo rate was 77%. The set of contrasts with levodopa+VBRT as the reference group and each of the other treatment groups was significant (all p's ≤ .05), with the exception of the levodopa+ClinMan comparison.
Consecutive Abstinence
The main effect of medication was significant, indicating that those treated with levodopa achieved a higher maximum number of consecutive cocaine-negative urines (levodopa, M = 5.2; placebo, M = 1.6), χ2 (1) = 3.86, p = 0.0487. The significant effect of therapy, χ2 (2) = 7.45, p = 0.0241, was driven by a higher number of consecutive negative urines in the VBRT group (M = 5.2) compared to the CBT group (M = 1.3), χ2 (1) = 7.87, p = 0.0050. Contrast tests of medication within each therapy platform condition again found the only significant difference in the VBRT group (levodopa, M = 11.85 versus placebo, M = 2.35; χ2 (1) = 12.84, p = 0.0009). Contrasts between levodopa+VBRT and each of the other treatment groups were significant (all p's ≤ .05), with the exception of the levodopa+ClinMan comparison. The percentage of subjects in each group that achieved varying durations of continuous abstinence during treatment is depicted in Figure 3.
Figure 3.
The percentage of subjects in each group achieving varying durations of continuous abstinence during treatment. Note. ClinMan = Clinical management. CBT = Cognitive behavioral therapy. VBRT = Voucher-based reinforcement therapy.
3.4. CBT session attendance and voucher earnings.
The mean percentage of CBT sessions attended across groups receiving this treatment component was 62.5% for placebo+CBT; 56.4% for placebo+VBRT; 61.6% for levodopa+CBT; and 83.4% for levodopa+VBRT. A logistic regression comparing session attendance was statistically significant for therapy, χ2 (1) = 4.04, p = 0.0446, medication, χ2 (1) = 7.98, p = 0.0047, and the interaction of therapy and medication, χ2 (1) = 8.49, p = 0.0036, revealing better CBT attendance in the levodopa+VBRT group. Within the VBRT conditions, cumulative earnings during treatment were greater for the levodopa group (M = US$291.35, S.D. = US$374.30) than for the placebo group (M = US$30.80, S.D. = US$58.4), t (19.9) = 3.08, p = 0.006. The longitudinal pattern of earnings was also assessed as a function of medication, time, and their interaction. Effects of medication F (1, 38) = 9.46, p = 0.0039, time, F (11, 38) = 2.32, p = 0.0268, and medication by time, F (11, 38) = 2.18, p = 0.0370 were observed. Simple effects analyses of the interaction revealed increases across time for levodopa, F (11, 38) = 3.05, p = 0.0052, but not for placebo. Evaluation of differences between medication groups over time showed that the levodopa group earned significantly more money at each week except the first.
3.5. Craving
BSCS scores differed as a function of medication, F (1, 75.7) = 4.47, p = 0.0378, and time, F (11, 40.3) = 6.13, p < 0.0001, reflecting decreasing craving scores as treatment progressed. Throughout treatment, those receiving levodopa reported significantly lower craving scores (levodopa, M = 2.8, SE = .30; placebo, M = 3.7, SE = .31). The interaction between medication and time, F (11, 40.3) = 1.81, p = 0.0842, suggested a slightly steeper decline in craving for cocaine in the levodopa group compared to the placebo group.
3.6. Medication Compliance
Under riboflavin or MEMS models, assessing the effects of medication, time, and their interaction, only significant effects of time were observed, F (5, 233) = 5.59, p <.0001, and F (5, 233) = 13.91, p <.0001, respectively, with both demonstrating decreased compliance during treatment. Riboflavin-based compliance rates ranged from 50% to 70% and tended to be higher than the more conservative MEMS-based rates (range 17% to 67%), consistent with discrepancies observed in other pharmacotherapy studies (Mooney et al., 2004).
3.7. Side Effects and Vital Signs
The “ever”-occurrence of 33 side effects in the treatment period, including seven levodopa-related side effects, (i.e., nausea, vomiting, anxiety/nervousness, depression, dizziness, problems concentrating, and twitching) were evaluated. Two side effects occurred significantly more often in levodopa treated patients compared to placebo: nausea, Fisher's exact test (d.f. = 1), p = 0.0359, (20.4% vs. 40.7%, respectively); and dizziness, Fisher's exact test (d.f. = 1), p = 0.0124, (13.0% vs. 35.2%, respectively). Two subjects discontinued treatment due to intolerance of medication; one received placebo while the other received levodopa. No effects of medication, time, or their interaction were observed on body weight, diastolic blood pressure, systolic blood pressure, or heart rate.
4. Discussion
This is the first study to detect a reliable treatment effect for levodopa and, in doing so, to show how the magnitude of this effect depends on conditions of the behavioral therapy platform. The most robust levodopa-placebo difference emerged in the behavioral therapy platform that included VBRT. Levodopa+VBRT was superior across cocaine use outcomes when compared to the other treatment combinations.
Levodopa-treated subjects had lower cocaine use rates, longer periods of continuous abstinence, and less craving compared to patients assigned to placebo. These findings stand in contrast to those reported in an earlier medication screening trial in which levodopa, (tested at a lower dose, 300/75 mg/d) showed no advantage over placebo (Shoptaw et al., 2005). Additionally, Mooney et al. (Mooney et al., 2007) documented the safety and feasibility of levodopa/carbidopa therapy (800/200 mg/d) in an initial safety trial and in a subsequent dose ranging study but reported no clear decrease in cocaine use as a function of levodopa dose. Given the apparent safety and tolerability of levodopa (Mooney et al., 2007), the present results are highly encouraging with regard to the potential utility of levodopa pharmacotherapy in reducing cocaine use when combined with appropriate behavioral interventions.
There is consensus in the scientific community that combining behavioral treatments and pharmacological agents provides more effective treatment than either constituent therapy alone in most cases (Carroll et al., 2004b; Onken et al., 1995; Stitzer and Walsh, 1997; Volkow et al., 2004). Carroll (Carroll et al., 2004b) however, specifically stated that determinations of optimal behavioral therapy platform-pharmacotherapy combinations should be evidence based, not arbitrarily determined. The process should involve consideration of the specific strengths and weaknesses of the medication, as well as the potential targets of the behavioral therapy platform (Carroll et al., 2004b). Levodopa's strengths, i.e., safety, tolerability and compliance, are offset by modest direct pharmacotherapeutic effects (Mooney et al., 2007; Shoptaw et al., 2005). Still, our finding that a high intensity behavioral therapy platform enhances the pharmacotherapy efficacy provides the evidence for a balanced, tenable therapy combination using the criteria specified by Carroll (Carroll et al., 2004b). That we did not observe a linear relationship between therapy “dose” or intensity and medication effect suggests that levodopa was effective because of its unique interaction with VBRT. Findings from this trial add to other recent reports showing a significant and specific synergism between contingency management and cocaine pharmacotherapy (Kosten et al., 2003; Poling et al., 2006; Schmitz et al., 1998).
Although not designed to investigate treatment mechanisms, the trial permits speculation that might direct future research to elucidate the underpinnings of enhanced effectiveness of the levodopa contingency management combination. One explanation comes from recent models of dopamine and reward saliency. Koob and others (Koob and Le Moal, 2001) have proposed that chronic cocaine use produces persistent diminution of brain reward function, resulting in a condition in which the saliency of drug-related cues far exceeds the saliency value of natural non-drug reinforcers (Ahmed et al., 2002; Ahmed and Koob, 1998; Koob and Le Moal, 1997, 2005). Contingency management increases availability of non-drug or environmental reinforcers directly competing with drug use. To the extent that processes related to reward saliency are mediated via dopamine transmission (e.g. Berridge and Robinson, 1998; Volkow, 2004), levodopa administration might compensate for dopamine-related deficits and thus enhance saliency of contingently delivered reinforcers, thus improving responsiveness to this behavioral intervention. Conversely, this proposed mechanism suggests that in the absence of alternative reinforcers the therapeutic efficacy of levodopa would be diminished, precisely the observation in the non-voucher conditions. If confirmed as the treatment mechanism, levodopa and other dopamine-augmentation strategies could be used as a way to enhance the efficacy of contingency management.
Early onset of levodopa treatment benefit suggests that it may also attenuate cocaine withdrawal symptoms, necessarily by the same dopaminergic mechanism. This is supported by the decreased self-reported cocaine craving in subjects receiving active medication. Theoretical arguments concerning dopaminergic perturbations contributing to persistent craving have been posited by Gawin, Dackis and others (Dackis and Gold, 1985; Gawin and Ellinwood, 1988). Empirical evidence for the perspective comes from McDougle et al. (McDougle et al., 1992) who, using a levodopa challenge paradigm, demonstrated increased dopamine responsivity during early cocaine abstinence. Wolfsohn (Wolfsohn and Angrist, 1990) initially reported rapid resolution of abstinence symptoms in 8 patients receiving levodopa on a detoxification unit, but failed to replicate these preliminary results in a later placebo-controlled study (Wolfsohn et al., 1993). Further studies that include measures of cocaine withdrawal symptoms are needed to clarify the extent to which reduction of withdrawal is a mediator of levodopa's effects on cocaine use.
Interestingly, our study did not find strong and reliable advantages of contingency management procedures relative to the non-voucher therapies. In non-medication studies of behavior therapy for cocaine dependence, the relative advantage of VBRT in reducing cocaine use has been robust (e.g., Higgins et al., 1994; Higgins et al., 1991; Rawson et al., 2006). Possible explanations may have to do with differences in the implementation of VBRT. In the present study contingent rewards were distributed once per week, whereas standard procedures recommend delivery of the earned reinforcer immediately following the target behavior, i.e., urine test results. Given that immediacy of contingent reinforcement is a robust predictor of behavioral response in both human laboratory (Roll et al., 2000) and treatment research (Lussier et al., 2006), the delayed procedure used here may have weakened the contingency management effect. Our contingency management procedure also differed in that it was not integrated with the other behavioral interventions (i.e., ClinMan, CBT) but rather delivered in parallel as a standalone intervention. Therapists delivering CBT did not address whether or not their patients were earning voucher rewards, which may have removed an important source of support and encouragement toward achieving targeted behaviors.
As with other cocaine clinical trials, the majority of individuals who began treatment did not complete it. Although we applied current intent-to-treat analytic strategies for handling missing data (Bryk and Raudenbush, 1992), high rates of attrition can complicate interpretation of true treatment effects (Nich and Carroll, 2002). In this study, small group sizes, combined with slightly higher retention in the levodopa + VBRT, may have introduced bias in the analysis of certain outcomes, particularly those that rely on length of time in treatment (e.g., continuous abstinence rates). Interpretability of the present findings is further affected by the additive design used in constructing the levels of behavior therapy. Because VBRT was added to a behavioral platform consisting of ClinMan+CBT, the data do not allow conclusions regarding the separate and independent effects of this component. Moreover, we cannot rule out the possibility that better CBT attendance in the VBRT group accounted for observed treatment differences.
Despite limitations of the current study, the results support the inclusion of levodopa into the treatment armamentarium for cocaine dependence, specifically as an adjunctive pharmacotherapy for patients receiving abstinence-based contingency management procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1992;101:147–158. [Google Scholar]
- Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. Therapy Manuals for Drug Addiction; USDHHS NIH: 1998. [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004a;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004b;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. A comparative trial of psychotherapies for ambulatory cocaine abusers: relapse prevention and interpersonal psychotherapy. Am J Drug Alcohol Abuse. 1991;17:229–247. doi: 10.3109/00952999109027549. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Cocores JA, Gold MS, Pottash ALC. Dopaminergic treatments in cocaine withdrawal. Soc Neurosci Abstr. 1989;15:251. [Google Scholar]
- Covi L, Hess JM, Kreiter NA, Haertzen CA. Effects of combined fluoxetine and counseling in the outpatient treatment of cocaine abusers. Am J Drug Alcohol Abuse. 1995;21:327–344. doi: 10.3109/00952999509002701. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS, Estroff TW, et al. Hyperprolactinemia in cocaine abuse. Soc Neurosci Abstr. 1984;10:1099. [Google Scholar]
- Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management--imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID -I / P, Version 2.0) Biometric Research Department; NY: 1995. [Google Scholar]
- Gawin FH, Ellinwood EH., Jr. Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Billett W. Bromocriptine-desipramine protocol in treatment of cocaine addiction. J Clin Pharmacol. 1987;27:549–554. doi: 10.1002/j.1552-4604.1987.tb03065.x. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Baumgartel PD, Marzio LR. Bromocriptine therapy in cocaine withdrawal. J Clin Pharmacol. 1987;27:267–270. doi: 10.1002/j.1552-4604.1987.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Arnoni G, Elk R, Rhoades H, Schmitz J. Baseline assessment, study entry, and stabilization: double-blind clinical trials in drug dependence. NIDA Res Monogr. 1997;175:158–181. [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Dougherty D, Creson D, Moeller FG, Elk R, Schmitz J, Davis C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J Clin Psychopharmacol. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective bonferroni test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR, editors. Relapse Prevention. Guilford Press; New York: 1985. [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: main and matching effects. J Consult Clin Psychol. 1998;66:832–837. doi: 10.1037//0022-006x.66.5.832. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Price LH, Palumbo JM, Kosten TR, Heninger GR, Kleber HD. Dopaminergic responsivity during cocaine abstinence: a pilot study. Psychiatry Res. 1992;43:77–85. doi: 10.1016/0165-1781(92)90143-q. [DOI] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Cacciola JS, Rutherford MJ, O'Brien CP, Koppenhaver J. Group counseling versus individualized relapse prevention aftercare following intensive outpatient treatment for cocaine dependence: initial results. J Consult Clin Psychol. 1997;65:778–788. doi: 10.1037//0022-006x.65.5.778. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mezinskis J, Dyrenforth S, Goldsmith JR, Cohen M, Somoz E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatric Annals. 1998;28:577–583. [Google Scholar]
- Mezinskis JP, Honos-Webb L, Kropp F, Somoza E. The measurement of craving. J Addict Dis. 2001;20:67–85. doi: 10.1300/J069v20n03_07. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Sayre SL, Green C, Rhoades H, Schmitz JM. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addictive Disorders & Their Treatment. 2004;3:165–173. [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88:214–223. doi: 10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nich C, Carroll KM. Intention-to-treat meets missing data: implications of alternate strategies for analyzing clinical trials data. Drug Alcohol Depend. 2002;68:121–130. doi: 10.1016/s0376-8716(02)00111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken LS, Blaine JD, Boren JJ. Medications and behavioral therapies: the whole may be greater than the sum of the parts. NIDA Res Monogr. 1995;150:1–4. [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Roy SN, Pradhan SN. Correlation of behavioral and neurochemical effects of acute administration of cocaine in rats. Life Sci. 1978;22:1737–1744. doi: 10.1016/0024-3205(78)90626-4. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Martin RA, Michalec E, Abrams DB. Brief coping skills treatment for cocaine abuse: 12-month substance use outcomes. J Consult Clin Psychol. 2000;68:515–520. doi: 10.1037//0022-006x.68.3.515. [DOI] [PubMed] [Google Scholar]
- Roll JM, Reilly MP, Johanson CE. The influence of exchange delays on cigarette versus money choice: a laboratory analog of voucher-based reinforcement therapy. Exp Clin Psychopharmacol. 2000;8:366–370. doi: 10.1037//1064-1297.8.3.366. [DOI] [PubMed] [Google Scholar]
- SAS . The SAS System for Windows (Version 9.13) SAS Institute Inc.; Cary, NC: 2006. [Google Scholar]
- Schmitz JM, Oswald LM, Jacks SD, Rustin T, Rhoades HM, Grabowski J. Relapse prevention treatment for cocaine dependence: group vs. individual format. Addict Behav. 1997;22:405–418. doi: 10.1016/s0306-4603(96)00047-0. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Exp Clin Psychopharmacol. 1998;6:162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, Ling W. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100(Suppl 1):78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Walsh SL. Psychostimulant abuse: the case for combined behavioral and pharmacological treatments. Pharmacol Biochem Behav. 1997;57:457–470. doi: 10.1016/s0091-3057(96)00436-4. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;(Suppl 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2003 National Survey on Drug Use and Health: National findings. Office of Applied Studies; Rockville, MD: 2004. (DHHS Publication No. SMA 04-3964, NSDUH Series H-25). [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Treatment Episode Data Set (TEDS): 1994-2004. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2006. [Google Scholar]
- Teoh SK, Mendelson JH, Mello NK, Weiss R, McElroy S, McAfee B. Hyperprolactinemia and risk for relapse of cocaine abuse. Biol Psychiatry. 1990;28:824–828. doi: 10.1016/0006-3223(90)90517-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. Drug dependence and addiction, III: Expectation and brain function in drug abuse. Am J Psychiatry. 2004;161:621. doi: 10.1176/appi.ajp.161.4.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Wolfsohn R, Angrist B. A pilot trial of levodopa/carbidopa in early cocaine abstinence. J Clin Psychopharmacol. 1990;10:440–442. [PubMed] [Google Scholar]
- Wolfsohn R, Sanfilipo M, Angrist B. A placebo-controlled trial of L-dopa/carbidopa in early cocaine abstinence. Neuropsychopharmacology. 1993;9:49–53. doi: 10.1038/npp.1993.42. [DOI] [PubMed] [Google Scholar]