Abstract
The success of gene therapy depends on the specificity of transgene delivery by therapeutic vectors. The present study describes the use of an adenovirus (Ad) fiber replacement strategy for genetic targeting of the virus to human CD40, which is expressed by a variety of diseased tissues. The tropism of the virus was modified by the incorporation into its capsid of a protein chimera comprising structural domains of three different proteins: the Ad serotype 5 fiber, phage T4 fibritin, and the human CD40 ligand (CD40L). The tumor necrosis factor-like domain of CD40L retains its functional tertiary structure upon incorporation into this chimera and allows the virus to use CD40 as a surrogate receptor for cell entry. The ability of the modified Ad vector to infect CD40-positive dendritic cells and tumor cells with a high efficiency makes this virus a prototype of choice for the derivation of therapeutic vectors for the genetic immunization and targeted destruction of tumors.
Mammalian viruses possess a number of features which make them rational prototypes as the means of gene delivery and expression for research and therapeutic purposes. Natural variability in the structures of virions, routes and modes of infection, and replication dynamics among closely related viruses clearly shows their inherent plasticity as biological systems. This preexisting diversity logically leads to the concept of directed evolution of viruses toward the goal of their practical utilization.
Gene therapy represents the field of biomedicine where the attempts to realize this concept have been most persistent, with the major goals being the efficient and safe delivery of gene therapy vectors to and their expression at the site of disease. In this regard, the derivation of gene vectors from human adenoviruses (Ads) represents one of the most systematic and successful efforts in changing the structure and infectious cycle of a viral agent to make it suitable for therapeutic use. While early developments in Ad vector technology resulted in a number of promising gene therapy vectors, the use of these agents in clinical trials revealed the need for considerable improvements in their safety, specificity, and efficacy.
One of the key problems with Ad-based therapies is the lack of selective transduction of diseased tissue, which results from the cell type-independent profile of expression of therapeutic transgenes by Ad and the presence of its primary receptor in various human tissues. The former issue is addressed by the use in the design of new Ad vectors of tissue- and tumor-specific elements of transcriptional control (1), the approach known as transcriptional targeting. To solve the latter problem, the strategy of transductional targeting, which is based on modification of the receptor specificity of a vector, has been proposed (22, 23).
Most of the Ad vectors designed for therapeutic use are derived from human Ads of serotypes 2 and 5 (Ad2 and Ad5, respectively), which are known to bind to target cells by virtue of the fiber protein positioned at the vertices of the icosahedral Ad capsid. Specifically, the carboxy-terminal domain of the fiber protein, termed the knob (13), anchors the virion on the cell surface by binding to the coxsackievirus and Ad receptor (CAR) (3, 44). Therefore, the presence of CAR on the cell surface is a key determinant of its accessibility or refractoriness to Ad. Numerous studies have shown that the profiles of CAR expression in normal and diseased tissues do not favor selective Ad-mediated gene delivery (20, 26, 30, 34), thereby necessitating the development of Ad vectors capable of selectively infecting target tissues by exploiting as surrogate receptors the unique cell surface markers expressed by these cells.
Genetic modifications of the Ad virion to generate such targeted vectors have focused primarily on alteration of the tropism of the virus through incorporation of the target receptor-specific ligands into its fiber. The major emphasis of these efforts was on the insertion of small peptide ligands into either the carboxy terminus or the so-called HI loop of the fiber. However, these endeavors met with only very modest success because of the limited capacity of the fiber knob to accommodate modifications (16, 51). The realization of these limitations recently resulted in the new strategy of transductional targeting of Ad, which capitalizes on the modular structure of the native fiber protein as a leading principle of its rational redesign toward the generation of a universal ligand-presenting molecule. In this strategy, the fiber knob domain, alone or together with the shaft domain (21, 27, 46), is replaced with a combination of two heterologous domains, one of which functions as a receptor-binding ligand and the other of which supports the functional structure of the entire protein chimera. Krasnykh et al. previously demonstrated the feasibility of this approach by replacing the Ad fiber protein within the virion with a protein chimera comprising an N-terminal portion of the fiber genetically fused with phage T4 fibritin (FF) (21). Targeting of the resultant Ad vector to an artificial receptor was achieved by incorporation into the FF chimera of a short peptide ligand.
In the present study, we demonstrated the versatility of this fiber replacement strategy by creating an Ad vector targeted to human CD40 by virtue of the incorporation of the CD40 ligand (CD40L) into its capsid. Our study showed that despite the significant size of the ligand used and its complex tertiary structure, both components of the targeting protein, the CD40L domain and the FF backbone, folded properly, thereby making the entire chimera fully functional. Importantly, for the first time, a pair of cell surface molecules which are normally involved in an intercellular interaction was used as a component of an alternative cell entry pathway for a targeted Ad vector. By demonstrating the efficient targeting of Ad with CD40L to human cancer cells and dendritic cells (DCs), we highlight the advantages offered by the fiber replacement strategy for the generation of tropism-modified therapeutic vectors.
MATERIALS AND METHODS
Cell lines.
Human embryonal kidney cell line 293 (11) was purchased from Microbix (Toronto, Ontario, Canada). Cell lines 293T/17 and T24 (human bladder carcinoma) were obtained from the American Type Culture Collection (ATCC; Manassas, Va.). Cell lines 293.CD40, 293.CD40L, 293F28, and 293FΔTAYT are derivatives of cell line 293 which express human CD40, human CD40L, Ad5 wild-type fiber, or a mutant form of the fiber with a deletion of amino acids 489 to 492, respectively; they were generated by transfection of 293 cells with plasmids pcDNA.CD40, pcDNA.CD40L, pVS2, and pVSΔTAYT and subsequent selection with a relevant antibiotic. Selection of the 293.CD40 and 293.CD40L clones was done with 1,000 μg of Geneticin (G418)/ml; the 293F28 and 293FΔTAYT clones were selected with 600 μg of Zeocin (Invitrogen, Carlsbad, Calif.)/ml. Cell clones expressing high levels of CD40 and CD40L were identified by Western blotting of cell lysates with a rabbit polyclonal anti-CD40 or anti-CD40L antibody (Ab) (Santa Cruz Biotechnology, Santa Cruz, Calif.); clones expressing wild-type and mutant fibers were detected with antifiber monoclonal Ab (MAb) 4D2 (15), provided by Jeffrey Engler (University of Alabama at Birmingham [UAB]). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 medium; derivatives of 293 cells were maintained in the same medium containing 100 μg of G418/ml or 100 μg of Zeocin/ml. The medium was supplemented with fetal calf serum (FCS) (10%), glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). FCS was purchased from Gibco-BRL (Gaithersburg, Md.); media and supplements were obtained from Mediatech (Herndon, Va.). All cells were propagated at 37°C in a 5% CO2 atmosphere.
Human DCs were derived from CD14-positive monocytes isolated from peripheral blood and were maintained in cultures according to a previously described protocol (45).
Genetic engineering.
To produce soluble human CD40L (sCD40L) in bacteria, the DNA sequence encoding the tumor necrosis factor (TNF)-like domain of CD40L (amino acids Gly116 through Leu261) was amplified from plasmid pcDL-SRαhCD40L (ATCC 79814) by “sticky-end” PCR (sePCR) (55) and fused with six histidine codons (six-His tag). This procedure was accomplished with two pairs of primers: primers CD40L.NdeI.F.long (TAT GGG CAG CAG CCA TCA TCA TCA TCA TCA CGG TGA TCA GAA TCC TCA AAT) and CD40L.NotI.R.short (GCT TAT CAG AGT TTG AGT AAG CCA AAG G) and primers CD40L.NdeI.F.short (5′-TGG GCA GCA GCC ATC ATC ATC ATC ATC ACG GTG ATC AGA ATC CTC AAA T) and CD40L.NotI.R.long (GGC CGC TTA TCA GAG TTT GAG TAA GCC AAA GG) (the sequences coding for the six-His tag are underlined). The PCR products were mixed in equimolar amounts, denatured, annealed, and ligated with NdeI-NotI-digested pET20b(+) (Novagen, Madison, Wis.). The resultant plasmid was designated pET.sCD40L.
To generate mutant forms of CD40L, CD40L/AV and CD40L/VA, in which the cysteine residues at positions 83 and 123 (Cys83 and Cys123) were replaced with alanine and valine or with valine and alanine, the same sePCR technique was used. This time, the DNA sequences were amplified by using pET.sCD40L as the template. To amplify the sequence between Cys83 and Cys123 and introduce the Ala and Val substitutions, primers with altered nucleotides (underlined) were used in pairs: primers Ala.F.long (CTT CGC TTC CAA TCG GGA AGC) and Val.R.short (ACA GGT TTG GCG GAA CTG TG) and primers Ala.F.short (GCT TCC AAT CGG GAA GCT TC) and Val.R.long (CCC GAC AGG TTT GG CGG AA). Similarly, to amplify the same sequence containing Val and Ala substitutions, sePCR was carried out with primers Val.F.long (CTT CGT TTC CAA TCG GGA AGC) and Ala.R.short (GCA GGT TTG GCG GAA CTG TG) and primers Val.F.short (GTT TCC AAT CGG GAA GCT TCG A) and Ala.R.long (CCC GGC AGG TTT GGC GGA A). The sequence located upstream from the mutant region was amplified with two pairs of primers: primers 6H/CD40L.NdeI.F.long (TAT GGG CAG CAG CCA TCA TCA TCA TCA TCA CGG TGA TCA GAA TCC TCA AAT) and R.long (GAA GGT GAC TTG GGC ATA GAT A) and primers 6H/CD40L.NdeI.F.short (TGG GCA GCA GCC ATC ATC ATC ATC ATC ACG GTG ATC AGA ATC CTC AAA T) and R.short (GTG ACT TGG GCA TAG ATA TAA TAG A). The sequence located downstream from this region was amplified with primers F.long (CGG GCA ACA ATC CAT TCA C) and CD40L.NotI.R.long (GGC CGC TTA TCA GAG TTT GAG TAA GCC AAA GG) and primers F.short (CAA CAA TCC ATT CAC TTG GGA) and CD40L.NotI.R.short (GCT TAT CAG AGT TTG AGT AAG CCA AAG G). PCR products representing the mutant region or each of the flanking sequences were mixed in pairs in equimolar amounts, denatured, annealed, mixed together, and ligated with NdeI-NotI-digested pET20b(+). This process resulted in pET.sCD40L/AV and pET.sCD40L/VA.
To incorporate DNA encoding the CD40-binding domain of human CD40L (Gly116 through Leu261) into the FF fusion gene (21), the corresponding sequence was amplified from pCDL-SRα-CD40L with primers sCD40L.F (GGG AGA TCT GGT GAT CAG AAT CCT CAA ATT GCG) and sCD40L.R (GGG AAT ATT AGA GTT TGA GTA AGC CAA AGG ACG) (BglII and SspI recognition sites are underlined). The PCR product was digested with BglII and SspI and cloned into BamHI-SwaI-digested pXK.FF.LL and pQE.6H.FF.LL, resulting in pXK.FF/CD40L and pQE.6H.FF/CD40L, respectively. Of note, plasmids pXK.FF.LL and pQE.6H.FF.LL contain the gene encoding the chimeric Ad5 fiber-phage T4 fibritin protein fused with the carboxy-terminal linker (21). The protein encoded by pQE.6H.FF.LL also contains an N-terminal six-His tag. The BamHI and SwaI sites located at the end of the linker sequence allow for the cloning of ligand-encoding sequences at the end of the chimeric gene.
To express the chimeric FF/CD40L protein in mammalian cells, the AgeI-MfeI fragment from pXK.FF/CD40L, containing the sequence of the FF/CD40L gene, was transferred into expression plasmid pVS2 (N. Korokhov, G. Mikheeva, A. Krendelshchikov, N. Belousova, V. Simonenko, V. Krendelshchikova, A. Pereboev, A. Kotov, O. Kotova, P. L. Triozzi, W. A. Aldrich, K.-M. Lo, P. T. Banerjee, S. D. Gillies, D. T. Curiel, and V. Krasnykh, submitted for publication) digested with the same restriction endonucleases. A recombinant Ad genome containing the firefly luciferase-expressing cassette in place of the E1 region and the FF/CD40L gene in place of the wild-type fiber gene was generated by homologous DNA recombination in Escherichia coli as previously described (4). To this end, the FspI-SalI fragment isolated from shuttle plasmid pXK.6H.FF.LL.CD40L and SwaI-linearized rescue plasmid pVK700 (2) were used as partners for recombination. The resultant plasmid was designated pVK719.
Plasmid pcDNA.CD40, encoding human CD40, was generated by replacing the HindIII-XbaI fragment within pcDNA3 (Invitrogen) with the cDNA of CD40 isolated as a HindIII-XbaI fragment from plasmid hCD40pcDM8 (40), provided by Ivan Stamenkovic. Similarly, plasmid pcDNA.CD40L, expressing human CD40L, was designed by cloning the BamHI fragment of pcDL-SRαhCD40L, which contains CD40L cDNA, into the BamHI site of pcDNA3.
To generate a gene encoding an Ad5 fiber protein with the previously described deletion of the TAYT sequence (positions 489 to 492 in the wild-type Ad5 fiber protein) (38) (F5Δ), an “inverse” PCR with plasmid pXK3.1 (2) and primers with the sequences AAC GCT GTT GGA TTT ATG CC and GCC TTC AGT AAG ATC TCC ATT TC was carried out. This PCR resulted in a linearized copy of pXK3.1 lacking the TAYT-encoding sequence. The sequence was circularized with T4 DNA ligase, resulting in pXK3.1ΔTAYT. The plasmid vector for the expression of the mutant fiber was constructed by ligating the BstXI-MfeI fragment of pXK3.1ΔTAYT with BstXI-MfeI-digested pVS2. The new plasmid was named pVSΔTAYT.
Viruses.
The virus containing FF/CD40L, Ad5Luc.FF/CD40L, was rescued by the two-step procedure developed by Von Seggern et al. (50). First, 293F28 cells, which stably express the wild-type Ad5 fiber, were transfected with PacI-digested pVK719. The rescued virus at this point was mosaic in the sense that the Ad virions randomly incorporated a mixture of wild-type fibers and FF/CD40L chimeras. After additional rounds of amplification on 293F28 cells, the virus was used for infection of either 293 or 293FΔTAYT cells to obtain versions containing only FF/CD40L chimeras or FF/CD40L chimeras in combination with the Ad5 mutant fiber (F5Δ).
All Ad vectors were isolated from infected cells and purified by equilibrium centrifugation in CsCl gradients as previously described (10). The viral particle titer was determined spectrophotometrically by the method of Maizel et al. (29) with a conversion factor of 1.1 × 1012 viral particles per absorbance unit at 260 nm.
Recombinant proteins.
The six-His-tagged sCD40L protein and its derivatives were expressed in E. coli BL21(DE3)(pLysS) essentially as described by Pullen et al. (36). The expression of recombinant proteins was induced in a 500-ml bacterial culture with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 25°C, after which the cells were harvested and frozen at −80°C. Frozen cells were resuspended in 25 ml of a buffer containing 20 mM bis-Tris (pH 6.8), 100 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, and 10% glycerol and lysed by sonication on ice. The lysates were cleared by centrifugation at 14,000 × g for 30 min at 4°C, mixed at a ratio of 1:5 with phosphate buffer (PB) (50 mM NaHPO4 [pH 8.0], 300 mM NaCl) containing 1 mM phenylmethylsulfonyl fluoride, and applied to a 3-ml Ni-nitrilotriacetic acid (NTA)-Sepharose (Qiagen) column. The column was washed with 15 ml of PB, and bound proteins were eluted with a linear gradient of imidazole (20 to 160 mM) in PB. Fractions containing sCD40L were combined; dialyzed against a buffer containing 25 mM morpholineethanesulfonic acid (pH 6.4), 1 mM EDTA, 1 mM dithiothreitol, 5% glycerol, and 150 mM NaCl; concentrated on a CentriPlus YM-30 concentrator (Millipore, Bedford, Mass.); filtered through a 0.22-μm-pore-size filter; and stored at 4°C.
Recombinant six-His-tagged FF/CD40L protein was expressed in E. coli SG13009 harboring pQE.FF/CD40L and then purified by immobilized ion metal affinity chromatography (IMAC) with NTA-Sepharose (Qiagen) essentially as described above for sCD40L. The protein was dialyzed against phosphate-buffered saline (PBS) and stored at 4°C.
To express the FF/CD40L protein in 293T/17 cells, the cells were transfected with pVS.FF/CD40L by using DOTAP liposomal transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's protocol. At 48 h after transfection, the cells were collected and lysed in cell culture lysis reagent (Promega, Madison, Wis.) for subsequent analysis by immunoblotting.
The expression and purification of the recombinant Ad5 fiber protein were described previously (8).
The concentrations of the proteins in purified preparations and in cell lysates were determined by using a DC protein assay (Bio-Rad, Hercules, Calif.).
Flow cytometry.
Cells of line 293 or 293.CD40 grown in cultures were detached from the plastic support by treatment with Versene solution (Mediatech) and washed with PBS containing 0.1% bovine serum albumin and 0.01% sodium azide. Aliquots of 5 × 105 cells were incubated for 1 h at 4°C with either anti-CD40 MAb G28.5 (25) or one of the recombinant sCD40L proteins diluted in PBS at a concentration of 5 or 1 μg/ml, respectively. Following a washing step, the cells probed with G28.5 were incubated for 1 h at 4°C with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse immunoglobulin G (Sigma, St. Louis, Mo.), while those probed with sCD40L were incubated with an FITC-conjugated anti-six-His MAb (Covance, Princeton, N.J.). After two additional washing steps, the cells were analyzed with a FACScan (Becton Dickinson, Mountain View, Calif.) at the UAB Cell Sorting Facility. Cells incubated with the secondary antibody only were used as a negative control.
Generation of conformation-specific anti-CD40L MAbs.
To generate hybridoma cell lines producing MAbs specific to the native configuration of the TNF-like domain of human CD40L, the standard protocol established at the UAB Hybridoma Shared Facility was used. The recombinant sCD40L protein described above was used for the immunization of mice and for the preliminary screening of primary clones by an enzyme-linked immunosorbent assay (ELISA). Positive clones were subjected to an additional round of cloning, and the specificity of the antibodies produced by the resultant lines was assessed by using them for CD40L binding in dot blot and fluorescence-activated cell sorting (FACS) applications. Specifically, the antibodies were initially screened in a dot blot assay with denatured, polyvinylidene difluoride (PVDF) membrane-bound sCD40L. Antibody species which showed no binding in this assay were subsequently used to probe cell-associated human CD40L expressed by 293.CD40L cells (see above); parental CD40L-negative 293 cells were used as a negative control. This procedure allowed for the selection of six hybridoma lines which produced MAbs reacting with the cell-bound CD40L protein but not with the denatured sCD40L protein. One of these lines, 2B3, was used for the preparative production of MAbs in mice. Antibodies were purified from ascitic fluid by using rProtein A-Sepharose Fast Flow columns (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's protocol.
Western blot analysis.
Aliquots of either purified Ad vectors equal to 1010 viral particles or cell lysates containing 1 μg of total soluble protein were denatured by boiling for 5 min in sample buffer and loaded onto a sodium dodecyl sulfate (SDS)-7.5% polyacrylamide gel. Upon separation, viral proteins were electroblotted onto a PVDF membrane and detected with antifiber MAb 4D2. The blots were developed by using an ECL Plus detection kit with a horseradish peroxidase-conjugated secondary anti-mouse antibody, both purchased from Amersham Pharmacia Biotech.
ELISA.
Anti-human CD40L MAb 2B3, which binds to the extracellular portion of CD40L in its native configuration, was generated at the UAB Hybridoma Core Facility by using purified sCD40L as an antigen. For the ELISA, 0.2-μg aliquots of 2B3 diluted in 50 mM carbonate-bicarbonate buffer (pH 9.6) were allowed to adsorb overnight at 4°C to wells of a 96-well Nunc-Maxisorp ELISA plate. The plate was blocked with blocking buffer (Tris-buffered saline [TBS], 0.05% Tween 20, 0.5% casein) for 2 h at 4°C and then washed six times with washing buffer (TBS, 0.05% Tween 20). Purified viruses diluted in TBS to concentrations ranging from 2 × 108 to 4 × 1011 particles/ml were added to the wells in 100-μl aliquots and allowed to bind to the antibody for 1 h at room temperature. The wells were washed six times with washing buffer, and the bound virions were probed with rabbit anti-Ad2 serum (ATCC). Following incubation for another hour, the wells were washed, incubated with goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (DAKO, Carpinteria, Calif.), washed again, and developed with o-phenylenediamine (Sigma) as recommended by the manufacturer. The absorbance of the samples at 490 nm was determined with a microtiter plate reader.
Gene transfer experiments.
Cells of lines 293, 293.CD40, and T24 grown in wells of 24-well plates to 90 to 100% confluence were washed with serum-free growth medium and then incubated on ice with 0.2 ml of either plain medium or medium containing a blocking agent. In the latter instance, recombinant Ad5 fiber knob (24) or sCD40L proteins were added to the medium at various concentrations (see the figure legends). One hour later, the cells were infected at a multiplicity of infection (MOI) of 10 or 100 viral particles per cell with an Ad vector, which was added in 0.2-ml aliquots of medium containing 4% FCS. After incubation on ice for 1 h, the medium containing the virus and the inhibitor was removed, and the cells were washed with medium containing 10% FCS. Fresh medium was added, and incubation was continued at 37°C for 22 h to allow reporter expression. The cells were washed with PBS and lysed in luciferase reporter lysis buffer (Promega). The luciferase activity in the cell lysates was measured according to the manufacturer's protocol. Each data point was set in triplicate and calculated as the mean of three determinations. In instances where gene transfer was done without the addition of a blocking agent, the virus was added to the cells in 0.4-ml aliquots of medium containing 2% FCS.
All incubation and washing steps in the gene transfer experiments involving DCs were done in suspension. To minimize standard deviations in the data, which could result from the loss of a fraction of the cells during the washing steps, the luciferase activity measured in the cell lysates was normalized to the protein concentration in the resultant cell lysates. Otherwise, the format of these studies was the same as that described above.
Labeling of Ad vectors with [methyl-3H]thymidine.
Cells of lines 293 and 293ΔTAYT grown in 175-cm2 flasks were infected with either Ad5Luc1 or Ad5Luc.FF/CD40L (MOI of 300 viral particles per cell) diluted in DMEM/F12 medium with 2% FCS. At 18 h postinfection, 1 mCi of [methyl-3H] thymidine (Amersham, Arlington Heights, Ill.) was added to each flask, and the cells were further incubated at 37°C until a complete cytopathic effect was observed. The cells were harvested, and viruses were purified from the cell lysates by centrifugation in CsCl gradients according to a standard protocol (10). The specific radioactivity of the labeled virus preparations measured in a liquid scintillation counter was in the range of 10−5 to 10−4 cpm per virion.
Ad-binding assay.
The binding of 3H-labeled Ad to cells was assayed according to a procedure described previously by Shayakhmetov and Lieber (39). Specifically, target cells (4 × 105 cells per aliquot) were incubated on ice for 1 h with 3H-labeled Ad. A total of 4,000 viral particles per cell were added to cells in 200-μl aliquots of DMEM/F12 medium with 2% FCS. The cells were pelleted by centrifugation for 5 min at 2,000 × g and washed twice with 0.5 ml of ice-cold DMEM/F12 with 2% FCS. After the final wash, the cell-associated radioactivity was measured in a scintillation counter. The number of viral particles bound per cell was calculated by using the specific radioactivity of the virus preparations, the total radioactivity of the samples, and the number of cells.
RESULTS
Disulfide-free form of human CD40L possesses CD40-binding capability.
To design an Ad vector targeted to CD40-expressing human tissues, we chose to modify the virus tropism by using the TNF-like domain of human CD40L, which had been shown to bind to CD40 (48). The carboxy-terminal location of this domain within the CD40L molecule and its globular, trimeric structure both predicted its successful functioning as a targeted ligand upon genetic fusion with the FF chimera previously designed by Krasnykh et al. for Ad targeting (21). Before using the TNF-like domain of human CD40L for the genetic modification of Ad tropism, we wished to investigate whether the disulfide bond present within the CD40L protein (19) is crucial for its binding to CD40. Should this bond be compulsory for the correct folding of CD40L, the genetic fusion of this protein with Ad5 protein components would be highly problematic, as Ad5 virions are assembled in the nucleoplasm, a reducing environment of which makes the formation of such bonds impossible.
To explore this matter, we chose to derive a recombinant protein comprising the TNF-like domain of human CD40L, sCD40L, and two versions of this molecule, sCD40L/AV and sCD40L/VA, in which the pair of cysteine residues forming the disulfide bond in the wild-type molecule was mutated. In order to minimize the effect of these mutations on the overall pattern of folding of sCD40L and its stability, we chose to replace these cysteines with either valine-alanine or alanine-valine pairs. This approach capitalized on the finding of Worn and Pluckthun that the structural configuration of these substitute amino acid pairs provides some stability to disulfide-deprived single-chain antibodies (53).
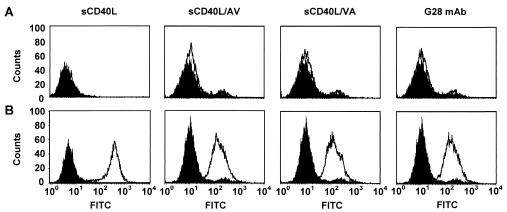
Open reading frames for all three proteins were fused with the sequence for an N-terminal six-His tag and expressed in E. coli BL21(DE3)(pLysS) by using plasmid vectors pET.6H.sCD40L, pET.6H.sCD40L/AV, and pET.6H.sCD40L/VA. SDS-polyacrylamide gel electrophoresis (PAGE) analysis of these proteins showed that they were predominantly expressed in a soluble form (Fig. 1). A functional test of the sCD40L proteins was carried out by flow cytometry, in which the 293.CD40 cell line derived from the 293 cells stably express human CD40. This analysis demonstrated that all three forms of sCD40L, the wild type and the mutants, were able to bind to cell surface-localized CD40 (Fig. 2). The shifts in the cell-associated fluorescence intensity observed with each of the sCD40Ls proteins were comparable to those seen when anti-CD40 MAb G28.5 was used as a CD40-specific probe. No binding of either sCD40Ls or G28.5 to CD40-negative 293 cells was observed, suggesting the specificity of binding to CD40 expressed on 293.CD40 cells.
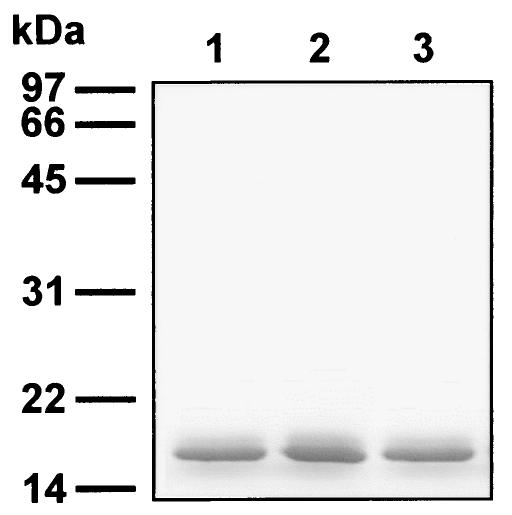
FIG. 1.
Expression of recombinant forms of human sCD40L protein in E. coli. Recombinant proteins corresponding to the TNF-like domain of either the native human CD40L, sCD40L (lane 1), or its mutated, cysteine-free forms, CD40L/AV and CD40L/VA (lanes 2 and 3, respectively), were expressed in E. coli by using plasmid vector pET20b(+). All of these molecules were designed to contain amino-terminal six-His tags. The proteins were purified from cleared bacterial lysates by affinity chromatography on Ni-NTA-Sepharose, denatured in a sample buffer, and analyzed by SDS-PAGE.
FIG. 2.
Specific binding of sCD40L proteins to CD40-expressing cells. 293 (A) or 293.CD40 (B) cells were probed with either anti-CD40 MAb G28.5 or one of the three recombinant proteins comprising the TNF-like domain of human CD40L (white profiles). sCD40L corresponds to the wild-type CD40L protein, while sCD40L/AV and sCD40L/VA are cysteine-free mutants. The bound proteins were detected with an FITC-conjugated secondary Ab. Control samples were incubated with the secondary Ab only (black profiles). The cells were sorted on a FACScan flow cytometer.
These results clearly showed that even in the absence of the internal disulfide bond, the TNF-like domain of CD40L retains its CD40-binding capacity and is thus suitable as a targeting ligand for an Ad vector.
A genetic fusion of sCD40L with the FF chimera is expressed as a stable trimeric protein.
We next wished to use the TNF-like domain as a targeting moiety in the context of a genetic fusion with the chimeric molecule comprising FF. This goal was rationalized by previous success in using the ligand-containing derivative of the FF chimera to replace the fiber in an Ad capsid for the purpose of vector targeting to an alternative receptor (21).
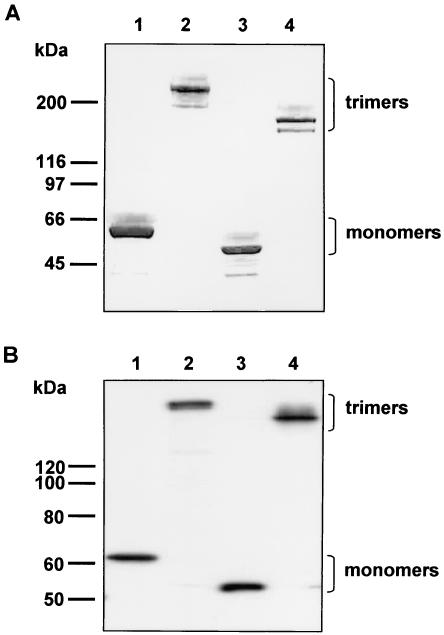
Therefore, the TNF-binding domain of CD40L was genetically fused to the carboxy terminus of the FF protein and expressed in E. coli. The ability of the resultant FF/CD40L molecule to self-assemble into homotrimers, which is an essential structural characteristic of the wild-type Ad5 fiber, was assessed by SDS-PAGE of the purified protein (Fig. 3A). A comparison of the electrophoretic mobilities of the fully denatured and native forms of FF/CD40L with similarly prepared samples of the wild-type Ad5 fiber confirmed that FF/CD40L is expressed as a full-size molecule which forms stable trimers.
FIG. 3.
Analysis of trimerization of recombinant FF/CD40L chimeras. FF/CD40L proteins (lanes 3 and 4) expressed in either E. coli (A) or 293T/17 cells (B) were analyzed by SDS-PAGE or Western blotting, respectively. Wild-type Ad5 fiber protein expressed in insect cells was used as a control (lanes 1 and 2). Samples comprising IMAC-purified proteins (A) or cleared cell lysates (B) were loaded on the gels in amounts normalized by total protein contents. Lanes 1 and 3 contain samples which were fully denatured by boiling in a sample buffer prior to loading on the gels. Samples in lanes 2 and 4 were not denatured and thus contain proteins in their native configurations.
The same results regarding the stability of FF/CD40L and its trimerization capacity were obtained in an experiment in which this protein was transiently expressed in mammalian cells. Since FF/CD40L contains the nuclear localization signal of the Ad5 fiber, it was expected to translocate to the nucleus and follow the biosynthetic and intracellular trafficking pathways characteristic of the wild-type fiber. The sequence encoding FF/CD40L was subcloned into mammalian expression plasmid pVS2, which was then used to transfect 293T/17 cells in order to direct the production of the chimera. Western blot analysis of the product of expression showed that, like bacterially expressed FF/CD40L, the protein produced by 293T/17 cells was stable and retained its trimerization capacity (Fig. 3B). The lack of any visible degradation of the chimera indicated its correct folding in the nuclei of transfected cells. These results suggested that the designed protein meets the criteria imposed by the structure of the Ad capsid and thus can be incorporated into a complete Ad particle.
The TNF-like domain of CD40L retains its CD40-targeting capability upon genetic incorporation into the Ad capsid.
As our results predicted the suitability of the FF/CD40L chimera for fiber replacement and Ad targeting, we next wished to derive an Ad vector incorporating this targeting protein and characterize its structure and tropism. The FF/CD40L gene was transferred into the genome of replication-incompetent, E1-deleted Ad expressing a luciferase reporter. This genome was generated and then used to rescue Ad5Luc.FF/CD40L as described in Materials and Methods. The rescue and subsequent propagation of the virus were carried out according to the two-step procedure developed by Von Seggern et al. (50); the first step was carried out with fiber-complementing cells, and the final propagation of the vector was carried out with 293 cells. The yield of the purified virus was equal to 1.8 × 104 viral particles per cell, which is well within the range of yields normally seen for unmodified Ad5 vectors.
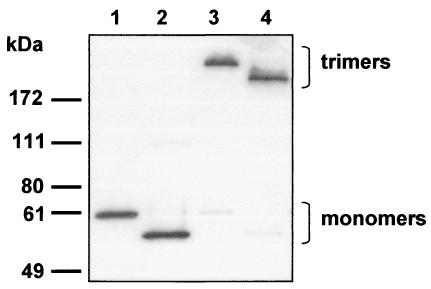
The presence of FF/CD40L within Ad5Luc.FF/CD40L capsids was confirmed by SDS-PAGE of purified virions, which showed that the efficiency of integration of FF/CD40L into the viral particles was similar to that of the wild-type fiber (Fig. 4). As predicted by our earlier results with transiently expressed FF/CD40L, no degradation of the chimera was seen.
FIG. 4.
Incorporation of FF/CD40L proteins into Ad particles. Samples of the modified Ad vector, Ad5LucFF/CD40L (lanes 2 and 4), or the unmodified control, Ad5Luc1 (lanes 1 and 3), containing 1010 viral particles were resolved by SDS-PAGE with (lanes 1 and 2) or without (lanes 3 and 4) boiling in a sample buffer. Proteins electroblotted onto a PVDF membrane were detected with antifiber MAb 4D2.
Having demonstrated the incorporation of the targeting protein into the virus, our next goal was to investigate whether the folding of the TNF-binding domain of CD40L contained within FF/CD40L was the same as that in native CD40L. This goal was addressed by probing purified Ad5Luc.FF/CD40L virions with conformation-specific anti-CD40L MAb 2B3 in an ELISA. Strong binding of Ad5Luc.FF/CD40L particles to this MAb clearly showed that, despite the absence of the disulfide bond within the CD40L portion of the targeting chimera, its native structure had not been compromised (Fig. 5).
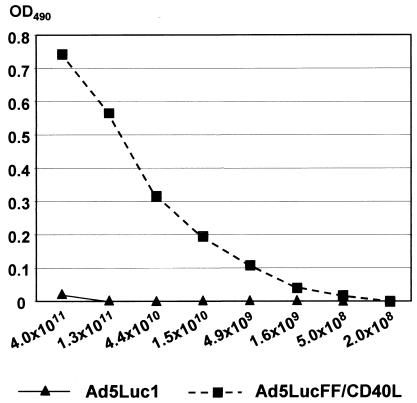
FIG. 5.
Structural integrity of the TNF-like domain of CD40L incorporated into the FF/CD40L chimera. A control virus containing unmodified Ad5 fibers, Ad5Luc1, or CD40-targeted Ad5LucFF/CD40L was incubated with the conformation-specific anti-CD40 MAb 2B3 adsorbed on an ELISA plate. Bound virions were detected with a polyclonal anti-Ad2 Ab. The amounts of each virus used are shown below the graph (number of particles). OD490, optical density at 490 nm.
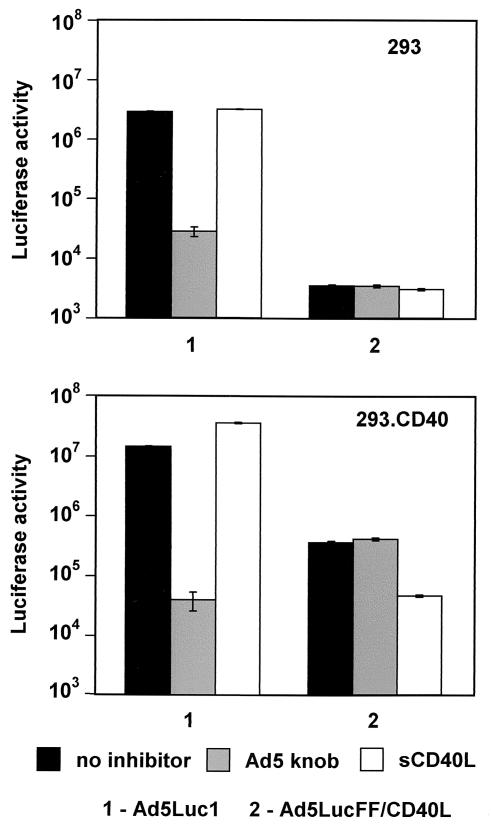
The ultimate test of the functionality of FF/CD40L as a CD40-targeting moiety was carried out by using Ad5Luc.FF/CD40L for gene transfer to CD40-expressing cells. To this end, 293.CD40 (CD40-positive) and 293 (CD40-negative) cells were infected with either Ad5Luc.FF/CD40L or the isogenic Ad5Luc1 vector, which contains the wild-type Ad5 fiber. To demonstrate the receptor specificity of Ad5Luc.FF/CD40L infection, we used recombinant Ad5 fiber knob and sCD40L proteins as specific blockers of CAR and CD40, respectively (Fig. 6). As expected, infection by Ad5Luc1, which relies on CAR as the primary binding receptor, was very efficient in both 293 and 293.CD40 cells. Predictably, it was blocked in the presence of the Ad5 fiber knob but was not affected by sCD40L. In marked contrast, a very significant difference in the efficiency of infection by Ad5Luc.FF/CD40L was seen in 293.CD40 versus 293 cells. Not only did CD40-positive cells express 100-fold more luciferase than receptor-negative ones, but also reporter expression in 293.CD40 cells was selectively inhibited by sCD40L. Since no inhibition of Ad5Luc.FF/CD40L infection by the Ad5 fiber knob was seen, in aggregate these results clearly show that the replacement of the fiber in the Ad5 virion with the FF/CD40L chimera resulted in the desired alteration of the tropism of the vector by rendering its cell entry mechanism CD40 dependent.
FIG. 6.
Receptor specificity and efficiency of transduction by a CD40-targeted Ad vector. CD40-negative 293 cells and CD40-positive 293.CD40 cells were transduced with Ad5LucFF/CD40L or untargeted Ad5Luc1 (control) at an MOI of 10 viral particles/cell. Prior to infection, the cells in the samples shown by the gray and white bars were preincubated with the recombinant Ad5 fiber knob and with sCD40L (each used at 100 μg/ml), respectively. Black bars correspond to luciferase activity in cells infected in the absence of inhibitors. Luciferase activity (relative light units) detected in the lysates of infected cells is the average of three measurements. Error bars indicate standard deviations.
Somewhat disappointing was the difference in the observed efficiencies of gene transfer to 293.CD40 cells for control vector Ad5Luc1 and targeted Ad5Luc.FF/CD40L, with the latter being 40-fold less efficient. One explanation for this observation is that while FF/CD40L is an efficient retargeting construct, in its present configuration it is less than a perfect fiber substitute as far as the overall structure and stability of Ad virions is concerned. The major goal of the experiments described below was to circumvent this problem of the suboptimal efficiency of Ad5Luc.FF/CD40L infection.
Incorporation of the mutant Ad5 fiber protein into Ad5Luc.FF/CD40L virions results in improved gene transfer without compromising the target specificity of the vector.
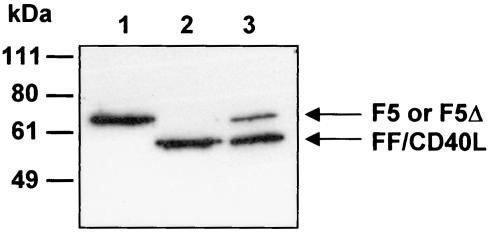
To circumvent the aforementioned problem, we put forward the hypothesis that the infectivity of Ad5Luc.FF/CD40L particles might be improved by incorporating a full-size Ad5 fiber. Obviously, the resultant virions had to be designed in a way which would not compromise the targeting of the vector to CD40. Therefore, we wished to test this concept by designing a mosaic version of Ad5Luc.FF/CD40L which, in addition to FF/CD40L,would also contain a full-size Ad5 fiber engineered to lack CAR-binding ability. This goal was accomplished by propagating Ad5Luc.FF/CD40L in 293FΔTAYT cells derived from 293 cells to constitutively express an Ad5 fiber whose binding to CAR was abolished by the previously described deletion (38). This additional vector amplification step resulted in a virus preparation designated Ad5Luc.FF/CD40L.F5Δ, which contained viral particles randomly incorporating both types of modified fibers (Fig. 7).
FIG. 7.
Integration of mutant F5Δ and FF/CD40L proteins into mosaic Ad5Luc.FF/CD40L.F5Δ virions. Aliquots of 1010 viral particles of Ad5Luc1 (lane 1), Ad5Luc.FF/CD40L (lane 2), and Ad5Luc.FF/CD40L.F5Δ (lane 3) were fully denatured, and viral proteins were separated by SDS-PAGE. The wild-type (F5) and mutant (F5Δ) Ad5 fibers as well as the FF/CD40L chimera were identified on the blots with MAb 4D2.
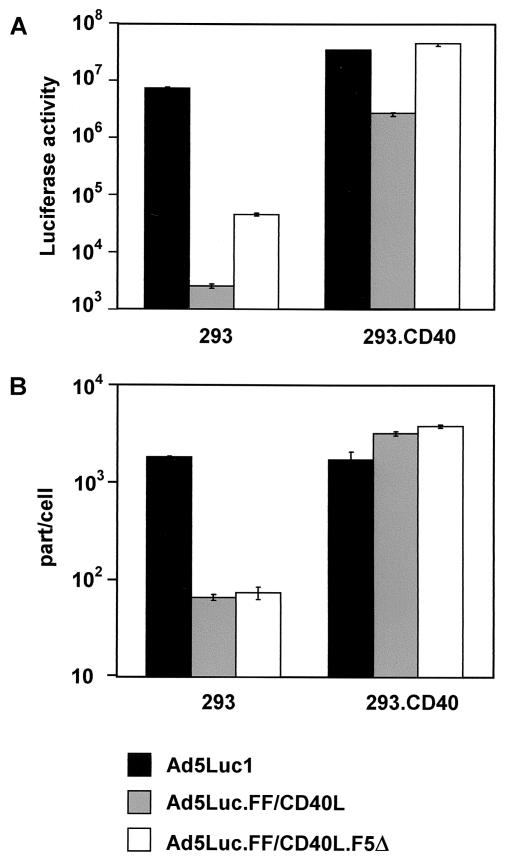
The infectivity of this mosaic vector was then compared to those of Ad5Luc1 and Ad5Luc.FF/CD40L, the latter vector being used in the original, one-fiber configuration. In keeping with our expectations, the results of this comparison (Fig. 8A) showed that the mosaic vector outperformed both Ad5Luc1 and Ad5Luc.FF/CD40L in infecting 293.CD40 cells; we noted a 17-fold increase in gene transfer over that observed with Ad5Luc.FF/CD40L. Interestingly, a nearly equivelent increase in gene transfer to CD40-negative 293 cells by Ad5Luc.FF/CD40L.F5Δ was also observed, raising concerns that the incorporation of the mutant Ad5 fiber could result in binding of the mosaic vector to cell surface molecules other than CD40 and CAR. These concerns were ruled out by a subsequent cell-binding experiment in which radiolabeled Ad vectors were allowed to bind to either 293 or 293.CD40 cells under conditions preventing the internalization of the viruses.
FIG. 8.
Comparison of the gene transfer and cell-binding profiles of Ad5 vectors. (A) Transduction of 293 and 293.CD40 cells with Ad5Luc1, Ad5Luc.FF/CD40L, or Ad5Luc.FF/CD40L.F5Δ was done essentially as described in the legend to Fig. 6, except that no inhibition of infection was attempted. (B) Radiolabeled viruses were allowed to bind to cells under conditions which prevented their internalization. After unbound virus was removed, the cell-associated radioactivity was measured with a scintillation counter. All of the measurements were done in triplicate. part/cell, particles per cell.
The data shown in Fig. 8B demonstrate that the binding of Ad5Luc.FF/CD40L.F5Δ virions to both 293 and 293.CD40 cells was indistinguishable from that of Ad5Luc.FF/CD40L particles, which lack the mutant fiber. Therefore, the observed increase in the infectivity of the mosaic virus was not due to enhanced binding to cells, and its target receptor specificity thus was not compromised.
CD40-targeted Ad vectors demonstrate increased infectivity for DCs and cancerous cells.
The experiments carried out with 293.CD40 cells demonstrated the CD40-dependent profile of infection by the targeted viruses containing the FF/CD40L protein as well as its efficacy, suggesting that, compared to the unmodified Ad5 vector, these viruses may be even more efficient in delivering transgenes to human cells which are CD40 positive but express CAR at low levels.
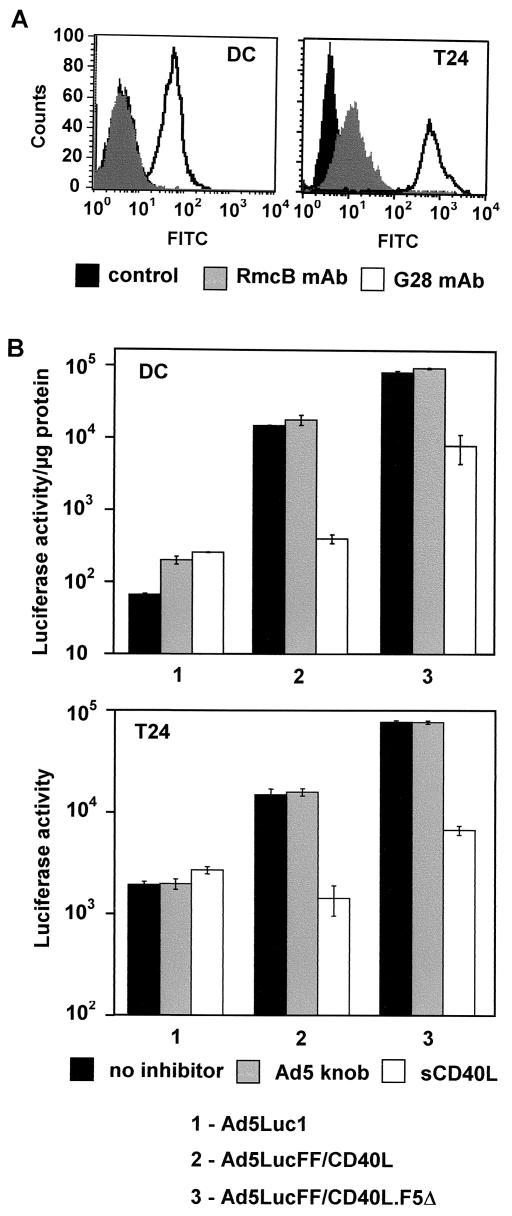
First, we tested these vectors on human DCs which had been shown previously to express CAR poorly but to be CD40 positive (42). These phenotypic features were also supported by our own FACS data (Fig. 9A). The target DCs were derived from CD14-positive monocytes isolated from peripheral human blood by the standard procedure used at the UAB Cell Processing Facility. These cells were transduced in vitro by each of the three Ad vectors, Ad5Luc1, Ad5Luc.FF/CD40L, and Ad5Luc.FF/CD40L.F5Δ, followed by a reporter expression assay. Luciferase activity in DCs treated with control vector Ad5Luc1 was at the background level, indicating the lack of any detectable transduction (Fig. 9B). In good agreement with the previously observed infectivities of CD40-targeted vectors for 293.CD40 cells, these viruses transduced DCs at much higher rates. Specifically, Ad5Luc.FF/CD40L- and Ad5Luc.FF/CD40L.F5Δ-infected DCs expressed the reporter at levels 220- and 1,250-fold higher than Ad5Luc1-treated cells.
FIG. 9.
CD40-mediated transduction of human cells with Ad5Luc.FF/CD40L. (A) The expression of CAR and CD40 by humanmonocyte-derived DCs and T24 bladder cancer cells was assessed by flow cytometry with relevant antibodies (see Materials and Methods for details). Of note, the control peak on the left panel overlaps the RmcB-generated signal and thus is not seen. (B) The infectivities of the targeted vectors, Ad5Luc.FF/CD40L and Ad5Luc.FF/CD40L.F5Δ, and that of the control vector, Ad5Luc1, were compared. Gray and white bars show samples blocked with 100 μg each of the Ad5 fiber knob and sCD40L/ml, respectively, prior to addition of the virus. Black bars correspond to luciferase activity in cells infected in the absence of inhibitors. Error bars indicate standard deviations from the average reporter activity seen in three identical samples.
CD40-targeted vectors also proved to be more efficient in transducing T24 bladder cancer cells, whose CD40-positive phenotype and low level of CAR expression have been reported elsewhere (17, 47) and also confirmed by us (Fig. 9A). However, in this instance, the difference in gene transfer between targeted and nontargeted vectors was less pronounced (Fig. 9B). As before, Ad5Luc.FF/CD40L.F5Δ was the most efficient, resulting in expression of the reporter at a level 40-fold higher than that obtained with control vector Ad5Luc1.
Consistent with the design of the targeted Ad vectors and our previous findings with 293.CD40 cells, transduction of both DCs and T24 cells by these viruses was efficiently inhibited by sCD40L while being unaffected by the Ad5 fiber knob. These additional data further confirmed the CAR-independent, CD40-mediated pathway of infection exploited by CD40L-modified Ad during cell entry.
DISCUSSION
Human CD40 is a type I transmembrane protein that belongs to the family of TNF receptors (reviewed in reference 48). It was discovered as a surface marker on B cells, where it activates proliferation, differentiation, and immunoglobulin production and induces the reexpression of telomerase activity in memory B cells to prolong their life span. Subsequently, the expression of CD40 was detected in DCs as well as in human tumors, including those of the breasts (14, 52), bladder (17), and ovaries (9, 12). Its involvement in the generation of the immune response and its localization on the cell surface make CD40 an attractive target receptor for therapeutic Ad vectors. Such vectors could be used either for genetic immunization against cancer or infectious agents or for the selective destruction of tumors by means of tumor-restricted virus replication. This particular concept of Ad targeting is further supported by the fact that the aforementioned types of CD40-positive cells are known to express CAR poorly and thus are infected by regular Ad5-based vectors inefficiently (7, 12, 18, 37, 42, 47).
Early attempts to realize this concept focused on the development of CD40-targeted Ad vectors whose tropism was modified by using the so-called protein bridge approach. In those instances, the capsid of Ad was not altered, and targeting of the vector to CD40 occurred through its association with either bispecific Ab conjugates comprising an anti-Ad Ab cross-linked with an anti-CD40 Ab (6, 42, 43) or a recombinant protein incorporating the extracellular portion of human CAR genetically fused with an anti-CD40 single-chain Ab (35). While efficient from the targeting standpoint, this approach proved to be technically cumbersome, as it required the production of several components of the vector complex, their conjugation and/or association to form the targeted vector complex, and additional purification steps.
These problems could be avoided if a CD40-specific vector could be designed by the genetic modification of Ad tropism, resulting in a one-component, self-replicative gene therapy agent. The existence of a natural ligand for CD40, CD40L, appears to facilitate this task. Furthermore, the high degree of similarity in the overall structures of the CD40L and Ad fiber proteins (5, 48) makes this approach even more attractive by suggesting that targeting protein chimeras comprising functional domains derived from each of the two proteins could be designed to target the vector. Of note is that both molecules consist of three major structural domains, whose order within the proteins is the same and whose functions parallel each other. Specifically, the N termini of each of the proteins function as anchoring moieties, the C-terminal domains are involved in receptor recognition, and the rod-like domains located in the middle serve to extend the receptor-binding domains away from either the cell surface (CD40L) or the virion (Ad fiber). However, the concept of targeting of Ad to CD40 with CD40L is best supported by the remarkable structural similarity between the TNF-like domain of CD40L and the Ad fiber knob domain (19, 49, 54). Both domains are trimeric and folded as β sandwiches, each formed by the two layers of β strands and similarly oriented relative to the threefold symmetry axis. This structural similarity translates into similar modes of interaction of CD40L and the Ad fiber with their cognate receptors.
Based on these considerations, we first attempted the design of CD40-targeted Ad vectors by direct replacement of the Ad5 fiber knob with the TNF-like domain of human CD40L. However, the results of these studies were largely negative: not only did all of our fiber-CD40L chimeras fail to trimerize, but also their transient expression in Ad5-infected cells prevented the assembly of progeny virions, making the rescue of the targeted vectors impossible (unpublished data). We have considered two hypotheses to explain this failure. First, the structure of the engineered fiber-CD40L proteins could be compromised by incorrect folding of the TNF-like domain of CD40L upon fusion with the fiber and localization to the cell nucleus. In this regard, it should be noted that cysteine residues at positions 83 and 123 of human CD40L are linked by a disulfide bond, whose formation requires the oxidative environment of the endoplasmic reticulum, as well as the involvement of disulfide isomerases. Whereas these requirements are met during the biosynthesis of native CD40L, they cannot be fulfilled upon fusion with the Ad fiber due to the nuclear localization of the resultant chimera caused by the nuclear localization signal present in the fiber tail (15). Therefore, the reducing environment of the nucleoplasm and the lack of disulfide isomerases in the nucleus could compromise the proper folding of the CD40L component of the targeting protein. The recently reported failures in the modification of Ad tropism with disulfide-constrained ligands, epidermal growth factor, and single-chain antibodies (28) clearly support this view.
The other potential cause of the problem with the fiber-CD40L fusion was that the stability of the trimeric TNF-like domain of CD40L was insufficient to maintain the integrity of the entire fusion, resulting in a monomeric protein which could not be incorporated into the Ad virion. This hypothesis is supported, albeit indirectly, by previous findings on the contribution of the CD40L stalk domain to the stability of the molecule (41) and the improved CD40 binding of recombinant sCD40L stabilized with GCN4 peptide (33). Furthermore, these reports suggested that the solution for the problem with the fiber-CD40L fusion may involve increasing the stability of the targeting protein chimera through the incorporation into its design of an additional stabilizing moiety.
To test these concepts, the present work started with the assessment of the contribution of the disulfide bond present within CD40L to the folding and functionality of this protein. Our data on the CD40-binding capacity of recombinant sCD40L proteins lacking Cys83 and Cys123 showed that this bond is not essential for the interaction of CD40L with CD40. As this finding proved that our hypothesis of improper folding of CD40L was incorrect, we next chose to improve the stability of the targeting protein chimera by making a triple fusion molecule comprising the previously designed FF backbone (21) and the TNF domain of CD40L. Upon expression and purification, the resultant FF/CD40L protein showed the expected trimerization properties and the ability to bind cell-anchored CD40.
We next demonstrated that the incorporation of the FF/CD40L chimera into the Ad virion does not affect the functional structure of its CD40-binding component, resulting in a vector capable of infecting target cells through a CD40-mediated pathway. However, comparison of the CD40-targeted virus with untargeted Ad containing wild-type fibers showed an unfavorable 40-fold difference in transduction efficiency on 293.CD40 cells, which express CAR and CD40 at high levels. Simultaneously, our experiments with radiolabeled Ad5LucFF/CD40L and Ad5Luc1 revealed that the binding of both viruses to 293.CD40 cells was equally efficient. That result led us to the hypothesis that complete deletion of the fiber in Ad5LucFF/CD40L affected its ability to accomplish a step in the infection process downstream from primary binding to the cell surface. For instance, this deletion could affect the dynamics of the escape of the virus from the endosome following internalization, as well as its intracellular trafficking. Previously published findings on the altered intracellular migration of Ad5 virions incorporating Ad serotype 7 fibers provide reasonable grounds for such an explanation (31, 32). To test this hypothesis, we constructed a mosaic version of Ad5LucFF/CD40L which, in addition to the FF/CD40L chimera, also contained an Ad5 fiber protein unable to bind to CAR due to a mutation in the knob domain. The presence of this mutated fiber protein indeed increased the infectivity of the CD40-targeted vector to the level seen for Ad5Luc1.
Subsequent use of Ad5LucFF/CD40L bearing either FF/CD40L alone or in combination with the mutated Ad5 fiber protein showed the superior efficacy of this vector on human monocyte-derived DCs, suggesting that it may serve as a prototype for the derivation of therapeutic vectors for genetic immunization. For instance, such vectors could be used ex vivo or in vivo for directed delivery of antigen-encoding genes to human DCs to induce the development of an antigen-specific immune response. Similarly, the fact that Ad5LucFF/CD40L proved to be far more efficacious than Ad5Luc1 in transducing human bladder tumor cells suggests that its conditionally replicative derivatives would be rational choices as gene therapeutic agents for fighting this type of cancer.
The present work shows for the first time that the Ad fiber replacement strategy may be used to derive efficient and selective Ad vectors targeted to specific cell surface molecules expressed by target cells. This approach makes possible the use of complex polypeptide ligands for Ad targeting and thus expands the range of target receptors to be exploited by therapeutic vectors.
Acknowledgments
We thank Ivan Stamenkovic for making the cDNA of human CD40 available to us. We are grateful to Joanne T. Douglas for help in preparing the manuscript.
This work was supported by a research contract with EMD Lexigen Research Center and Merck KGaA and by NIH grants P50 CA89019, 1R41CA 91608-01, and R01 CA86881. David T. Curiel is an equity holder in VectorLogics, Inc.
REFERENCES
- 1.Alemany, R., C. Balague, and D. T. Curiel. 2000. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 18:723-727. [DOI] [PubMed] [Google Scholar]
- 2.Belousova, N., V. Krendelchtchikova, D. T. Curiel, and V. Krasnykh. 2002. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J. Virol. 76:8621-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Chartier, C., E. Degryse, M. Gantzer, A. Dieterle, A. Pavirani, and M. Mehtali. 1996. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70:4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chroboczek, J., R. W. Ruigrok, and S. Cusack. 1995. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 199:163-200. [DOI] [PubMed] [Google Scholar]
- 6.de Gruijl, T. D., S. A. Luykx-de Bakker, B. W. Tillman, A. J. van den Eertwegh, J. Buter, S. M. Lougheed, G. J. van der Bij, A. M. Safer, H. J. Haisma, D. T. Curiel, R. J. Scheper, H. M. Pinedo, and W. R. Gerritsen. 2002. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J. Immunol. 169:5322-5331. [DOI] [PubMed] [Google Scholar]
- 7.Dietz, A. B., and S. Vuk-Pavlovic. 1998. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood 91:392-398. [PubMed] [Google Scholar]
- 8.Dmitriev, I., V. Krasnykh, C. R. Miller, M. Wang, E. Kashentseva, G. Mikheeva, N. Belousova, and D. T. Curiel. 1998. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 72:9706-9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghamande, S., B. L. Hylander, E. Oflazoglu, S. Lele, W. Fanslow, and E. A. Repasky. 2001. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 61:7556-7562. [PubMed] [Google Scholar]
- 10.Graham, F. L., and L. Prevec. 1991. Manipulation of adenovirus vectors. Methods Mol. Biol. 7:109-128. [DOI] [PubMed]
- 11.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 12.Hakkarainen, T., A. Hemminki, A. V. Pereboev, S. D. Barker, C. K. Asiedu, T. V. Strong, A. Kanerva, J. Wahlfors, and D. T. Curiel. 2003. CD40 is expressed on ovarian cancer cells and can be utilized for targeting adenoviruses. Clin. Cancer Res. 9:619-624. [PubMed] [Google Scholar]
- 13.Henry, L. J., D. Xia, M. E. Wilke, J. Deisenhofer, and R. D. Gerard. 1994. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J. Virol. 68:5239-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano, A., D. L. Longo, D. D. Taub, D. K. Ferris, L. S. Young, A. G. Eliopoulos, A. Agathanggelou, N. Cullen, J. Macartney, W. C. Fanslow, and W. J. Murphy. 1999. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood 93:2999-3007. [PubMed] [Google Scholar]
- 15.Hong, J. S., and J. A. Engler. 1991. The amino terminus of the adenovirus fiber protein encodes the nuclear localization signal. Virology 185:758-767. [DOI] [PubMed] [Google Scholar]
- 16.Hong, J. S., and J. A. Engler. 1996. Domains required for assembly of adenovirus type 2 fiber trimers. J. Virol. 70:7071-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobson, E., G. Jonsson, P. Bjorck, and S. Paulie. 1998. Stimulation of CD40 in human bladder carcinoma cells inhibits anti-Fas/APO-1 (CD95)-induced apoptosis. Int. J. Cancer 77:849-853. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan, J. M., Q. Yu, S. T. Piraino, S. E. Pennington, S. Shankara, L. A. Woodworth, and B. L. Roberts. 1999. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J. Immunol. 163:699-707. [PubMed] [Google Scholar]
- 19.Karpusas, M., Y. M. Hsu, J. H. Wang, J. Thompson, S. Lederman, L. Chess, and D. Thomas. 1995. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure 3:1426. [PubMed] [Google Scholar]
- 20.Kim, M., K. R. Zinn, B. G. Barnett, L. A. Sumerel, V. Krasnykh, D. T. Curiel, and J. T. Douglas. 2002. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur. J. Cancer 38:1917-1926. [DOI] [PubMed] [Google Scholar]
- 21.Krasnykh, V., N. Belousova, N. Korokhov, G. Mikheeva, and D. T. Curiel. 2001. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J. Virol. 75:4176-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasnykh, V., and J. T. Douglas. 2002. Targeted adenoviral vectors. I. Transductional targeting, p. 205-245. In D. T. Curiel and J. T. Douglas (ed.), Adenoviral vectors for gene therapy. Academic Press, Inc., San Diego, Calif.
- 23.Krasnykh, V. N., J. T. Douglas, and V. W. van Beusechem. 2000. Genetic targeting of adenoviral vectors. Mol. Ther. 1:391-405. [DOI] [PubMed] [Google Scholar]
- 24.Krasnykh, V. N., G. V. Mikheeva, J. T. Douglas, and D. T. Curiel. 1996. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 70:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledbetter, J. A., and E. A. Clark. January 1993. Ligands and methods for augmenting B-cell proliferation. U.S. patent 5,128,368.
- 26.Li, Y., R. C. Pong, J. M. Bergelson, M. C. Hall, A. I. Sagalowsky, C. P. Tseng, Z. Wang, and J. T. Hsieh. 1999. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 59:325-330. [PubMed] [Google Scholar]
- 27.Magnusson, M. K., S. S. Hong, P. Boulanger, and L. Lindholm. 2001. Genetic retargeting of adenovirus: novel strategy employing “deknobbing” of the fiber. J. Virol. 75:7280-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnusson, M. K., S. See Hong, P. Henning, P. Boulanger, and L. Lindholm. 2002. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J. Gene Med. 4:356-370. [DOI] [PubMed] [Google Scholar]
- 29.Maizel, J. V., Jr., D. O. White, and M. D. Scharff. 1968. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36:115-125. [DOI] [PubMed] [Google Scholar]
- 30.Miller, C. R., D. J. Buchsbaum, P. N. Reynolds, J. T. Douglas, G. Y. Gillespie, M. S. Mayo, D. Raben, and D. T. Curiel. 1998. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 58:5738-5748. [PubMed] [Google Scholar]
- 31.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris, A. E., R. L. Remmele, Jr., R. Klinke, B. M. Macduff, W. C. Fanslow, and R. J. Armitage. 1999. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154). J. Biol. Chem. 274:418-423. [DOI] [PubMed] [Google Scholar]
- 34.Okegawa, T., Y. Li, R. C. Pong, J. M. Bergelson, J. Zhou, and J. T. Hsieh. 2000. The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 60:5031-5036. [PubMed] [Google Scholar]
- 35.Pereboev, A. V., C. K. Asiedu, Y. Kawakami, S. S. Dong, J. L. Blackwell, E. A. Kashentseva, P. L. Triozzi, W. A. Aldrich, D. T. Curiel, J. M. Thomas, and I. P. Dmitriev. 2002. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 9:1189-1193. [DOI] [PubMed] [Google Scholar]
- 36.Pullen, S. S., M. E. Labadia, R. H. Ingraham, S. M. McWhirter, D. S. Everdeen, T. Alber, J. J. Crute, and M. R. Kehry. 1999. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 38:10168-10177. [DOI] [PubMed] [Google Scholar]
- 37.Rea, D., F. H. Schagen, R. C. Hoeben, M. Mehtali, M. J. Havenga, R. E. Toes, C. J. Melief, and R. Offringa. 1999. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J. Virol. 73:10245-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing Adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 39.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamenkovic, I., E. A. Clark, and B. Seed. 1989. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 8:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su, L., E. A. Garber, and Y. M. Hsu. 2001. CD154 variant lacking tumor necrosis factor homologous domain inhibits cell surface expression of wild-type protein. J. Biol. Chem. 276:1673-1676. [DOI] [PubMed] [Google Scholar]
- 42.Tillman, B. W., T. D. de Gruijl, S. A. Luykx-de Bakker, R. J. Scheper, H. M. Pinedo, T. J. Curiel, W. R. Gerritsen, and D. T. Curiel. 1999. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J. Immunol. 162:6378-6383. [PubMed] [Google Scholar]
- 43.Tillman, B. W., T. L. Hayes, T. D. DeGruijl, J. T. Douglas, and D. T. Curiel. 2000. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 60:5456-5463. [PubMed] [Google Scholar]
- 44.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Triozzi, P. L., R. Khurram, W. A. Aldrich, M. J. Walker, J. A. Kim, and S. Jaynes. 2000. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer 89:2646-2654. [DOI] [PubMed] [Google Scholar]
- 46.van Beusechem, V. W., A. L. van Rijswijk, H. H. van Es, H. J. Haisma, H. M. Pinedo, and W. R. Gerritsen. 2000. Recombinant adenovirus vectors with knobless fibers for targeted gene transfer. Gene Ther. 7:1940-1946. [DOI] [PubMed] [Google Scholar]
- 47.van der Poel, H. G., B. Molenaar, V. W. van Beusechem, H. J. Haisma, R. Rodriguez, D. T. Curiel, and W. R. Gerritsen. 2002. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J. Urol. 168:266-272. [DOI] [PubMed] [Google Scholar]
- 48.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]
- 49.van Raaij, M. J., N. Louis, J. Chroboczek, and S. Cusack. 1999. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 A resolution. Virology 262:333-343. [DOI] [PubMed] [Google Scholar]
- 50.Von Seggern, D. J., S. Huang, S. K. Fleck, S. C. Stevenson, and G. R. Nemerow. 2000. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J. Virol. 74:354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wickham, T. J., E. Tzeng, L. L. Shears II, P. W. Roelvink, Y. Li, G. M. Lee, D. E. Brough, A. Lizonova, and I. Kovesdi. 1997. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 71:8221-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wingett, D. G., R. E. Vestal, K. Forcier, N. Hadjokas, and C. P. Nielson. 1998. CD40 is functionally expressed on human breast carcinomas: variable inducibility by cytokines and enhancement of Fas-mediated apoptosis. Breast Cancer Res. Treat. 50:27-36. [DOI] [PubMed] [Google Scholar]
- 53.Worn, A., and A. Pluckthun. 1998. An intrinsically stable antibody scFv fragment can tolerate the loss of both disulfide bonds and fold correctly. FEBS Lett. 427:357-361. [DOI] [PubMed] [Google Scholar]
- 54.Xia, D., L. J. Henry, R. D. Gerard, and J. Deisenhofer. 1994. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 2:1259-1270. [DOI] [PubMed] [Google Scholar]
- 55.Zeng, G. 1998. Sticky-end PCR: new method for subcloning. BioTechniques 25:206-208. [DOI] [PubMed] [Google Scholar]