Abstract
Surface proteins of Staphylococcus aureus are linked to the bacterial cell wall by sortase, an enzyme that cleaves polypeptides at the threonine of the LPXTG motif. Surface proteins can be released from staphylococci by treatment with hydroxylamine, resulting in the formation of threonine hydroxamate. Staphylococcal extracts, as well as purified sortase, catalyze the hydroxylaminolysis of peptides bearing an LPXTG motif, a reaction that can be inhibited with sulfhydryl-modifying reagents. Replacement of the single conserved cysteine at position 184 of sortase with alanine abolishes enzyme activity. Thus, sortase appears to catalyze surface-protein anchoring by means of a transpeptidation reaction that captures cleaved polypeptides as thioester enzyme intermediates.
Surface proteins of Staphylococcus aureus are anchored to the bacterial cell wall by a mechanism requiring a C-terminal sorting signal with a conserved LPXTG motif (1). Cleavage between the threonine and the glycine of the LPXTG motif liberates the carboxyl of threonine to form an amide bond with the amino group of the pentaglycine crossbridge (2, 3), thereby tethering the C terminus end of surface proteins to the bacterial peptidoglycan (4–7). The LPXTG motif is conserved in more than 100 surface proteins of Gram-positive pathogens, suggesting that anchoring of these polypeptides occurs by a universal mechanism (1). Although surface protein anchoring can be followed by pulse-labeling polypeptides in vivo (8, 9), the transpeptidation reaction has thus far not been measured in vitro. Furthermore, sortase, the enzyme that is thought to catalyze this reaction, has not yet been purified and characterized.
To identify the sortase gene, we recently screened a collection of temperature-sensitive staphylococcal mutants for a defect in surface-protein anchoring (cell wall sorting) (10). A mutant S. aureus strain that displayed a severe sorting defect was transformed with a plasmid library of staphylococcal genomic DNA, and individual clones were screened for complementation of surface-protein anchoring. One gene, named srtA (surface protein sorting A), complemented the sorting defect of the temperature-sensitive variant (10). Overexpression of srtA in wild-type staphylococci was sufficient to increase the rate of surface-protein anchoring, suggesting that SrtA is involved in the cell wall sorting reaction (10). Nevertheless, this work left unresolved whether srtA encodes sortase, the enzyme that has been proposed to catalyze the transpeptidation reaction that links surface proteins to the staphylococcal cell wall.
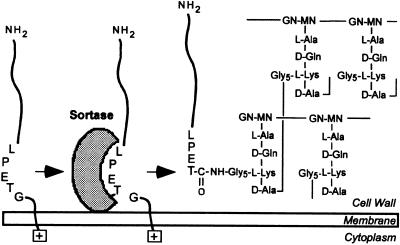
Surface-protein anchoring in S. aureus can be inhibited with sulfhydryl-modifying reagents such as methanethiosulfonate and organic mercurial, indicating that sortase must be a sulfhydryl (cysteine)-containing enzyme (11). We wondered whether staphylococcal sortase captures surface proteins after their cleavage at the LPXTG motif as acyl-enzyme intermediates. In this model, the sulfhydryl of sortase may function as a nucleophile at the peptide bond between threonine and glycine, thereby forming a thioester with the carboxyl of threonine and releasing the amino group of the cleaved C-terminal-sorting signal (Fig. 1). If so, an acyl-enzyme intermediate between surface proteins and sortase should be sensitive to hydroxylaminolysis (12, 13). Lipmann first used hydroxylamine (NH2OH) to demonstrate the existence of acyl-enzyme intermediates, as this strong nucleophile attacks thioester bonds to form hydroxamate with carboxyl groups, thereby regenerating the enzyme active-site sulfhydryl (14). We show here that hydroxylamine treatment causes the formation of a C-terminal threonine hydroxamate of surface proteins, which are thereby released into the culture medium. Staphylococcal extracts, as well as purified SrtA, catalyze the hydroxylaminolysis of peptides bearing an LPXTG motif. Replacement of the single conserved cysteine with alanine abolished enzymatic activity, suggesting that sortase catalyzes a transpeptidation reaction by means of the formation of a thioester acyl-enzyme intermediate.
Figure 1.
Sortase catalyzes the anchoring of surface proteins to the staphylococcal cell wall. Surface proteins bearing sorting signals are cleaved between the threonine (T) and the glycine (G) of their LPXTG motif, and the carboxyl of threonine is amide-linked to the amino of the pentaglycine crossbridge within the staphylococcal cell wall. GN-MN, N-acetylglucosamine-N-acetylmuramic acid.
Experimental Procedures
Hydroxylaminolysis of Surface Proteins in Vivo.
S. aureus OS2 (spa−:ermC) or S. aureus BB270 (wild type) carrying a plasmid encoding staphylococcal enterotoxin B (SEB) fused to a fragment of staphylococcal protein A (SPA)490–524 (5, 9) were grown in minimal medium until OD600 reached 0.5. A 1-ml aliquot of the staphylococci culture (109 cells) was pulse-labeled with 100 μCi of Pro-Mix for 1 min, chase solution (50 μl of 100 mg/ml casamino acids, 20 mg/ml methionine and cysteine) was added, and cells were incubated at 37°C for 5 min. A 0.5-ml aliquot was centrifuged at 15,000 × g for 5 min, and the supernatant was precipitated with 0.5 ml of 10% trichloroacetic acid (TCA). Another 0.5-ml culture aliquot was directly precipitated with 0.5 ml of 10% TCA and suspended in 1 ml of 0.5 M Tris⋅HCl (pH 7.0), and peptidoglycan was digested with 100 μg of lysostaphin (15) for 1 hr at 37°C. Proteins were precipitated with TCA, washed in acetone, dried and then boiled in SDS. Aliquots were subjected to immunoprecipitation with anti-SEB and analyzed by PAGE and PhosphorImager (Molecular Dynamics).
Purification of Surface Proteins.
Staphylococci [1013 BB270(pSEB-MH6-CWS) cells] (5) were incubated in 200 ml of 50 mM Tris⋅HCl (pH 7.0), with or without 0.1 M NH2OH, for 60 min. Samples were centrifuged at 10,000 × g for 15 min, and the supernatants were subjected to affinity chromatography on a nickel nitrilotriacetic acid (Ni-NTA) column and eluted with 0.5 M imidazole. SEB-MH6-CWS was precipitated with trifluoroacetic acid (TFA), washed in acetone, dried, and cleaved with cyanogen bromide (CNBr) in 70% formic acid (5). C-terminal peptides were suspended in 6 M guanidine⋅hydrochloride (pH 8.0), purified by affinity chromatography on Ni-NTA, eluted in 4 ml of 6 M guanidine⋅hydrochloride and 0.2 M acetic acid, desalted over a C18 cartridge, and dried. Pellets were solubilized in 50 μl of buffer B [8 M urea/0.1 M NaH2PO4/0.01 M Tris⋅HCl (pH 8.0)] and subjected to RP-HPLC on a C18 column with a linear gradient from 1% to 90% acetonitrile (CH3CN) in 0.1% TFA in 90 min. Matrix-assisted laser desorption ionization MS (MALDI-MS), electrospray ionizaton MS (ESI-MS), and affinity chromatography were performed as previously described (4).
Hydroxylaminolysis of LPXTG Peptide in Staphylococcal Extracts.
Staphylococci [1013 S. aureus OS2 cells in 50 ml 50 mM Tris⋅HCl (pH 7.5)] were disrupted in a bead-beater instrument, and the crude extract was centrifuged at 1,500 × g for 15 min to remove beads and unbroken cells. A 10-μl aliquot of the supernatant (10 mg/ml protein) was used as enzyme preparation. Reactions were assembled in a volume of 260 μl containing 50 mM Tris⋅HCl and 150 mM NaCl (pH 7.5). The concentration of LPXTG peptide substrate 4-(4-dimethylaminophenylazo)benzoic acid (Dabcyl)-QALPETGEE-5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid (Edans) was 10 μM; of p-hydroxymecuribenzoic acid (pHMB) was 5 mM; and of NH2OH was 0.2 M. Incubations were carried out for 1 hr at 37°C, followed by centrifugation at 15,000 × g for 5 min. Supernatant was analyzed in a fluorometer using 350 nm for excitation and 495 nm for recordings.
Purification of SrtAΔN Protein.
The primers orf6N-ds-B (5′-AAAGGATCCAAACCACATATCGATAATTATC-3′) and orf6C-B (5′-AAGGATCCTTATTTGACTTCTGTAGCTACAA-3′) were used to PCR amplify the srtA sequence from the chromosome of S. aureus OS2. The DNA fragment was digested with BamHI, inserted into pQE30 (Qiagen, Chatsworth, CA)-cut BamHI to generate pHTT14, transformed into Escherichia coli XL-1 Blue, and selected on Luria agar with ampicillin (100 μg/ml). E. coli XL-1 Blue (pHTT14) (1012 cells) in 30 ml of buffer C [50 mM Tris⋅HCl/150 mM NaCl/10% glycerol (pH 7.2)] were lysed in a French pressure cell at 14,000 psi (1 psi = 6.89 kPa). The extract was centrifuged at 29,000 × g for 30 min, and the supernatant was applied to 1 ml of Ni-NTA resin, preequilibrated with buffer C. The column was washed with 40 ml of buffer C, and SrtAΔN protein was eluted in 4 ml of buffer C with 0.5 M imidazole at a concentration of 3 μg/μl. The mutant protein SrtAΔN, C184A was generated by PCR amplification of pHTT14 with the primers C184A-1 (5′-CAATTAACATTAATTACTGCTGATGATTACAATGAAAAG-3′) and C184A-2 (5′-CTTTTCATTGTAATCATCAGCAGTAATTAATGTTAATTG-3′). The PCR product was digested with DpnI and transformed into E. coli XL-1 Blue, and the mutant plasmid pHTT16 was isolated and analyzed. When purified proteins were subjected to ESI-MS, we observed an average mass of 22139.02 Da (±2.37 Da) (SrtAΔN) and 22103.22 Da (±3.05 Da) (SrtAΔN, C184A), respectively. These measurements are consistent with the predicted average mass of SrtAΔN (22133.93 Da) and SrtAΔN, C184A (22101.86 Da).
Hydroxylaminolysis of LPXTG Peptide by Purified SrtAΔN.
Reactions were assembled in a volume of 260 μl containing 50 mM Tris⋅HCl buffer, 150 mM NaCl (pH 7.5), and, as indicated, 5 μM SrtAΔN in 50 mM Tris⋅HCl (pH 7.5), 10 μM LPXTG peptide (Dabcyl-QALPETGEE-Edans), 10 μM GLXTP peptide (Dabcyl-QAGLETPEE-Edans), 5 mM [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET), 0.2 M NH2OH, 5 mM pHMB, or 10 mM DTT. Incubations were carried out for 1 hr at 37°C. Samples were analyzed in a fluorometer using 350 nm for excitation and 495 nm for recordings.
Results
Hydroxylamine Release of Surface Proteins into the Medium of S. aureus Cultures.
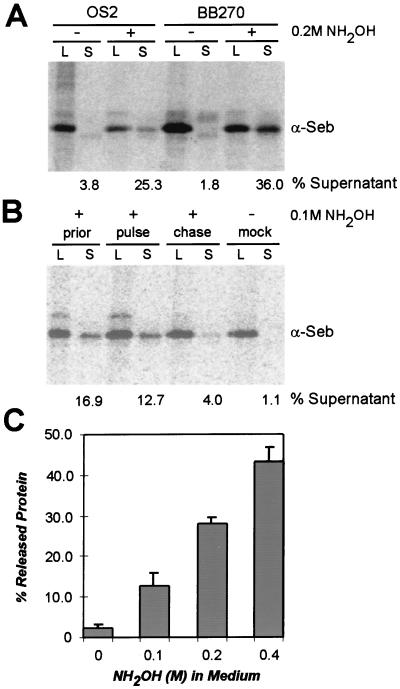
To examine whether hydroxylamine can release surface proteins from cell-bound acyl-enzyme intermediate complexes, staphylococci were pulse-labeled with [35S]methionine in either the presence or absence of 0.2 M NH2OH (Fig. 2). Labeled cultures were divided into two aliquots. One sample was centrifuged, and proteins released into the culture medium were recovered in the supernatant (S). The other culture aliquot was treated with lysostaphin (L) (15), an endopeptidase that cuts the pentaglycine crossbridges of peptidoglycan and releases all cell wall-anchored surface protein of staphylococci (9). Surface proteins (SEB-SPA490–524) of mock-treated staphylococci were cell wall anchored; however, treatment with 0.2 M NH2OH caused 25.3% of all labeled SEB-SPA490–524 to be released into the extracellular medium (Fig. 2A). This observation was not strain-specific, as S. aureus OS2 (spa−:ermC) and S. aureus BB270 (wild type) displayed similar amounts of surface protein release.
Figure 2.
Release of surface protein from S. aureus by treatment with hydroxylamine. (A) Surface-protein (SEB-SPA490–524) anchoring to the bacterial cell wall was measured by pulse-labeling staphylococci with [35S]methionine in duplicate. One sample was precipitated with TCA, and anchored surface protein was released by lysostaphin-digestion of the cell wall envelope (L). The other sample was centrifuged, and surface protein released into the culture medium (S, supernatant) was precipitated with TCA. Surface protein was quantified by immunoprecipitation with anti-SEB (α-Seb), followed by SDS/PAGE and PhosphorImager analysis. Surface protein release was measured as the percent of SEB-SPA490–524 in the culture medium, as compared with the total amount after lysostaphin solubilization. Hydroxylamine (0.2 M NH2OH) was added 15 sec prior to labeling of S. aureus strains OS2 (spa−:ermC) or BB270 (wild type). (B) Hydroxylamine was added 15 sec prior to labeling (prior), during pulse-labeling (pulse), or 5 min after the addition of the chase to S. aureus OS2 cultures (chase). (C) Increasing amounts of hydroxylamine added 15 sec prior to labeling of S. aureus OS2 cultures caused increasing amounts of surface-protein release.
Hydroxylaminolysis of acyl-enzyme intermediates should occur only during or shortly after the pulse-labeling of staphylococci, as SEB-SPA490–524 is rapidly anchored to the cell wall (9). Addition of hydroxylamine either before or during the pulse with [35S]methionine released surface proteins into the extracellular medium (16.9% and 12.7%, respectively) (Fig. 2B). Very little SEB-SPA490–524 was detected in the culture medium when hydroxylamine was added after the pulse (4%), indicating that hydroxylaminolysis occurs during surface protein sorting to the cell wall, but does not affect mature, anchored polypeptides. Increasing the amount of hydroxylamine prior to pulse-labeling caused increased amounts of surface protein to be released into the culture medium (Fig. 2C). Addition of 0.5 M or greater amounts of NH2OH prevented metabolic labeling of staphylococci with [35S]methionine (data not shown).
Hydroxylaminolysis Causes the Formation of C-Terminal Threonine Hydroxamate.
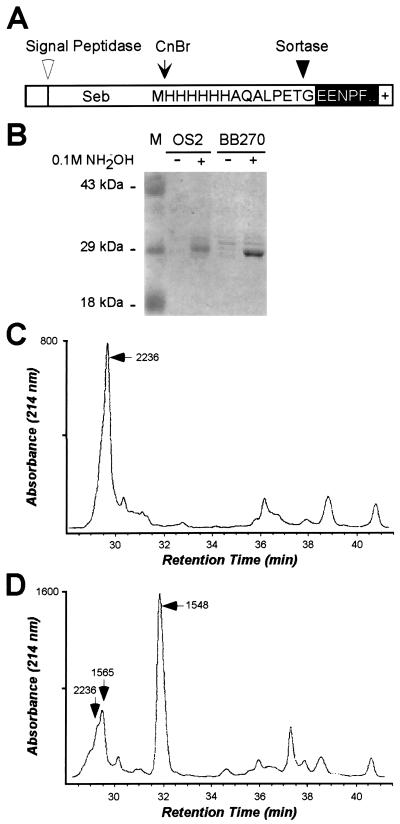
If sortase acyl-enzyme intermediates can be resolved with NH2OH, the released surface proteins should contain hydroxamate at the threonine of the LPXTG motif. To test this prediction, 1013 staphylococci expressing the surface protein SEB-MH6-CWS (4) were incubated in the presence or absence of 0.1 M NH2OH. The cells were sedimented by centrifugation, and SEB-MH6-CWS (Fig. 3A) was purified from the culture medium by affinity chromatography on Ni-NTA. Coomassie-stained SDS/PAGE revealed the presence of released surface protein (Fig. 3B). Polypeptides were cleaved at methionine with CNBr, and C-terminal peptides containing the H6 tag were purified by a second affinity chromatography step on Ni-NTA (4). RP-HPLC of peptides recovered from mock-treated bacteria revealed an absorbance peak at 30 min (25% CH3CN) (Fig. 3C), which, when examined by ESI-MS, contained a compound of 2236 Da. This measurement is consistent with the structure of NH2-l-Ala-d-iGln-l-Lys(NH2-H6AQALPET-Gly5)-d-Ala-COOH (predicted mass 2235), a C-terminal peptide that is likely the product of cell wall degradation and, together with other peptidoglycan fragments, released into the culture medium (1).
Figure 3.
Characterization of surface protein released from staphylococci by hydroxylamine treatment. (A) The drawing shows the primary structure of SEB-MH6-CWS and the cleavage sites for signal peptidase, cyanogen bromide (CnBr), and sortase. (B) Staphylococci (1013 cells of strains BB270 or OS2) expressing the surface protein SEB-MH6-CWS were incubated in either presence (+) or absence (−) of 0.1 M NH2OH. Cells were sedimented by centrifugation, and the supernatant was subjected to affinity purification on Ni-NTA resin. Surface protein was eluted with imidazole and analyzed on Coomassie-stained SDS/PAGE. The position of molecular mass standards (M) is indicated in kDa. (C) Purified SEB-MH6-CWS surface protein released from mock-treated S. aureus BB270 cells was cleaved with CNBr at methionine residues. C-terminal peptides were purified by affinity chromatography and analyzed on RP-HPLC. The absorption peak at 30 min was analyzed by ESI-MS to contain a compound with an average mass of 2236 Da, consistent with the structure of surface protein linked to murein tetrapeptide. (D) RP-HPLC chromatogram of C-terminal anchor peptides released from S. aureus BB270 cells by means of treatment with 0.1 M NH2OH. The absorption peak at 32 min was analyzed by ESI-MS to harbor a compound with average mass 1548 Da. Edman degradation revealed the peptide sequence NH2-H6AQALPET* (T* is an unknown structure, see text for detail). Arrows point to the elution of compounds with mass 1548 (NH2-H6AQALPET*), 1565 (NH2-H6AQALPET〉; T〉 is threonine hydroxamate), and 2236 Da.
RP-HPLC of peptides released with 0.1 M NH2OH generated an absorbance peak at 32 min (27% CH3CN) (Fig. 3D) containing a compound with average mass of 1548 Da. Edman degradation revealed the peptide sequence NH2-H6AQALPET*, in which the thirteenth cleavage cycle released a phenylthiohydantoin moiety of unknown structure. The predicted mass of NH2-H6AQALPET〉 (T〉 indicates threonine hydroxamate) is 1565 Da, 17 Da more than the observed mass of 1548 Da. RP-HPLC fractions were scanned for the presence of ion signals with an average mass of 1548, 1565, or 2236. Mock-treated staphylococci released only the C-terminal peptide with mass 2236, whereas hydroxylamine-treated bacteria released peptides of 1548 Da, 1565 Da, and 2236 Da (Fig. 3D). Thus, NH2OH treatment of staphylococci released surface protein hydroxamate, suggesting that sortase forms an acyl-enzyme intermediate with cleaved surface protein.
The amounts of purified C-terminal peptide with m/z 1565 were insufficient for Edman degradation. Therefore, we compared the structure of the peptides with m/z 1548 and 1565 by tandem mass spectrometry using the triply charged parent ions at m/z 517.2 and 522.9, respectively. Collisionally induced dissociation produced daughter ion spectra consistent with the peptide sequences NH2-H6AQALPET〉 and NH2-H6AQALPET* (the structure of T* is unknown) (data not shown).
Staphylococcal Extracts Catalyze Hydroxylaminolysis at the LPXTG Motif.
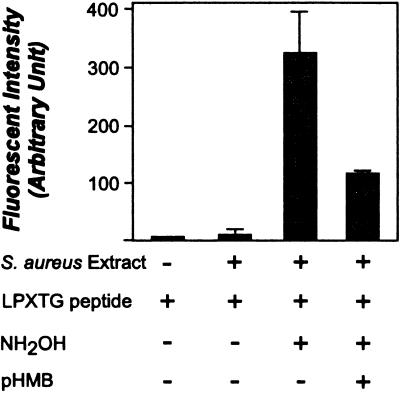
If NH2OH releases surface proteins from staphylococci in vivo, sortase may catalyze the cleavage of LPXTG motif-bearing peptides in the presence of NH2OH in vitro. Fluorescence of the Edans fluorophore within the peptide Dabcyl-QALPETGEE-Edans is quenched by the close proximity of Dabcyl (16). When the peptide is cleaved and the fluorophore is separated from Dabcyl, an increase in fluorescence is observed (17). Incubation of the LPXTG peptide with crude staphylococcal extracts caused only a small increase in fluorescence (Fig. 4). However, the addition of 0.1 M NH2OH to staphylococcal extracts resulted in an increase in fluorescence intensity (Fig. 4). This activity appears to be specific for sortase, as it can, at least in part, be inhibited by preincubation of staphylococcal extracts with pHMB or MTSET (18), known inhibitors of the sorting reaction (11). These results suggest that sortase catalyzes the hydroxylaminolysis of LPXTG peptide in vitro.
Figure 4.
Hydroxylaminolysis of LPXTG peptide by staphylococcal extracts in vitro. Staphylococcal extracts were incubated with the sorting substrate Dabcyl-QALPETGEE-Edans (LPXTG), and peptide cleavage was monitored as an increase in fluorescence. The addition of 0.2 M NH2OH increased peptide cleavage, whereas the addition of pHMB, a known inhibitor of sortase, inhibited cleavage.
Purified Soluble SrtAΔN Catalyzes Hydrolysis and Hydroxylaminolysis at the LPXTG Motif.
When expressed in E. coli and analyzed by centrifugation of crude lysates, the staphylococcal SrtA protein sedimented with membranes (data not shown). Crude E. coli extracts containing recombinant SrtA catalyzed cleavage of LPXTG peptide in vitro (data not shown). To obtain a soluble enzyme and examine its properties, the NH2-terminal membrane anchor segment of SrtA (residues 2–25) was replaced with a six-histidine tag (SrtAΔN). SrtAΔN was expressed in E. coli XL-1 Blue and purified by affinity chromatography from cleared lysates (Fig. 5A). When incubated with the LPXTG peptide and measured as an increase in fluorescence, SrtAΔN catalyzed cleavage of the substrate (Fig. 5B). Addition of 0.2 M NH2OH to this reaction resulted in an increase in fluorescence, indicating that cleavage of the LPXTG peptide occurred more efficiently. Hydroxylaminolysis of LPXTG peptide depended on the sulfhydryl of SrtAΔN, as preincubation with either MTSET or pHMB abolished all enzymatic activity.
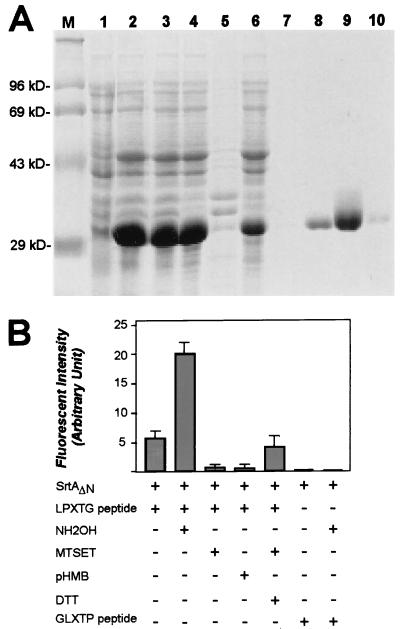
Figure 5.
Purification and characterization of sortase (SrtA). (A) E. coli XL-1 Blue(pHTT14) expressing SrtAΔN, in which the NH2-terminal membrane anchor of sortase (SrtA) has been replaced with a six-histidine tag, was lysed by French press. SrtAΔN was purified by affinity chromatography on Ni-NTA and analyzed on Coomassie-stained SDS/PAGE. Lanes: 1, uninduced culture; 2, 1 mM isopropyl β-d-thiogalactoside-induced culture; 3, French press extract; 4, the supernatant of centrifuged French press extracts; 5, the sediment of French press extracts; 6, flow-through of affinity chromatography on Ni-NTA; 7, column wash; and 8–10, 1-ml fractions eluted with 0.5 M imidazole. (B) Purified SrtAΔN was incubated with the LPXTG peptide, and cleavage was monitored as an increase in fluorescence. The reaction was inhibited by the addition of MTSET or organic mercurial (pHMB), whereas the addition of 0.2 M NH2OH accelerated cleavage. MTSET-treated SrtAΔN could be rescued by incubation with 10 mM DTT. The peptide Dabcyl-QAGLETPEE-Edans (GLXTP) served as a control for cleavage specificity of purified SrtAΔN.
Methanethiosulfonate forms disulfide with sulfhydryl groups that can be reversed by reducing agents such as DTT (19). MTSET-inactivated SrtAΔN was incubated in the presence of 10 mM DTT, which restored 89% of its activity (Fig. 5B). If hydroxylaminolysis of LPXTG peptide by SrtAΔN is a measure of the transpeptidation reaction that links surface protein to the staphylococcal cell wall, alterations of the LPXTG motif should prevent peptide cleavage. Dabcyl-QAGLETPEE-Edans, in which the LPXTG motif sequence has been changed without altering the overall amino acid composition, was incubated with SrtAΔN in either the presence or absence of 0.2 M NH2OH (Fig. 5B). No increase in fluorescence was observed, indicating that this peptide did not serve as a substrate for hydroxylaminolysis by SrtAΔN.
Cysteine-184 of SrtA Is Necessary for Sortase Activity.
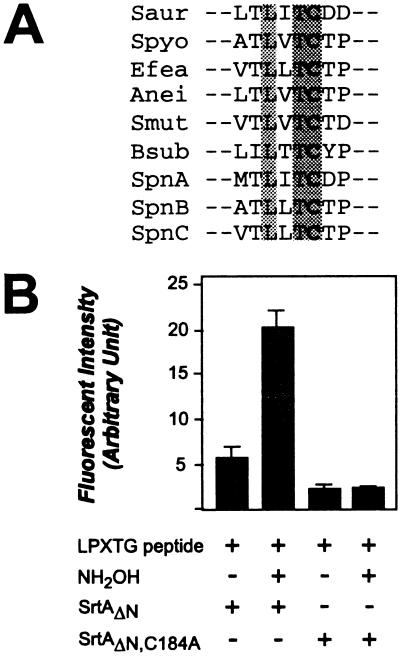
If sortase forms a thioester linkage with cleaved surface protein, replacement of the single conserved cysteine at position 184 with alanine should abolish all enzymatic activity. We purified such a mutant enzyme (SrtAΔN, C184A) and, when it was incubated with LPXTG peptide, observed only a very small increase in fluorescence. This increase may be caused by the binding of the LPXTG substrate to SrtAΔN, C184A. However, addition of 0.2 M NH2OH to this reaction did not cause a further increase in fluorescence, indicating that the LPXTG peptide was not cleaved. Thus, cysteine-184 of SrtA is necessary for both efficient hydrolysis and hydroxylaminolysis of LPXTG-bearing peptides (Fig. 6).
Figure 6.
Cysteine-184 of SrtA is necessary for sortase activity. (A) Alignment of S. aureus SrtA (Saur), i.e., residues 179–186 comprising cysteine-184, with corresponding amino acids of sortase homologs from Streptococcus pyogenes (Spyo), Enterococcus faecalis (Efea), Actinomyces naeslundii (Anei), Streptococcus mutans (Smut), Bacillus subtilis (Bsub), and Streptococcus pneumoniae (SpnA, SpnB, and SpnC). (B) Purified SrtAΔN, C185A contains a replacement of cysteine-184 with alanine. When incubated with LPXTG peptide, SrtAΔN, C184A caused only a small increase in fluorescence, which may be because of the binding of substrate to the mutant enzyme. The addition of NH2OH to SrtAΔN, C184A did not generate an increase in fluorescence, indicating that no cleavage of LPXTG peptide occurred.
Discussion
Hydroxylamine has been used to characterize several transpeptidation reactions (13, 20, 21). The best studied example is bacterial transpeptidases, which catalyze the crosslinking of cell wall peptides in the peptidoglycan, a reaction that is inhibited by penicillin (22–24). Transpeptidases or penicillin-binding proteins cleave the cell wall precursor at d-Ala-d-Ala and capture the carboxyl of the wall peptide as an ester with the active site hydroxyl of serine (25, 26). The acyl-enzyme intermediate is resolved by the nucleophilic attack of the amino within the cell wall crossbridge (pentaglycine in staphylococci), thereby allowing the formation of crosslinked wall peptides and regenerating the enzyme active-site hydroxyl (26, 27). Hydroxylamine can release the physiological acyl-enzyme intermediate as well as ester-linked penicilloyl, as this compound is a much stronger nucleophile than either the amino of the pentaglycine crossbridge or water (12). Although hydroxylamine can attack both ester and thioester bonds, the thioester enzyme intermediates appear to be more sensitive to hydroxylaminolysis (13) .
The observation that surface-protein anchoring is sensitive to sulfhydryl-modifying reagents (11) prompted us to examine hydroxylaminolysis of sortase acyl-enzyme intermediates. We show here that hydroxylamine releases surface proteins from staphylococci. Two polypeptide species were purified from the culture medium, surface protein threonine hydroxamate (T〉) and the more abundant form with a loss of 17 Da (T*). Although the structure of T* is unknown, we think it is likely that this compound is generated from T〉 during our purification scheme, which includes treatment with TFA, formic acid, guanidine⋅hydrochloride, and urea. In vitro, hydroxylaminolysis and hydrolysis of LPXTG peptide were sensitive to inhibition with the sulfhydryl reagents. Protein sequence comparison revealed the striking conservation of cysteine-184, as well as flanking amino acid residues, within sortase enzymes from several different Gram-positive bacteria (Fig. 6A). Replacement of cysteine-184 with alanine abolished sortase activity, suggesting that the cysteine sulfhydryl may function as an active-site nucleophile.
We propose a model in which surface proteins of S. aureus are linked to the cell wall by a transpeptidation reaction. Sortase (SrtA) cleaves polypeptides between the threonine and the glycine of the LPXTG motif, resulting in the formation of a hydroxylamine-sensitive thioester linkage between the carboxyl of threonine and the enzyme sulfhydryl (Eq. 1). In vivo, the acyl-enzyme intermediate is resolved by the nucleophilic attack of the amino group within the pentaglycine crossbridge (Eq. 2). Recent observations suggest that the pentaglycine crossbridge of the lipid II precursor may function as a nucleophile for the sorting reaction (11); however, hydroxylamine can substitute for pentaglycine both in vivo and in vitro.
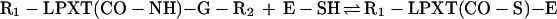 |
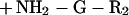 |
1 |
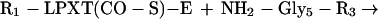 |
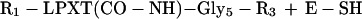 |
2 |
Purified sortase (SrtA) catalyzed hydrolysis of LPXTG-bearing peptide, suggesting that in vitro even water can function as a nucleophile to resolve the acyl-enzyme intermediate. As a spontaneous release of surface protein into the culture medium cannot be measured during pulse-labeling experiments, it appears that the availability of pentaglycine amino groups at the anchoring site of surface proteins prevents hydrolysis from occurring in vivo. The availability of purified, soluble sortase (SrtAΔN) and an in vitro assay for the hydroxylaminolysis of LPXTG peptide should allow the screening for compounds that inhibit anchoring of surface proteins in Gram-positive bacteria. As surface proteins are believed to be essential for the pathogenesis of bacterial disease (1), such compounds may be useful for the therapy of human infections caused by Gram-positive bacteria that have gained resistance to all known antibiotics (28).
Acknowledgments
We thank Drs. Dominique Missiakas [University of California, Los Angeles (UCLA)], Peter Model, Marjorie Russel (Rockefeller University), and members of our laboratory for discussion and critical reading of this manuscript. H.T.T. was supported by the Predoctoral Training Program in Microbial Pathogenesis at UCLA (AI07323). S.K.M. was supported by the Predoctoral Training Program in Genetic Mechanisms at UCLA (T32GM07104). The W. M. Keck Foundation provided support toward instrument purchase. Work in the laboratory of O.S. is supported by Grant AI33987 from the National Institutes of Health-National Institute of Allergy and Infectious Diseases, Infectious Disease Branch.
Abbreviations
- TCA
trichloroacetic acid
- SEB
staphylococcal enterotoxin B
- Ni-NTA
nickel nitrilotriacetic acid
- TFA
trifluoroacetic acid
- MALDI-MS
matrix-assisted laser desorption ionization MS
- ESI-MS
electrospray ionization MS
- Dabcyl
4-(4-dimethylaminophenylazo)benzoic acid
- Edans
5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid
- MTSET
[2-(trimethylammonium)ethyl]methanethiosulfonate
- pHMB
p-hydroxymecuribenzoic acid
- SPA
staphylococcal protein A
- T*
threonine modified to an unknown structure (presumably threonine hydroxamate − 17 Da)
- T〉
threonine hydroxamate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Navarre W W, Schneewind O. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarre W W, Schneewind O. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 3.Schneewind O, Fowler A, Faull K F. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 4.Ton-That H, Faull K F, Schneewind O. J Biol Chem. 1997;272:22285–22292. doi: 10.1074/jbc.272.35.22285. [DOI] [PubMed] [Google Scholar]
- 5.Ton-That H, Labischinski H, Berger-Bächi B, Schneewind O. J Biol Chem. 1998;273:29143–29149. doi: 10.1074/jbc.273.44.29143. [DOI] [PubMed] [Google Scholar]
- 6.Navarre W W, Ton-That H, Faull K F, Schneewind O. J Biol Chem. 1998;273:29135–29142. doi: 10.1074/jbc.273.44.29135. [DOI] [PubMed] [Google Scholar]
- 7.Navarre W W, Ton-That H, Faull K F, Schneewind O. J Biol Chem. 1999;274:15847–15856. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- 8.Schneewind O, Model P, Fischetti V A. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 9.Schneewind O, Mihaylova-Petkov D, Model P. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazmanian S, Liu G, Ton-That H, Schneewind O. Science. 1999;285:760–765. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 11.Ton-That H, Schneewind O. J Biol Chem. 1999;274:24316–24320. doi: 10.1074/jbc.274.34.24316. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence P, Strominger J L. J Biol Chem. 1970;245:3653–3659. [PubMed] [Google Scholar]
- 13.Kozarich J W, Tokuzo N, Willoughby E, Strominger J L. J Biol Chem. 1977;252:7525–7529. [PubMed] [Google Scholar]
- 14.Lipmann F, Tuttle L C. J Biol Chem. 1945;161:415–416. [PubMed] [Google Scholar]
- 15.Schindler C A, Schuhardt V T. Proc Natl Acad Sci USA. 1964;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G T, Matayoshi E, Huffaker H J, Krafft G A. Tetrahedron Lett. 1990;31:6493–6496. [Google Scholar]
- 17.Matayoshi E D, Wang G T, Krafft G A, Erickson J. Science. 1989;247:954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- 18.Smith D J, Maggio E T, Kenyon G L. Biochemistry. 1975;14:764–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- 19.Pathak R, Hendrickson T L, Imperiali B. Biochemistry. 1995;34:4179–4185. doi: 10.1021/bi00013a005. [DOI] [PubMed] [Google Scholar]
- 20.Shao Y, Xu M-Q, Paulus H. Biochemistry. 1996;35:3810–3815. doi: 10.1021/bi952592h. [DOI] [PubMed] [Google Scholar]
- 21.Ramalingnam S, Maxwell S E, Medof M E, Chen R, Gerber L D, Udenfriend S. Proc Natl Acad Sci USA. 1996;93:7528–7533. doi: 10.1073/pnas.93.15.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strominger J L, Izaki K, Matsuhashi M, Tipper D J. Fed Proc. 1967;26:9–18. [PubMed] [Google Scholar]
- 23.Ghuysen J-M. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 24.Waxman D J, Strominger J L. Annu Rev Biochem. 1983;52:825– 869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen J R, Strominger J L. Proc Natl Acad Sci USA. 1978;75:84–88. doi: 10.1073/pnas.75.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yocum R R, Waxman D J, Rasmussen J R, Strominger J L. Proc Natl Acad Sci USA. 1979;76:2730–2734. doi: 10.1073/pnas.76.6.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tipper D J, Strominger J L. Proc Natl Acad Sci USA. 1965;54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieradzki K, Roberts R B, Haber S W, Tomasz A. N Engl J Med. 1999;340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]