Abstract
People can solve problems in more than one way. Two general strategies involve (A) methodical, conscious, search of problem-state transformations, and (B) sudden insight, with abrupt emergence of the solution into consciousness. This study elucidated the influence of initial resting brain-state on subjects' subsequent strategy choices. High-density electroencephalograms (EEGs) were recorded from subjects at rest who were subsequently directed to solve a series of anagrams. Subjects were divided into two groups based on the proportion of anagram solutions derived with self-reported insight versus search. Reaction-time and accuracy results were consistent with different cognitive problem-solving strategies used for solving anagrams with versus without insight. Spectral analyses yielded group differences in resting-state EEG supporting hypotheses concerning insight-related attentional diffusion and right-lateralized hemispheric asymmetry. These results reveal a relationship between resting-state brain activity and problem-solving strategy, and, more generally, a dependence of event-related neural computations on the preceding resting-state.
Keywords: Attention, Creativity, Hemispheric Asymmetry, Insight, Problem Solving, Resting State
Systematic, relatively stable, patterns of resting-state brain activity are associated with aspects of personality, intelligence, psychopathology, and neurological disorder (Davidson, 2003; John et al., 1988; Kumari et al., 2004; Thatcher et al., 2005), perhaps reflecting subtle differences in neuroanatomy or neurotransmitter levels (John et al., 1988). The existence of such associations suggests the possibility that resting-state neural activity may also be correlated with individual differences in the event-related, goal-oriented, cognitive processes that people use to negotiate the world around them, such as those used in problem solving.
The present study examined the hypothesis that resting-state neural activity influences the cognitive strategies people use to solve problems, in particular, the general strategies which result in problem solutions derived either by methodical search or by sudden insight. Determining whether the tendency to solve problems by search versus insight is influenced by resting-state activity would clarify whether the relevant neural computations are selected and engaged only once processing of a problem has begun, or whether preexisting biases in more fundamental neural processes influence the likelihood of using one strategy or the other. More generally, this study examined the hypothesis that event-related, goal-directed, neural computations are influenced by characteristics of the preceding resting state.
Cognition and the Resting State
Most research on human cognition has focused on directed, goal-oriented, thought. In contrast, a relatively small body of research has focused on the spontaneous, undirected, thought that occurs during a resting state when a person is given no particular task to perform (Christoff et al., 2004). Results from functional neuroimaging studies have shown that resting-state activity is decomposable into a number of separate networks (Damoiseaux et al. 2006) and that some of these networks include brain areas also recruited during the performance of tasks involving higher cognitive functions (Andreasen et al., 1995; Christoff et al., 2004). This suggests that spontaneous thought during rest may involve some of the same thought processes engaged during problem solving.
The neural correlates of the resting state are not identical to the default state identified by Raichle and colleagues (Raichle et al., 2001). The default state consists of the network of brain regions that are more active during the resting state than during the performance of a task. Activity in this network is attenuated during active engagement in a task. Spontaneous thought during rest engages additional brain areas that are also active during task performance but are not part of the default-state network and are therefore not attenuated during task engagement (Christoff et al., 2004).
Problem Solving by Search versus Insight
There are two general cognitive strategies which people use to solve problems. Search involves systematic evaluation of possible problem states intervening between the current state and the goal state, and the use of available operators to transform one state into another. The use of a search (or “analytic”) strategy involves systematic evaluation of problem states which lie on different possible paths linking the starting state and the goal state. These intermediate states and paths are computed by deliberate, predominantly conscious, manipulation of problem elements (Ericsson & Simon, 1993; Newell & Simon, 1972).
Another general strategy for problem solving involves insight (Bowden et al., 2005; Maier, 1931; Sternberg & Davidson, 1995; Wagner et al., 2004). Insight is the sudden awareness of the solution to a problem (i.e., the “Aha!” phenomenon) with little or no conscious access to the processing leading up to that solution (Metcalfe & Wiebe, 1987; Smith & Kounios, 1996). The notion of sudden insight is related to the distinction between discrete, all-or-none, information processing and continuous or incremental processing (Kounios, 1993; Kounios et al., 1987; Meyer et al., 1988; Sergent & Dehaene, 2004; cf. Lang et al., 2006), and has been identified as an important characteristic of creative thought (Andreasen, 2005; Ansburg & Hill, 2003; Friedman & Förster, 2005).
Research contrasting problem solving by insight and by analytic “noninsight” search strategies has identified distinguishing cognitive and neural mechanisms (Bowden & Jung-Beeman, 2003; Bowden et al., 2005; Fleck, in press; Friedman & Förster, 2005; Gilhooly & Murphy, 2005; Jung-Beeman et al., 2004; Kounios et al. 2006; Metcalfe & Wiebe, 1987; Smith & Kounios, 1996; Sternberg & Davidson, 1995). For example, the sudden awareness of insight solutions to verbal problems corresponds to a burst of high-frequency (gamma-band) oscillatory electroencephalogram (EEG) activity associated with an increase in functional magnetic resonance (fMRI) signal in the right anterior superior-temporal gyrus (Jung-Beeman et al., 2004). This finding is consistent with a special role for the right hemisphere (RH) in problem solving by insight, a hypothesis further supported by behavioral studies examining response times to lateralized visual presentation of potential solution words (Bowden & Jung-Beeman, 2003). These studies have demonstrated that the representation of a correct solution is activated at a subconscious level in the RH prior to conscious retrieval, but only for solutions associated with the “Aha!” experience characteristic of insight when they do become conscious (Bowden et al., 2005). The subconscious nature of the RH activity leading up to an insight suggests that analytic and insight processing can occur in parallel (cf. Kounios, 1993).
Individual Differences
Differences among individuals in the tendency to solve problems with an insight versus an analytic strategy may be associated with more fundamental characteristics of information processing. For instance, psychometric measures of creativity and measures of real-world creative achievement are associated with a habitual tendency toward diffuse rather than focused attention, which results in ineffective filtering of distracting or irrelevant environmental stimuli (Carson et al., 2003; Mendelsohn & Griswold, 1966; Rowe et al., 2007). One view describes creativity as the ability to utilize nonprepotent remote associations of problem elements in order to discover nonobvious solutions to a problem (Mednick, 1962). Diffuse attention facilitates access to remote associations because it enhances awareness of peripheral environmental stimuli that could serve as cues that trigger retrieval of such associations (Seifert et al., 1995). Furthermore, diffuse attention in the perceptual domain is associated with diffuse attention in the conceptual domain (Friedman & Förster, 2005; Rowe et al., 2007). Such diffuse conceptual attention allows a concept in semantic memory to activate both remote and close associates to approximately the same degree rather than according to a steeper gradient of association in which a concept activates similar concepts more closely than remotely related ones (Beeman et al., 1994; Faust & Lavidor, 2003; Folley & Park, 2005; Howard-Jones et al., 2005; Jung-Beeman et al., 2004; Mednick, 1962; Stringaris et al., 2006).
The tendency to solve problems with insight may also be associated with both structural and functional hemispheric asymmetry. Behavioral (Bowden & Jung-Beeman, 2003; Friedman & Förster, 2005), electrophysiological (Jung-Beeman et al., 2004), and neuroimaging (Jung-Beeman et al., 2004) studies suggest a special role for the right hemisphere (RH) in solving problems with insight. These findings are consistent with a hemispheric model of semantic processing in which the RH primarily processes remote associations of concepts, while the left hemisphere (LH) primarily processes close associations (Beeman et al., 1994; Faust & Lavidor, 2003; Folley & Park, 2005; Howard-Jones et al., 2005; Jung-Beeman, 2005; Jung-Beeman et al., 2004; Stringaris et al., 2006). This processing asymmetry may be a byproduct of specific architectonic differences between LH language areas and their RH homologues (Jung-Beeman et al., 2005; Hutsler & Galuske, 2003).
Two Hypotheses
Prior research suggests two hypotheses about resting-state brain activity and problem solving strategy. The first hypothesis involves electroencephalogram oscillations in the high-alpha (10−13 Hz) and low-beta (13−18 Hz) frequency bands. Occipital alpha, especially in the 10−13 Hz high-alpha band, has been shown to reflect an inhibitory gating mechanism regulating the intake of visual information (Ray & Cole, 1985; Worden et al., 2000). In contrast, evidence suggests that occipital beta reflects an Wróbel, 2000). Selective visual attention on the cortical level has been proposed to function comparably to lateral inhibition, with a center-increase/surround-decrease in cortical activity linked to thalamic gating (Pfurtscheller, 2003; Pfurtscheller & Lopes da Silva, 1999). An increase in activity at the center (beta) could therefore be coupled with inhibition of activity in surrounding regions associated with alpha synchronization.
These findings lead to the prediction that subjects exhibiting a tendency to solve problems with insight will have a tendency toward diffuse deployment of visual attention manifested as a reduction in resting-state occipital alpha (i.e., more general activation of visual processing areas resulting in broader intake of visual information) relative to subjects exhibiting a tendency to solve problems with an analytic noninsight strategy. Furthermore, subjects tending to solve problems analytically should have greater focused visual attention associated with increased occipital beta (i.e., more neural activity in specific visual pathways processing focally attended information) and increased occipital alpha (i.e., more inhibition of brain areas processing non-attended visual information) than subjects tending to solve problems with insight.1
The second hypothesis was that there would be hemispheric asymmetry indicative of greater RH activity and/or less LH activity in frontal, temporal, and parietal association areas implicated in semantic processing (Jung-Beeman et al., 2005) for subjects tending to solve problems with insight compared to subjects tending to solve problems without insight. This would be manifested in low-alpha (8−10 Hz) band activity reflecting inhibition or idling of association cortex24 and higher-frequency (i.e., higher beta- and gamma-band) oscillations which have been linked to cognitive processes such as the transient feature-binding associated with the activation of perceptual or conceptual representations (Jung-Beeman et al., 2004; Pulvermüller, 2001; Tallon-Baudry & Bertrand, 1999) and which are proportional to hemodynamic measures of neural activity (Kounios et al., 2006; Laufs et al., 2003).
Insight Self-Reports
Comparing resting-state neural activity of subjects who tend to solve problems with insight to resting-state neural activity of subjects who tend to solve problems with a noninsight analytic strategy necessitates determining which strategy each subject uses for each problem. Historically, the most common approach to studying insight has been to compare subjects' solving of problems traditionally classified as insight problems to that of noninsight problems (Sternberg & Davidson, 1995). Much has been learned from this approach. However, what is often not recognized is that most problems can be solved either by insight or by analytical processing (Bowden et al., 2005). Therefore, an examination of neural correlates of the solution of problems traditionally considered to be insight problems could include brain activity corresponding to some solutions achieved without insight. Our research has taken an alternative approach by using problems that can be solved with or without insight and sorting the solutions by subjects' trial-by-trial binary judgments of the solving method used.
The classical distinction between insight and noninsight problems rests largely on the differential phenomenology associated with the solutions, with the use of subjective reports of the solving experience dating back to the beginnings of insight research (Maier, 1931). In the modern era, Kaplan and Simon (1990) argued that the subjective “Aha!” experience is the defining feature of insight. More generally, the use of verbal protocols has been widely adopted in problem-solving research. Ericsson and Simon (1993) presented data to support the validity of such self-reports (both concurrent and retrospective) when these reports do not require subjects to formulate detailed interpretations of their cognitive strategies. Much work has also generally validated the use of self-reports in brain-imaging research (e.g., Baars, 2003; Kirchoff & Buckner, 2006; Lutz et al., 2002).
For the present study, in order to examine individual differences in the tendency to solve with insight, it was necessary to assess whether individuals solve particular problems with or without insight. Real-time verbalization of subjects' thoughts during problem solving is not compatible with artifact-free electroencephalogram (EEG) measurement. However, an appropriate modification of this approach has proven successful in a number of studies (Bowden et al., 2005; Jung-Beeman et al., 2004; Kounios et al., 2006; Bowden & Jung-Beeman 2003; Maier, 1931). This approach requires subjects to solve each problem, and then, immediately after reporting the solution, report whether or not that solution had been derived with insight (defined in terms of the suddenness of the awareness of the solution). This procedure circumvents the problem of electroencephalogram contamination by verbalization-related muscle artifact.
Several lines of evidence support the notion that such self-reports accurately reflect the sudden availability of the solution to a problem, rather than reflecting ancillary phenomena such as an affective response to the solution (Jung-Beeman et al., 2004). For example, the main neural correlate of insight self-reports is a burst of gamma-band EEG activity recorded over the right anterior superior-temporal lobe beginning at approximately the moment when the solution becomes available (i.e., approximately .3 sec before a manual response is made to indicate that a solution is available for report) and not as a later affective or novelty response to the availability of this solution. [.3 sec is approximately the time needed to access available response information and execute a button-press response (Smith & Kounios, 1996).] This gamma-band response corresponds to an increase in BOLD activity in the right anterior superior-temporal gyrus, an area of association cortex that has not been associated with affective or novelty processing (Jung-Beeman, 2005). Moreover, in addition to an increase in hemodynamic activity in this area at about the time that the solution becomes available, there is also earlier activity in this area beginning at about the time the problem is initially displayed, further supporting the notion that signal changes in this area reflect engagement of cognitive processes, not affective or novelty responses. Most importantly, below we present behavioral results consistent with the notion that self-reports of insight reflect the cognitive phenomenon of solutions becoming available in a sudden, discrete, fashion.2
Overview
High-density electroencephalograms (EEGs) were recorded from subjects during rest (first with eyes closed, then with eyes open) before they were told the nature of the subsequent task. They were then directed to solve a series of anagrams. For each anagram, subjects pressed a button immediately upon deriving the solution and then reported whether or not that solution had been derived with insight (defined in terms of the suddenness of the awareness of the solution) (Bowden & Jung-Beeman, 2003; Bowden et al., 2005; Jung-Beeman et al., 2004; Kounios et al., 2006). Using the ratio of insight solutions to noninsight solutions, subjects were classified as High Insight (HI) or Low Insight (LI). Power spectra for subjects' resting-state EEGs were computed and group comparisons of the EEG power values for each frequency band were computed in order to determine whether resting-state brain activity associated with individuals tending to solve problems with insight differed from resting-state brain activity associated with individuals tending to solve problems without insight.
Methods
Subjects
Twenty-six right-handed, native English-speaking, subjects participated. Subjects were divided into HI and LI groups by performing a median split on the ratio of insight to noninsight correct anagram-solutions. The HI group (mean age: 21.3 years, sd: 4.8) had a mean ratio of 3.5 (sd: 2.3) and included 6 males and 7 females. The low-insight (LI) group (mean age: 22.5, sd: 3.4) had a mean ratio of .8 (sd: .4) and included 7 males and 6 females. All subjects signed an informed consent form. The study was approved by Drexel University's Institutional Review Board.
Stimulus Materials
The stimuli were 180 anagrams (109 four-letter and 71 five-letter anagrams), preceded by a practice block of 14 anagrams. The anagrams were generated using a computer program described by Vincent et al. (2006). Each anagram had only one solution. The mean bigram sum of the solutions was 5,954.91 (sd: 2,555.31). The mean word frequency (Francis & Kucera, 1982) for the solutions was 54.75 per million (sd: 93.79).
Procedure
After obtaining consent, but before giving task instructions, we measured 128-channel EEG (sampling rate: 256 Hz, bandpass: .2−100 Hz, digitally-linked mastoid reference) from subjects for 3.5 minutes during eyes-closed rest and then for 3.5 minutes during eyes-open rest preceding work on a set of anagrams. Subjects were told to relax and not move their eyes, but were allowed to blink normally (during the eyes-open recording). After the resting-state EEGs were acquired, subjects were given instructions concerning the anagram task. On each trial, a .5-sec fixation plus-sign warning signal was followed by an anagram displayed at the center of a video monitor (replacing the plus sign) until the subject either responded with a computer-mouse button-press or the trial timed out (at 16 sec after the onset of the anagram). Subjects were instructed to press a button with their right index finger immediately upon deriving the solution (thereby terminating display of the anagram), and .5 sec later they viewed a message which prompted them to verbalize the solution. After each solution (correct and incorrect), subjects were prompted to press a button to indicate whether the solution was (a) derived with insight, (b) derived without insight, or (c) not sure. Insight was explained to subjects as occurring when the solution pops into awareness suddenly (i.e., an “Aha! moment”), as opposed to resulting from deliberate, conscious, rearrangement of the letters of the anagram. All subjects indicated that they were intuitively familiar with this distinction. Subjects were also told to make both insight and noninsight responses (across trials). This was done in order to minimize ceiling or floor effects resulting from response bias or stereotyped responding. When a subject did not respond with a button-press by the 16-sec deadline, the trial was terminated and the next trial initiated. Subjects were given a block of 14 practice trials before proceeding to the experimental trials.
EEG Analyses
All EEG analyses were performed with EMSE 5.1 (www.sourcesignal.com). Eye-blinks were corrected offline using an adaptive filter constructed separately for each subject. EEG segments containing other artifacts were detected by visual inspection and excised. Channels with excessive artifact were replaced by linear interpolation using neighboring electrodes. Power spectra were then computed and mean EEG power was computed at each electrode for each subject for the following frequency bands: delta (1.00 − 3.75 Hz), theta (4.00 − 7.75 Hz), low-alpha (8.00 − 9.75 Hz), high-alpha, (10.00 −12.75 Hz), beta-1 (13.00 − 17.75 Hz), beta-2 (18.00 − 24.75 Hz), beta-3 (25.00 − 29.75 Hz), gamma-1 (30.00 − 39.75 Hz), gamma-2 (40.00 − 49.75 Hz), and gamma-3 (50.00 − 58.00 Hz). Mean EEG power values for each subject were log-transformed and converted to z-scores (across electrodes, separately for each frequency band) prior to subjecting them to repeated-measures analysis of variance (ANOVA) in order to determine whether the HI and LI groups exhibited different resting-state EEG effects. Normalization by conversion to z-scores eliminates global power differences between subjects and between groups that could result from differences in arousal or skull thickness, thereby revealing regional differences in brain activation (Gevins & Smith, 2000; Kounios & Holcomb, 1994).
A repeated-measures analysis of variance (ANOVA) was performed on normalized EEG power values for each frequency band (using the Huynh-Feldt correction for nonsphericity where appropriate). Each ANOVA had an Anterior-Posterior factor (AP, 4 levels), a Hemisphere factor (H, 2 levels), a Dorsal-Ventral factor (DV, 2 levels), an Eyes-Closed/Open factor (E, 2 levels), and a High/Low Insight group factor (I, 2 levels). The electrodes used in the ANOVAs were F1/2, F7/8, C1/2, T7/8, P1/2, P7/8, O1/2, and O9/10 (according to the nomenclature of the extended International 10−20 System). The present report is primarily concerned with interactions of the electrode factors with the Insight factor because such interactions indicate a differential spatial pattern of neural activity at rest for HI and LI subjects. Relevant ANOVA results for each frequency band are listed in Table 1. The Insight factor interacted with one or more electrode factors in each analyzed frequency band.
Table 1.
Significant interactions from ANOVAs, separately for each EEG frequency band (I: Insight factor; AP: Anterior-Posterior factor; DV: Dorsal-Ventral factor; H: Hemisphere factor; E: Eye-Status).
|
Frequency Band |
Interaction |
Significance |
|---|---|---|
| Delta | AP × I | F[3,72] = 3.756, p = .021* |
| AP × DV × I | F[3,72] = 3.173, p = .034* | |
| E × H × I | F[1,24] = 3.645, p = .068 | |
| AP × H × I | F[3,72] = 2.852, p = .051 | |
| |
DV × H × I |
F[1,24] = 4.843, p = .038* |
| Theta | AP × I | F[3,72] = 3.619, p = .025* |
| AP × DV × I | F[3,72] = 2.434, p = .072 | |
| |
AP × H × I |
F[3,72] = 3.098, p = .037* |
| Low-Alpha | AP × I | F[3,72] = 2.936, p = .049* |
| AP × DV × I | F[3,72] = 2.486, p = .067 | |
| |
AP × H × I |
F[3,72] = 5.681, p = .003** |
| High-Alpha | AP × I | F[3,72] = 4.811, p = .004** |
| |
AP × H × I |
F[3,72] = 3.457, p = .039* |
| Beta-1 | AP × I | F[3,72] = 7.011, p < .001*** |
| |
E × AP × DV × I |
F[3,72] = 2.492, p = .079 |
| Beta-2 |
AP × I |
F[3,72] = 4.349, p = .007** |
| Beta-3 | E × DV × I | F[1,24] = 3.522, p = .073 |
| |
E × AP × DV × H × I |
F[3,72] = 3.209, p = .032* |
| Gamma-1 |
E × DV × I |
F[1,24] = 4.812, p = .038* |
| Gamma-2 | E × AP × I | F[3,72] = 2.360, p = .100 |
| |
E × DV × I |
F[1,24] = 7.888, p = .01** |
| Gamma-3 | E × DV × I | F[1,24] = 6.453, p = .018* |
p < .05
p < .01
p < .001
Topographic Mapping
As follow-up tests to significant ANOVAs, we computed an independent-samples HI-minus-LI t-test for each electrode and then plotted topographic maps showing the scalp distribution of these t-scores. These maps only show regions in which the t-scores lie outside of the range of −1.711 to +1.711, thereby showing scalp regions in which the HI—LI difference was in the top or bottom 5% of the t-distribution. No corrections for multiple comparisons were performed because the t-tests were follow-up tests to the ANOVAs and were intended to elucidate the specific patterns of effects driving significant ANOVA interactions. Though such ANOVAs have more statistical power because they pool variance across electrodes, they do not give precise descriptions of the topography of obtained effects. In contrast, statistical parametric mapping of t-score topographies gives more precise information about the spatial distribution of effects, but has less statistical power because it does not pool variance across electrodes. Nevertheless, due to the convolutions of the neocortex, an EEG effect measured at a scalp electrode is not necessarily generated in cortical tissue precisely underneath that electrode. Hence, such topographic mapping affords relatively low-resolution spatial imaging.
Results
Behavioral Results
Mean performance
On average, subjects solved 70.0% (sd: 8.1) of the anagrams correctly. Of these, 56.1% (sd: 19.2) were solved with insight. The HI and LI groups correctly solved approximately the same percentage of anagrams (HI: 68.9%; LI: 70.9%; t[24] = .60, p = .55). Mean reaction times for correctly solved anagrams were not significantly different for the HI (5.19 sec, sd: 0.86) and LI groups (5.10 sec, sd: 0.93) (t[24] = .28, p = .78). Mean error rates (i.e., proportion of responses that were incorrect) were low and similar for the two groups (HI: 3.7%, sd: 3.3; LI: 4.9%, sd: 2.8) (t[24] = 1.05, p = .30). The mean percentage of trials which were time-outs (i.e., without responses) did not differ significantly for the HI (27.4%, sd: 8.48) and LI groups (25.2%, sd: 8.80) (t[24] = −0.64, p = .53). The HI and LI groups did not differ in percentage of correct solutions labeled by subjects as “not sure” whether derived with or without insight (HI: 1.8%, sd: 4.2; LI: 2.9%, sd: 6.0; t[24] = 1.08, p = .29). These results demonstrate roughly equivalent overall performance for the HI and LI groups, indicating comparable anagram-solving ability. Nevertheless, these results mask strategy differences described next.
Individual differences
Previous research has shown that problem solving with insight yields solutions in an all-or-none fashion, while solving without insight is incremental and yields partial response-information prior to the completion of processing (Smith & Kounios, 1996). This predicts that subjects using an insight strategy will not have access to information about the correct response before the insight solution enters awareness and will therefore tend to time-out without responding when confronted with an imminent response deadline. In contrast, subjects using an analytic strategy are more likely to have some partial information about the correct response before the final solution is derived and are therefore more likely to guess as the response deadline approaches. This predicts that the tendency to solve anagrams with insight should be associated with the tendency to make errors of omission (i.e., timeouts), and the tendency to solve anagrams by a noninsight strategy should be associated with the tendency to make errors of commission (i.e., incorrect responses). Consistent with this prediction, increased use of an analytic strategy was, across subjects, correlated with an increase in errors of commission (i.e., incorrect responses); in contrast, increased use of an insight strategy was correlated with an increase in errors of omission (i.e., no-response time-outs) (see Figure 1). Specifically, the correlation between (a) the proportion of correct responses labeled as resulting from insight (I/[I+NI], where I is the number of insight solutions and NI is the number of noninsight solutions for a given subject) and (b) the proportion of errors of commission in unsolved problems (E/[E+TO], where E is the number of errors of commission, and TO is the number of timeouts, i.e., errors of omission) was −.528 (p = .0035, one-tailed – see Figure 1, top panel). In addition, the tendency to solve with insight rather than noninsight processing (I/[I+NI]) was positively correlated with the frequency of timeouts (r = .387, p = .028, one-tailed – see Figure 1, middle panel), showing that subjects who tended to solve with insight also tended to timeout without responding. There was also a marginally significant negative correlation between the tendency to solve with insight (I/[I+NI]) and the overall frequency of error responses, (r = −.327; p = .055, one-tailed – see Figure 1, bottom panel), suggesting that the tendency to solve with insight was inversely related to the tendency to make errors of commission.3
1. Correlations between behavioral measures.

Each circle represents an individual subject. Dotted lines represent best-fitting regression lines associated with correlations given in the text. Top panel: plot of relationship between proportion of correct solutions (i.e., insight plus noninsight, [I+NI]) associated with insight, and proportion of unsolved trials (i.e., errors plus timeouts, [E+TO]) on which a subject made an error response. Middle panel: plot of relationship between the proportion of correct solutions associated with insight (I/[I+N]) and the frequency (i.e., percentage) of trials on which a subject timed out without responding (TO). Bottom panel: plot of relationship between proportion of correct solutions associated with insight (I/[I+N]) and the ratio of the number of trials on which a subject made an error response (E) to the number of trials on which a subject made a correct response (I+NI).
Additionally, subjects did not seem to change their strategy (insight vs. noninsight) over the course of the experiment. Comparing the relative proportions of insight and noninsight solutions for the first and second halves of the experiment, an Experiment-Half(2) × Solution-Type(2) ANOVA found a nonsignificant interaction between these two factors (F[1,24] = .282; p = .6).
In sum, focused correlational analyses supported the notion that insight self-reports actually reflect different problem-solving strategies. Alternate explanations do not easily account for these results. For example, it could be argued that HI subjects simply responded more conservatively and tended to wait until they were certain of the correct response before they pressed the button to indicate that they had achieved the correct solution, hence their tendency to timeout rather than respond incorrectly. However, this scenario does not explain why HI subjects, when confronted with an imminent deadline, would timeout rather than respond with a potential solution that had not been completely verified, or even respond with a random guess. Furthermore, it predicts group differences in response time, which were not evident.
Posterior Oscillations Related to Visual Information Processing
EEG group differences were initially examined by analysis of variance (ANOVA) described in the Methods section and with relevant results summarized in Table 1. These analyses showed that the HI and LI groups differed significantly in the distribution of EEG power across all standard frequency bands. Follow-up tests based on statistical parametric mapping are described below.
The first set of analyses focused on predictions concerning the magnitude of high-alpha and beta-1 oscillations. It was predicted that HI subjects would exhibit less occipital high-alpha activity (indicating less attentional gating in the visual system) and less beta-1 activity (suggesting less focal attention) relative to LI subjects.
High-alpha (10−12.75 Hz)
Although eye status (open versus closed) did have a significant effect on the magnitude of high-alpha, eye status and insight did not significantly interact (Table 1). Figure 2 therefore presents the topography of the HI-LI difference collapsed across eye conditions. This figure reveals that the LI group had more high-alpha than the HI group measured over occipital cortex. This finding indicates that the LI group had less activity in visual cortex relative to the HI group. The left-occipital focus of this effect suggests that this suppression occurred primarily in LH visual cortex (Worden et al., 2000).
2. Topographic maps of t-scores of EEG-power comparisons (High-Insight group minus Low-Insight group) for the high-alpha (top panel), beta-1 eyes-closed (EC, middle panel), and beta-1 eyes-open (EC, bottom panel) frequency bands.

The red end of the t-score scale represents scalp regions in which EEG power for the High-Insight group is significantly greater than EEG power for the Low-Insight group. Blue values represent scalp areas in which the Low-Insight group has greater power than the High-Insight group. Colored regions on the topographic maps represent the top and bottom 5% of the t-score distribution. Red dots on the maps indicate positions of the electrodes.
Beta-1 (13.00 − 17.75 Hz)
In the beta-1 band, eye-condition interacted with insight (Table 1). The topography of the HI-LI difference is therefore displayed separately for the two eye-status conditions (Figure 2). With eyes closed, LI subjects showed greater beta-1 EEG power over the occipital midline than did HI subjects. With eyes open, the greater occipital beta-1 power for LI subjects had a broader topographic distribution encompassing bilateral occipital sites. The greater occipital beta for LI subjects indicates more focused visual attention (Bekisz & Wróbel, 2003; Pfurtscheller, 2003; Pfurtscheller & Lopes da Silva, 1999; Wróbel, 2000). This effect had a broader occipital scalp topography and much stronger statistical significance in the eyes-open condition than in the eyes-closed condition (a maximum t-score of −4.43 versus −3.30), consistent with the notion that this beta-1 effect is associated with visual attention. Right superior-parietal electrodes also showed a relatively small effect in the opposite direction (HI>LI), suggesting greater RH parietal beta-1 activity (see discussion of hemispheric asymmetry below).
There was also a relatively small effect measured over right inferior-frontal cortex in the beta-1 band with eyes closed (HI<LI). This effect was stronger at higher frequencies and is discussed below.
Hemispheric Asymmetry
The second set of analyses focused on predictions concerning differences between the two groups in resting-state functional hemispheric asymmetry. It was predicted that HI subjects would exhibit more RH activity and/or less LH activity relative to LI subjects. These predictions were examined in the low-alpha, beta-2, beta-3, and gamma bands.
Low-alpha (8.00 − 9.75 Hz)
The low-alpha frequency band reflects cortical inhibition and is inversely proportional to hemodynamic measures of neural activity (Kounios et al., 2006). Eye-condition did not interact with insight in this frequency band (Table 1). Figure 3 displays the topography of the HI—LI difference collapsed across eye conditions. There was greater low-alpha power for HI subjects at left inferior-frontal and anterior-temporal electrodes, and greater power for LI subjects focused at right dorsal-frontal electrodes and extending laterally. The difference at left inferior-frontal and anterior temporal electrodes reflects greater neural activity for LI subjects, while the difference at right dorsal-frontal electrodes reflects greater neural activity for HI subjects. The low-alpha results are consistent with the hemispheric hypothesis.
3. Topographic map of t-scores of EEG-power comparisons (High-Insight group minus Low-Insight group) for the low-alpha frequency band.
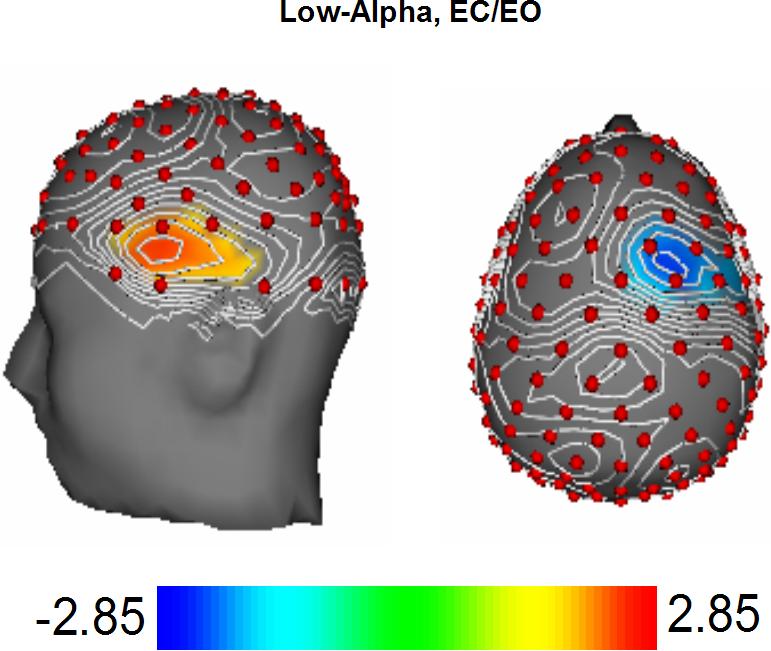
This figure uses the same conventions used in Figure 2.
Beta-2 (18.00 − 24.75 Hz)
Eye condition did not interact with insight in the beta-2 band (Table 1). Figure 4 (top panel) displays the topography of the HI—LI difference collapsed across eye conditions. Beta-band power is proportional to hemodynamic measures of neural activity (Laufs et al., 2003). There was greater beta-2 power for HI subjects at right inferior-frontal and anterior-temporal electrodes, and greater power for LI subjects at left occipital and parietal electrodes. The right frontal-temporal activity (HI>LI) is consistent with previous findings associating these areas with the processing of remote semantic associates (Jung-Beeman, 2005; Jung-Beeman et al., 2004; Folley & Park, 2005; Howard-Jones et al., 2005; Stringaris et al., 2006). Beta- and gamma-band activity can sometimes be contaminated by electromyograph (EMG) signals associated with muscle activity. While the contribution of EMG to this result cannot be completely ruled out, the unilateral and highly focal nature of this effect argues against the notion that it constitutes muscle rather than brain activity. The left occipital activity (LI>HI) is consistent with hypothesized greater focused visual attention in LI subjects. This left-occipital beta-2 effect, together with the left-occipital low-alpha effect (both LI>HI), suggests a center-increase/surround-decrease pattern of cortical activity (Pfurtscheller, 2003; Pfurtscheller & Lopes da Silva, 1999). In sum, the beta-2 results are consistent with the hemispheric hypothesis.
4. Topographic maps of t-scores of EEG-power comparisons (High-Insight group minus Low-Insight group) for the beta-2 EC/EO (eyes closed and open, top panel), beta-3 eyes-closed (EC, middle panel), and beta-3 eyes-open (EO, bottom panel) frequency bands.

This figure uses the same conventions used in Figure 2.
Beta-3 (25.00 − 29.75 Hz)
Eye condition interacted with insight in the beta-3 band (Table 1). The bottom two panels of Figure 4 display the topography of the HI—LI difference separately for the eyes-closed and eyes-open conditions. In the eyes-closed condition, there was greater beta-3 power for HI subjects at right frontal-temporal electrodes, and more power for LI subjects at left parietal electrodes. In the eyes-open condition, there was greater power for HI subjects at right parietal and temporal-occipital electrodes. The beta-3 effects are consistent with the hemispheric hypothesis.
Gamma-1 (30.00 − 39.75 Hz)
Eye condition interacted with insight in the gamma-1 band (Table 1). Figure 5 displays the topography of the HI--LI difference separately for the eyes-closed and eyes-open conditions. In the eyes-closed condition, there was greater power for HI subjects at right inferior-frontal and left temporal electrodes, and slightly more power for LI subjects at right inferior-parietal electrodes. In the eyes-open condition, there was more gamma-band power for HI subjects at electrodes in a broad area focused at right-parietal electrodes, and a weaker, more focal, left-parietal area. The larger bilateral gamma for HI subjects in the eyes-closed condition suggests a possible contribution from jaw-muscle EMG activity. (EMG artifact tends to be bilateral and occur at peripheral frontal and temporal electrodes.) Whether or not that bilateral activity reflects a contribution from EMG contamination, it disappeared when the eyes were opened, yielding a strong RH parietal asymmetry (HI>LI), consistent with the hemispheric hypothesis.
5. Topographic maps of t-scores of EEG-power comparisons (High-Insight group minus Low-Insight group) for the gamma-1 eyes-closed (EC, top panel) and gamma-1 eyes-open (EO, bottom panel) frequency bands.

This figure uses the same conventions used in Figure 2.
The results for the two higher-frequency gamma bands (40.00 − 49.75 Hz and 50.00 − 58.00 Hz) were very similar to those of the 30.00 − 39.75 Hz band, and are not detailed here.
Other Results
Previous research did not afford specific predictions concerning the topography or magnitude of possible group differences in the delta (1.00 − 3.75 Hz) or theta (4.00 − 7.75 Hz) frequency bands. Nevertheless, ANOVAs on EEG power values (Table 1) did reveal the effects described below.
The ANOVA of delta-band EEG power did not yield a significant interaction that included eye-condition as a factor. Topographic mapping of the group difference for this frequency band (not shown here) revealed a slightly left-lateralized frontal-central difference (HI>LI). The scalp topography of this effect suggests a medial-frontal source, though the oscillatory frequency of this effect is lower than the theta-band activity usually associated with, for instance, activity in anterior cingulate cortex (Luu et al., 2004).
The ANOVA of theta-band EEG power did not yield a significant interaction that included eye-condition as a factor (Table 1). Theta results include weak effects (HI>LI) measured at frontal-central and left-prefrontal electrodes. The frontal-central effect is likely “spill-over” from the stronger frontal-central delta-band effect described above. Similarly, the left prefrontal effect is likely spill-over from the stronger low-alpha effect discussed above, as low-alpha, rather than theta, effects are typical at lateral frontal electrodes (Gevins & Smith, 2000).
Discussion
The present study demonstrates that goal-oriented, event-related, cognitive processing is not completely determined by goals or task demands. Individual differences in resting-state brain activity also influence such neural computations. Specifically, subjects' preferred strategy for solving a series of anagrams (insight versus search), was influenced by characteristics of their prior resting state. This phenomenon is fundamentally different from the previous demonstration of a relationship between problem-solving strategy and transient preparatory activity immediately preceding the presentation of an anticipated problem (Kounios et al., 2006).
The results were organized around two hypotheses. The first was based on previous research demonstrating that highly creative individuals exhibit diffuse attention allowing input of a greater range of environmental stimuli, in contrast to less creative individuals who tend to focus their attention more narrowly, thereby sampling a smaller range of environmental stimuli (Carson et al., 2003; Folley & Park, 2005; Friedman & Förster, 2005; Mendelsohn & Griswold, 1966; Rowe et al., 2007). It was therefore predicted that HI subjects would have less resting-state occipital alpha-band activity, reflecting less inhibition of the visual system, and that LI subjects have more occipital beta activity, consistent with heightened focused attention. These predictions were supported by the results.
The second hypothesis was that HI and LI subjects would exhibit different patterns of resting-state hemispheric asymmetry at electrodes over lateral association cortex. This hypothesis was based on prior findings that creative cognition recruits RH association areas involved in semantic information processing relatively more than does noncreative cognition (Bowden & Jung-Beeman 2003; Folley & Park, 2005; Friedman & Förster, 2005; Howard-Jones et al., 2005; Jung-Beeman et al., 2004; Stringaris et al., 2006). The results provided broad support for the hypothesis that during a resting state HI subjects would show generally greater RH activity and less LH activity relative to LI subjects, with the most prominent effects being greater activity for HI subjects at right dorsal-frontal (low-alpha band), right inferior-frontal (beta and gamma bands) and right parietal (gamma band) electrodes, and greater activity at left inferior-frontal and left anterior-temporal electrodes for LI subjects in the low-alpha band.
Importantly, the behavioral results demonstrated that the HI and LI groups used different cognitive strategies to solve the anagrams. Consistent with the notion that insight processing yields information about the correct response in a discrete, all-or-none, fashion, while noninsight processing yields partial response information before the processing of a problem has been completed (Smith & Kounios, 1996), the present results showed that subjects who tended to solve problems with self-reported insight tended to make errors of omission, while subjects who tended to solve the problems with self-reported noninsight processing tended to make errors of commission.
Though these results demonstrate a dependence of task-related processing on characteristics of the prior resting-state, they do not directly address the issue of whether the relevant aspects of the resting-state are stable over time. It is possible that this resting activity varies over the course of hours or days. In general, though, the relative stability of individual differences in resting-state EEG is well known (John et al., 1988) and resting-state networks isolated with fMRI have also shown stability across testing sessions (Damoiseaux et al., 2006). Furthermore, accumulating evidence suggests a substantial genetic contribution to individual differences in resting-state EEG (Smit et al., 2005). It is likely that individual differences in resting-state brain activity are influenced by individual differences in neuroanatomy, microcircuitry, and neurotransmitter systems, all of which may be influenced by genetics and past experience. Nevertheless, contextual factors can perturb resting-state EEG (Angelakis 2004). Resting-state activity is likely transiently influenced by various contextual factors, such as an immediately preceding task, an anticipated task, and thoughts triggered by environmental stimuli. Future research will ascertain the relative influence of context-dependent and context-independent aspects of resting-state brain activity on goal-directed cognitive strategies.
The present results open up important avenues for research into the neural bases of cognition. For example, research on problem solving has often used complex problems whose solutions take too long for these problems to be used as stimuli in functional neuroimaging experiments. The demonstration that resting-state brain activity correlates with individuals' preferred strategy for solving simple problems suggests that the present approach could be used to correlate resting brain activity with strategies for solving (or the ability to solve) more complex problems, even in real-world situations. More generally, individual differences in resting-state activity may bear systematic relationships to a variety of event-related cognitive processes involved in attention, memory, thinking, and language processing. Investigation of such relationships, where they exist, may complement studies that directly examine event-related cognition. Furthermore, this research suggests the possibility that contextual manipulations designed to systematically influence resting-state activity may, in turn, influence cognitive strategies in useful ways.
Acknowledgments
This research was supported by NIDCD grants DC-04818 (to JK) and DC-04052 (to MJ-B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ongoing auditory-system alpha-band oscillations (e.g., the tau rhythm) are not easily detectable with scalp electrodes, possibly due to the geometry of auditory cortex (Hari, 1999). We therefore confined our discussion to visual attention.
A study by Schooler, Ohlsson, and Brooks (1993) suggested that concurrent verbalization during the solution of insight problems has a negative impact on solution rates for these problems (i.e., verbal overshadowing of insight). Although verbal overshadowing has been reported in other research (Brandimonte et al., 1997; Fallshore & Schooler, 1995) the effect occurred when participants were asked to verbalize concurrently with tasks that were primarily nonverbal in nature (e.g., visual imagery and memory for faces). Subsequent research using both concurrent verbalization and retrospective reports has failed to find verbal overshadowing effects during the solution of insight and noninsight problems (Fleck, in press; Fleck & Weisberg, 2004) yielding no differences in solution rates or solving times.
One outlier subject, identified by visual inspection of the scatterplots, was deleted from these correlational analyses because this outlier substantially suppressed the correlations. Prior to this deletion, the statistical significances of the 3 correlations described were .097 < p < .255.
References
- Andreasen NC. The creating brain: The neuroscience of genius. Dana Press; Washington, DC: 2005. [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Lubar JF, Stathopoulou S, Kounios J. Peak alpha frequency: An electroencephalographic measure of cognitive preparedness. Clinical Neurophysiology. 2004;115:887–897. doi: 10.1016/j.clinph.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Ansburg PI, Hill K. Creative and analytic thinkers differ in their use of attentional resources. Personality and Individual Differences. 2003;34:1141–1152. [Google Scholar]
- Baars BJ. How brain reveals mind: Neural studies support the fundamental role of conscious experience. Journal of Consciousness Studies. 2003;10:100–114. [Google Scholar]
- Beeman M, Friedman RB, Grafman J, Perez E, Diamond S, Lindsay MB. Summation priming and coarse semantic coding in the right hemisphere. Journal of Cognitive Neuroscience. 1994;6:26–45. doi: 10.1162/jocn.1994.6.1.26. [DOI] [PubMed] [Google Scholar]
- Bekisz M, Wróbel A. Attention-dependent coupling between beta activities recorded in the cat's thalamic and cortical representations of the central visual field. European Journal of Neuroscience. 2003;17:421–426. doi: 10.1046/j.1460-9568.2003.02454.x. [DOI] [PubMed] [Google Scholar]
- Bowden EM, Jung-Beeman M. Aha! Insight experience correlates with solution activation in the right hemisphere. Psychonomic Bulletin &. Review. 2003;10:730–737. doi: 10.3758/bf03196539. [DOI] [PubMed] [Google Scholar]
- Bowden EM, Jung-Beeman M, Fleck J, Kounios J. New approaches to demystifying insight. Trends in Cognitive Sciences. 2005;9:322–328. doi: 10.1016/j.tics.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Brandimonte MA, Schooler JW, Gabbino P. Attenuation of verbal overshadowing through color retrieval cues. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:915–931. doi: 10.1037//0278-7393.23.4.915. [DOI] [PubMed] [Google Scholar]
- Carson SH, Peterson JB, Higgins DM. Decreased latent inhibition is associated with increased creative achievement in high-functioning individuals. Journal of Personality and Social Psychology. 2003;85:499–506. doi: 10.1037/0022-3514.85.3.499. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Gabrieli JDE. Neural basis of spontaneous thought processes. Cortex. 2004;40:623–630. doi: 10.1016/s0010-9452(08)70158-8. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Simon HA. Protocol analysis: verbal reports as data. (revised edition). MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Fallshore M, Schooler JW. Verbal vulnerability of perceptual expertise. Journal of Experimental Psychology: Learning, Memory and Cognition. 1995;21:1608–1623. doi: 10.1037//0278-7393.21.6.1608. [DOI] [PubMed] [Google Scholar]
- Faust M, Lavidor M. Semantically convergent and semantically divergent priming in the cerebral hemispheres: Lexical decision and semantic judgment. Cognitive Brain Research. 2003;17:585–597. doi: 10.1016/s0926-6410(03)00172-1. [DOI] [PubMed] [Google Scholar]
- Fleck JI. Working memory demands in insight versus analytic problem solving. European Journal of Cognitive Psychology. in press. [Google Scholar]
- Fleck JI, Weisberg RW. The use of verbal protocols as data: An analysis of insight in the candle problem. Memory & Cognition. 2004;32:990–1006. doi: 10.3758/bf03196876. [DOI] [PubMed] [Google Scholar]
- Folley BS, Park S. Verbal creativity and schizotypal personality in relation to prefrontal hemispheric laterality: A behavioral and near-infrared optical imaging study. Schizophrenia Research. 2005;80:271–282. doi: 10.1016/j.schres.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis W, Kucera H. Frequency analysis of English usage Boston: Houghton Mifflin. 1982.
- Friedman RS, Förster J. Effects of motivational cues on perceptual asymmetry: Implications for creativity and analytical problem solving. Journal of Personality and Social Psychology. 2005;88:263–275. doi: 10.1037/0022-3514.88.2.263. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cerebral Cortex. 2000;10:829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Murphy P. Differentiating insight from non-insight problems. Thinking and Reasoning. 2005;11:279–302. [Google Scholar]
- Hari R. Magnetoencephalography as a tool of clinical neurophysiology. In: Niedermeyer E, Lopes da Silva F F, editors. Electroencephalography: Basic principles, clinical applications, and related fields. Williams, & Wilkins; Philadelphia: Lippincott: 1999. pp. 1107–1134. [Google Scholar]
- Howard-Jones PA, Blakemore S-J, Samuel EA, Summers IR, Claxton G. Semantic divergence and creative story generation: An fMRI investigation. Cognitive Brain Research. 2005;25:240–250. doi: 10.1016/j.cogbrainres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hutsler J, Galuske RAW. Hemispheric asymmetries in cerebral cortical networks. Trends in Neurosciences. 2003;26:429–435. doi: 10.1016/S0166-2236(03)00198-X. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS, Fridman J, Easton P. Neurometrics: Computer-assisted differential diagnosis of brain dysfunctions. Science. 1988;239:162–169. doi: 10.1126/science.3336779. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, Reber PJ, Kounios J. Neural activity when people solve verbal problems with insight. PLoS Biology. 2004;2:500–510. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomical correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kounios J. Process complexity in semantic memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:338–351. [Google Scholar]
- Kounios J, Osman AM, Meyer DE. Structure and process in semantic memory: New evidence based on speed-accuracy decomposition. Journal of Experimental Psychology: General. 1987;116:3–25. doi: 10.1037//0096-3445.116.1.3. [DOI] [PubMed] [Google Scholar]
- Kounios J, Frymiare JL, Bowden EM, Fleck JI, Subramaniam K, Parrish TB, Jung-Beeman M. The prepared mind: Neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychological Science. 2006;17:882–890. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- Kounios J, Holcomb PJ. Concreteness effects in semantic processing: ERP evidence supporting dual-coding theory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Williams SCR, Gray J. Personality predicts brain responses to cognitive demands. Journal of Neuroscience. 2004;24:10636–10641. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Kanngieser N, Jaskowski P, Haider H, Rose M, Verleger R. Precursors of insight in event-related brain potentials. Journal of Cognitive Neuroscience. 2006;18:2152–2166. doi: 10.1162/jocn.2006.18.12.2152. [DOI] [PubMed] [Google Scholar]
- Laufs HH, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences USA. 2003;19:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Lachaux J-P, Martinerie J, Varela FJ. Guiding the study of brain dynamics by using first-person data: Synchrony patterns correlate with ongoing conscious states during a simple visual task. Proceedings of the National Academy of Sciences USA. 2002;99:1586–591. doi: 10.1073/pnas.032658199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Maier NRF. Reasoning in humans: II. The solution of a problem and its appearance in consciousness. Journal of Comparative Psychology. 1931;12:181–194. [Google Scholar]
- Mednick SA. The associative basis of the creative process. Psychological Review. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- Mendelsohn GA, Griswold BB. Assessed creative potential, vocabulary level, and sex as predictors of the use of incidental cues in verbal problem solving. Journal of Personality and Social Psychology. 1966;4:423–421. doi: 10.1037/h0023783. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Wiebe D. Intuition in insight and noninsight problem solving. Memory & Cognition. 1987;15:238–246. doi: 10.3758/bf03197722. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Irwin DE, Osman AM, Kounios J. The dynamics of cognition and action: Mental processes inferred from speed-accuracy decomposition. Psychological Review. 1988;95:183–237. doi: 10.1037/0033-295x.95.2.183. [DOI] [PubMed] [Google Scholar]
- Newell A, Simon HA. Human problem solving. Prentice Hall; Englewood Cliffs, NJ: 1972. [Google Scholar]
- Pfurtscheller G. Induced oscillations in the alpha band: Functional meaning. Epilepsia. 2003;44(Suppl 12):2–8. doi: 10.1111/j.0013-9580.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology. 1999;110:1842–185. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain reflections of words and their meaning. Trends in Cognitive Sciences. 2001;5:517–524. doi: 10.1016/s1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228:750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences USA. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler JW, Ohlsson S, Brooks K. Thoughts beyond words: When language overshadows insight. Journal of Experimental Psychology: General. 1993;122:166–183. [Google Scholar]
- Seifert CM, Meyer DE, Davidson N, Patalano AL, Yaniv I. Demystification of cognitive insight: Opportunistic assimilation and the prepared-mind perspective. In: Sternberg RJ, Davidson JE, editors. The nature of insight. MIT Press; Cambridge, MA: 1995. pp. 65 – 124. [Google Scholar]
- Sergent C, Dehaene S. Is consciousness a gradual phenomenon? Evidence for an all-or-none bifurcation during the attentional blink. Psychological Science. 2004;15:720–728. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, de Geus EJC. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Smith RW, Kounios J. Sudden insight: All-or-none processing revealed by speed-accuracy decomposition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1443–1462. doi: 10.1037//0278-7393.22.6.1443. [DOI] [PubMed] [Google Scholar]
- Sternberg RJ, Davidson JE. The nature of insight. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Stringaris A, Medford N, Giora R, Giampietro VC, Brammer MJ, David AS. How metaphors influence semantic relatedness judgments: The role of the right-frontal cortex. NeuroImage. 2006;33:784–793. doi: 10.1016/j.neuroimage.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Northa D, Bivera C. EEG and intelligence: Relations between EEG coherence, EEG phase delay, and power. Clinical Neurophysiology. 2005;116:2129–2141. doi: 10.1016/j.clinph.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Vincent RD, Goldberg YK, Titone DA. Anagram software for cognitive research that enables specification of psycholinguistic variables. Behavior Research Methods. 2006;38:196–201. doi: 10.3758/bf03192769. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. Journal of Neuroscience. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróbel A. Beta activity: A carrier for visual attention. Acta Neurobiologiae Experimentalis. 2000;60:247–260. doi: 10.55782/ane-2000-1344. [DOI] [PubMed] [Google Scholar]


