Abstract
It is well established that peripheral administration of interleukin-1 (IL-1) and lipopolysaccharide (LPS) can activate the hypothalamo-pituitary-adrenocortical (HPA) axis, alter brain catecholamine and indoleamine metabolism, and affect behavior. However, the mechanisms of these effects are not fully understood. Stimulation of afferents of the vagus nerve has been implicated in the induction of Fos in the brain, changes in body temperature, brain norepinephrine, and some behavioral responses. In the present study, the IL-1β - and LPS-induced changes in certain behaviors, HPA axis activation, and catecholamine and indoleamine metabolism were studied in mice following subdiaphragmatic vagotomy. IL-1β and LPS induced the expected decreases in sweetened milk, food intake, and locomotor activity, and the responses to IL-1β, but not LPS, were slightly attenuated in vagotomized mice. Subdiaphragmatic vagotomy also attenuated the IL-1β - and LPS-induced increases in plasma ACTH and corticosterone, but the attenuations of the responses to IL-1β were only marginally significant. There were also slight reductions in the responses in catecholamine and serotonin metabolism, and the increases in brain tryptophan in several brain regions. These results indicate that the vagus nerve is not the major pathway by which abdominal IL-1β and LPS effect behavioral, HPA and brain catecholamine and indoleamine responses in the mouse. These results resemble those we observed in subdiaphragmatically vagotomized rats, but in that species the subdiaphragmatic vagotomy markedly attenuated the ACTH and corticosterone responses, and prevented the hypothalamic noradrenergic activation, as well as the fever. Overall the results indicate that the various responses to peripheral IL-1 and LPS involve multiple mechanisms including vagal afferents, and that there are species differences in the relative importance of the various mechanisms.
Keywords: vagus, vagotomy, interleukin-1, feeding, activity, endotoxin, norepinephrine, dopamine, serotonin, tryptophan, ACTH, corticosterone
INTRODUCTION
One of the more significant discoveries of the past 20 years was that administration of the cytokine, interleukin-1 (IL-1), to animals had the ability to induce marked physiological and behavioral responses. These responses include changes in body temperature [1], activation of the hypothalamo-pituitary-adrenocortical (HPA) axis [2] and certain other endocrine responses [3], activation of the immediate early gene product Fos in various areas of the brain [4,5], activation of brain noradrenergic and serotonergic systems [6-8], and a range of behavioral responses [9]. Similar responses are also induced by administration of endotoxin (lipopolysaccharide, LPS)[10]
Very many studies have addressed the mechanisms of these various responses, and a number of them have implicated a role for afferents of the vagus nerve in the brain’s responses to peripherally administered IL-1 and LPS. The initial observation was that lesioning of the vagus nerve below the diaphragm prevented the Fos responses in the brain to LPS [11]. This was followed by numerous observations that similar lesions prevented a variety of behavioral responses to IL-1 and LPS [12], and noradrenergic responses to IL-1 in the rat [13].
In a recent study in the rat, we observed that subdiaphragmatic vagotomy prevented the hyperthermia following intraperitoneal administration of IL-1β as well as the increased norepinephrine secretion in the hypothalamus indicated by in vivo microdialysis. It also reduced, but did not prevent, the shivering response and the increases in plasma ACTH and corticosterone [14]. Very few such studies have been performed in mice, so we have now studied the effect of subdiaphragmatic vagotomy on the behavioral, neurochemical and neurochemical responses to peripherally administered IL-1β and LPS. A preliminary account of these results was presented at the 2004 annual meeting of the Society for Neuroscience [15].
MATERIAL AND METHODS
Experimental animals
The experiments were performed using male virus antigen-free CD-1 mice obtained from Charles River (VAF+ from Colony R16 of the Raleigh-Durham facility). The animals were housed individually, under controlled environmental conditions of temperature (22 ± 2°C), humidity (55 ± 5%) and on a 12:12 light cycle (lights on at 07:00 AM). Water and Purina rat chow were available ad libitum.
Materials
Mouse interleukin-1β was purchased from R&D Systems Inc (Minneapolis, MN), and injected intraperitoneally (ip) at a dose of 100 ng/mouse in 0.1 ml sterile physiological saline. Bacterial endotoxin (LPS) was purchased from Sigma Chemical Company (St. Louis, MO: L3755), and injected ip at a dose of 1 µg/mouse in 0.1 ml saline. All other chemicals were analytical grade from Sigma Chemical Company.
Experimental procedures
Subdiaphragmatic Vagotomy
Mice were anaesthetized with Innovar plus (3 mg fentanyl, 210 mg droperidol, 150 mg midazolam dissolved in 174 ml of water) at a dose of 10 µl/g body weight ip. They were placed on their backs and a 3 cm long incision was made in the abdomen. The liver was carefully moved to the right side to expose the esophagus. Then under a surgical microscope, both branches of the vagus nerve (gastric and hepatic) were exposed and small fragments (about 0.7 mm long) were dissected out. The abdominal muscles and the skin were then sutured with surgical silk, and antibiotic ointment (Neosporin) applied to the wound. The animals were then placed individually in their home cages and allowed to recover from the surgery for 10 days. Sham-operated mice that were subjected to all the surgical procedures except the removal of the vagal nerve sections were used as controls. Four separate batches of subdiaphragmatically vagotomized mice were prepared.
The effectiveness of the vagotomy was assessed in two ways: post mortem stomach weights, and in two of the experiments by injecting FluoroGold as a tracer. FluoroGold (Hydroxystilbamidine: Biotium Inc., Hayward, CA) tracer [16] was injected at a dose of 0.3 mg/mouse ip. Three days later the caudal brain stem was excised and frozen. Sections were then cut on a freezing microtome, and fluorescence assessed using a fluorescence microscope. The excitation wavelength used was 350nm, and the sections were viewed using an Olympus U- MWU filter. Only mice that failed to show FluoroGold fluorescence in the caudal brain stem were considered to be properly vagotomized and have been included in the data presented. In fact, none of the mice that were vagotomized showed any such fluorescence in the experiments presented, while all of the sham-operated animals showed such fluorescence (see Figure 1).
Figure 1.

Effect of vagotomy on fluorescence observed in a coronal section of the brain stem. The fluorescence in sham-operated mice appears to be associated with the dorsal motor nucleus of the vagus. Mice were injected with Fluorogold, and the brain stem sectioned 72 h later. The bar indicates 350 microns.
Milk Intake
Mice were habituated for at least three days to drink milk from 20 ml glass bottles fitted with metal spouts [17]. The weighed bottles were placed in the cages at around 11 AM for 30 min, then removed and reweighed. Only mice that drank at least 1.5 g of milk in the session on the final day of habituation were used for the experiment. On the day of experiment, animals were injected ip with saline, or 100 ng IL-1β or 1 µg LPS dissolved in saline. Then, bottles of sweetened milk were placed in the cages 30 or 90 minutes after IL-1β, or 120 minutes after LPS injections. The mice were allowed to drink for 30 min, and milk consumption measured by reweighing the bottles at the end of this period [17].
Food intake
At the end of the milk drinking session, a weighed amount of Purina food pellets were placed in each animal’s cage. Twenty-four hours later, the remaining food pellets were removed and weighed [17]. The mice were also weighed at this time. Spillage of food pellets was rare, but any obvious spillage was noted, and those data excluded from the analysis.
Locomotor activity
Spontaneous locomotor activity was quantified in an open field, a box 59 × 59 cm with its floor divided into sixteen squares and illuminated with white light (20 Lux at the floor level). Activity was scored as line crossings. Four squares were defined as the center and the 12 squares along the walls as the periphery. Line crossing was considered when all four paws were removed from one square and entered another. Rears were scored when an animal either stood free on its hind legs or climbed a wall. The test was started by placing the mouse in the center of the box. Behavior was scored 90 min after injection of IL-1β for 6 min.
Neurochemical Analysis
Mice were killed by decapitation 120 minutes after injection of IL-1β, LPS or saline. Trunk blood was collected in Eppendorf tubes containing 20 µl of 0.1 M EDTA (disodium salt), and then centrifuged to separate the plasma for measurement of corticosterone and ACTH. The plasma was stored at -70°C until such analyses. The brains were quickly removed from the skull and the following brain regions excised: hypothalamus, parietal cortex, hippocampus and brainstem. They were weighed and frozen on dry ice as previously described [18]. The frozen samples were ultrasonicated in 0.1M HClO4 containing 1 mM EDTA and analyzed by HPLC with electrochemical detection (for details see [19]).
Determination of plasma ACTH and Corticosterone
Plasma ACTH and corticosterone were determined using radioimmunoassay kits from ICN (Irvine, CA) as described by the manufacturer.
Experimental Design
Four separate batches of operated mice were prepared. Neurochemical and HPA determinations were made following IL-1β in every batch, and also following LPS in the third and fourth batches. The first two batches were used to assess milk intake, food intake and body weight following treatment with both IL-1β and LPS. Animals were randomly divided into four experimental groups: sham-operated or vagotomized mice were injected with sterile physiological saline or Subsequently, they were randomized and rotated within groups such that each animal received IL-1β either 2 or 3 times. Each mouse was also tested with LPS but only once. Only IL-1β was used in the terminal experiment in which brain and blood samples were collected. The third batch of mice was tested after IL-1β in the open field. The fourth batch of mice was not tested behaviorally. In the terminal experiments for the third and fourth batches, mice were randomly divided into six experimental groups: sham-operated or vagotomized mice were injected with saline, IL-1β or LPS. Thus no mouse received LPS more than once, and when IL-1β treatments were repeated, this occurred at least one week after the previous treatment.
Statistical Analysis
All data are reported as the mean ± standard error of the mean. Two-way analysis of variance (ANOVA) was performed using SuperANOVA followed by Fisher’s Least Significant Difference test. For the neurochemical experiments involving six groups of mice, the ANOVA was performed first with a 2 × 3 design: vagotomized or sham-operated, and the three treatment groups: saline, IL-1β and LPS, to determine any main effects of the vagotomy, and then repeated in a 2 × 2 design, separately for the IL–1β and LPS (vs. saline) treatments.
RESULTS
Verification of Vagotomy
Vagotomy was verified from stomach weights in every batch of vagotomized mice, and using ip FluoroGold as a tracer in the experiments from which the open field and neurochemical and endocrine data are presented. Stomach weights in vagotomized mice averaged 844 ± 95 mg as compared to 444 ± 26 mg in sham-operated mice (F(1,28)=16.0, P < 0.001). Figure 1 shows examples of the fluorescence from sections of brain stem in the region of the dorsal motor nucleus of the vagus from subdiaphragmatically vagotomized and sham-operated mice. In all cases, sham-operated mice showed pronounced fluorescence in the brain stem, whereas none was observed in the vagotomized mice.
Milk Intake
In all experiments, injections of IL-1β and LPS decreased milk intake in both subdiaphragmatically vagotomized and sham-operated mice (Figure 2). When milk intake was tested 30 min following IL-1β, ANOVA did not reveal significant differences in milk intake between sham-operated and vagotomized animals (F(1,34) = 0.02), but there was a statistically significant effect of the IL-1β (F(1,34) = 167, P < 0.001), and a significant interaction between the surgery and the IL-1β (F(1,34) = 12.73, P < 0.001). The latter indicates some attenuation of the hypophagic effect of IL-1β in the vagotomized mice. Similar results were obtained when milk intake was assessed 90 min after IL-1. ANOVA indicated no effect of the vagotomy ( F(1,34) = 3.39), but there were significant effects of IL-1β (F(1,34) = 5.00, P < 0.01), and the interaction between vagotomy and the IL-1β treatment (F(1,34) = 8.09, P < 0.01). These effects of vagotomy on the responses to IL-1β appeared to be largely attributable to the decreases observed in basal (saline) milk intake. In LPS-treated mice, ANOVA indicated a significant decrease in milk intake following the injection (F(1,34) = 35.4, P < 0.001), but no effect of the vagotomy (F(1,34) = 0.94), nor was there a significant interaction between surgery and the LPS (F(1,34) = 2.20). Thus vagotomy appeared to attenuate the effects of IL-1β on milk intake, but the similar effect of LPS was not statistically significant.
Figure 2.

Effect of IL-1β (100 ng/mouse, ip) or LPS (1 µg/mouse, ip) on milk intake in vagotomized and sham-operated mice measured 30, 90 min (IL-1β) or 120 min (LPS) after the injections. Data from 11 vagotomized and 8 sham-operated mice in each treatment group. *Significantly different from saline: *p < 0.05; **p < 0.01; significantly different from sham †p < 0.05; †† p < 0.01. N
Food Intake
IL-1β and LPS treatments also decreased food pellet intake over the following 24 h (Figures 3 and 4). Following IL-1β, ANOVA did not reveal significant differences in food intake between sham-operated and vagotomized animals (F(1,54) = 1.89), but there was a statistically significant effect of the IL-1β (F(1,54) = 15.0, P < 0.001), and a significant interaction between the surgery and the IL-1β (F(1,54) = 4.89, P < 0.05). Thus IL-1β treatment decreased the food intake, and this effect was significantly attenuated by the vagotomy (Figure 3). Similar results were obtained following LPS (Figure 4). ANOVA indicated that LPS decreased food intake (F(1,34) = 9.7, P < 0.01), but there was no significant effect of the surgery (F(1,34) = 1.73), and no significant interaction between the surgery and LPS treatments (F(1,34) = 0.073).
Figure 3.
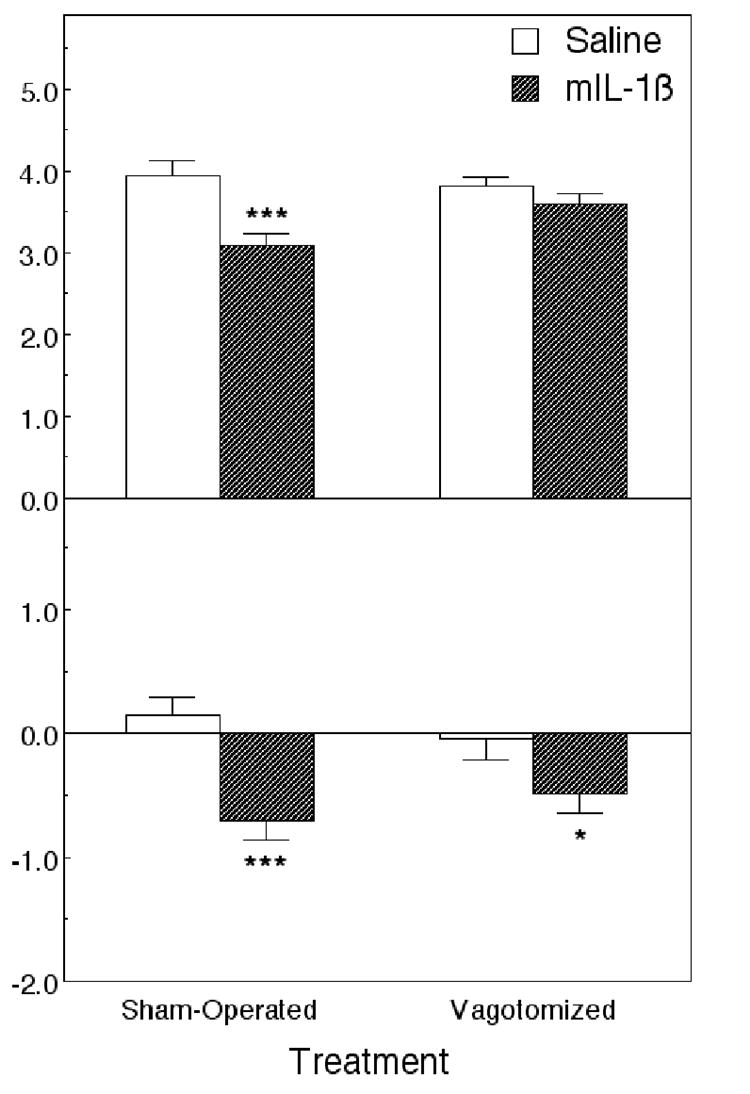
Effect of IL-1β (100 ng/mouse, ip) on food intake and changes in body weight in vagotomized and sham-operated mice 22 h after the injection. Data from 11 vagotomized and 8 sham-operated mice in each treatment group. *Significantly different from saline: *p < 0.05; ***p < 0.001.
Figure 4.

Effect of LPS (1 µg/mouse, ip) on food intake and changes in body weight 22 h after the injection. Data from 11 vagotomized and 8 sham-operated mice in each treatment group. * Significantly different from saline: *p < 0.05; **p < 0.001.
Body Weight
IL-1β and LPS treatments slightly reduced the body weights of the mice 24 h later (Figures 3 and 4). ANOVA did not reveal significant differences in body weight between sham-operated and vagotomized animals (F(1,54) = 0.00). There was a statistically significant effect of the IL-1β (F(1,54) = 16.1, P < 0.001), but no significant interaction between the surgery and the IL-1 β (F(1,54) = 1.61) (Figure 3). Injection of LPS decreased the body weight (F(1,34) = 7.1, P < 0.01; Figure 4). ANOVA did not show significant differences caused by the surgery (F(1,34) = 0.71), and there was no significant interaction between the surgery and the LPS treatment (F(1,34) = 0.011).
Activity in the Open Field
Vagotomy alone slightly, but significantly, decreased the activity in the center and the periphery of the open field (F(1,28) = 6.44, P < 0.05 and 8.98, P < 0.01, respectively; Figure 5). IL-1β depressed the number of crossings in the periphery (F(1,28) = 13.4, P < 0.001) and the total number of crossings F(1,28) = 12.7, P < 0.001). These effects were somewhat more pronounced in the sham-operated mice, but there was no statistically significant interaction between surgery and the treatment (F(1,28) = 0.15, 0.76 and 0.73 for central, peripheral and total line-crossings, respectively). Rears were significantly decreased by IL-1β (F(1,28 = 22.9, P < .0001), but there was no significant effect of vagotomy (F(1,28) = 2.16) and no vagotomy × IL-1β interaction.
Figure 5.

Effect of IL-1β (100 ng/mouse, ip) on 6-min open field activity observed 90 min after the injection. Data from 16 vagotomized and 18 sham-operated mice in each treatment group. * Significantly different from saline: *p < 0.05; **p < 0.01.
Plasma ACTH and Corticosterone Concentrations
Injection of IL-1β and LPS induced significant elevations of plasma concentrations of ACTH and corticosterone (Figure 6). ANOVA indicated statistically significant effects of the IL-1β and LPS treatments on plasma ACTH (F(2,41) = 14.1, P < 0.001) and corticosterone (F(2,45) = 16.1, P < 0.001). There was no statistically significant effect of vagotomy on ACTH (F(1,41) = 2.26) or corticosterone (F(1,45) = 2.36). However, vagotomy significantly altered the elevations of plasma ACTH and corticosterone concentrations in response to ip IL-1β /LPS as indicated by significant interactions between surgery and the IL-1β/LPS treatments (ACTH: F(2,41) = 5.55, P < 0.01; corticosterone: F(2,45) = 4,37, P < 0.02). When separate analyses were performed for IL-1β and LPS, IL-1β treatment significantly elevated plasma ACTH ( F(1,28) = 21.9, P < 0.0001) and corticosterone (F(1,29) = 28.4. P < 0.0001). LPS treatment also significantly elevated plasma ACTH (F(1,27) = 29.2, P < 0.0001) and corticosterone (F(1,29) = 29.7, P < 0.0001). However, IL-1β had only a marginal interaction with vagotomy (ACTH: F(1,28) = 3.09, P = 0.09; corticosterone: F(1,29) = 3.65, P = 0.07), whereas the interaction of vagotomy with LPS was statistically significant (ACTH: F(1,27 = 20.2, P < 0.0001; corticosterone: F(1,30) = 12.0, P < 0.002). Similar results were obtained in the other three experiments.
Figure 6.

Effect of IL-1β (100 ng/mouse, ip) or LPS (1 µg/mouse, ip) on plasma corticosterone (ng/ml of plasma) and ACTH (pg/ml of plasma) Blood was collected by decapitation 120 min after the injections. N = 9-11 for the vagotomized, and n = 7 for the sham-operated mice. *Significantly different from saline: *p < 0.05; **p < 0.01; ***p < 0.001.
Neurochemical Responses
Responses of the noradrenergic, dopaminergic, and serotonergic systems on the prefrontal cortex, the hypothalamus, the hippocampus and the brain stem to ip IL-1β and LPS were examined in four separate experiments (in two of these only IL-1β was tested). The results of all of these experiments were quite similar; Figures 7-10 show the results of one of the four experiments. Table I lists the P-values from an analysis of variance encompassing all four neurochemical experiments.
Figure 7.

Effect of IL-1β (100 ng/mouse, ip) or LPS (1 µg /mouse, ip) on brain MHPG:NE ratios. Mice were decapitated 120 min after injection. N = 5-6 for vagotomized and n = 6 for sham-operated mice. *Significantly different from saline: *p < 0.05; **p < 0.01. F-values: (IL-1β then LPS) parietal cortex: F(1,15) = 17.6, P < 0.001, F(1,16) = 11.6, P < 0.005; hypothalamus: F(1,18) = 18.8, P < 0.001, F(1,19) = 12.0, P < 0.005; hippocampus: F(1,15) = 6.90, P < 0.02, F(1,19) = 0.9 ns; and brain stem: F(1,18) = 23.0, P < 0.0001, F(1,19) = 14.7, P < 0.001).
Figure 10.

Effect of IL-1β (100 ng/mouse, ip) or LPS (1 µg/mouse, ip) on brain tryptophan concentrations (ng/mg wet tissue). Mice were decapitated 120 min after the injections. N = 5-6 for the vagotomized and n = 6 for the sham-operated mice. *Significantly different from saline (or from sham-saline in the case of vagotomy-saline): *p < 0.05; **p < 0.01. F-values: (IL-1β then LPS) parietal cortex: F(1,15) = 10.1, P < 0.01, F(1,16) = 5.49, P < 0.05; hypothalamus: F(1,18) = 12.1, P < 0.005, F(1,19) = 6.92 P < 0.02; hippocampus: F(1,18) = 10.8, P < 0.005, F(1,19) = 4.82, P < 0.05; and brain stem: F(1,18) = 4.23, P = 0.053, F(1,19) =6.41, P < 0.05).
Table I.
P-values for the statistical significance of the various factors (IL-1, LPS, Vagotomy) on the neurochemical measures (MHPG/NE, DOPAC/DA, 5-HIAA/5-HT and Tryptophan) made in the four brain regions (parietal cortex, hypothalamus, hippocampus and brain stem). The values were obtained by combining the data from all four neurochemical experiments. VagX = Vagotomized
| MHPG/NE | ||||
|---|---|---|---|---|
| Parietal Cortex | Hypothalamus | Hippocampus | Brainstem | |
| VagX | 0.03 | |||
| IL-1 | 0.001 | 0.0001 | 0.0001 | 0.0001 |
| LPS | 0.0001 | 0.0001 | 0.001 | |
| VagX × IL-1 | 0.03 | (.09) | ||
| VagX × LPS | ||||
| DOPAC/DA | ||||
| Parietal Cortex | Hypothalamus | Hippocampus | Brainstem | |
| VagX | 0.04 | |||
| IL-1 | 0.002 | 0.01 | ||
| LPS | (0.09) | |||
| VagX × IL-1 | (0.09) | |||
| VagX × LPS | ||||
| 5-HIAA/5-HT | ||||
| Parietal Cortex | Hypothalamus | Hippocampus | Brainstem | |
| VagX | ||||
| IL-1 | 0.0001 | 0.0014 | 0.004 | 0.0001 |
| LPS | 0.002 | |||
| VagX × IL-1 | ||||
| Vagx × LPS | .03 | |||
| TRP | ||||
| Parietal Cortex | Hypothalamus | Hippocampus | Brainstem | |
| VagX | (0.2) | 0.002 | ||
| IL-1 | 0.002 | 0.0001 | 0.0001 | 0.0001 |
| LPS | 0.001 | 0.0003 | 0.0001 | |
| VagX × IL-1 | (0.08) | |||
| VagX × LPS | ||||
Norepinephrine (NE)
IL-1β and LPS increased MHPG:NE ratios (an index of NE metabolism considered to reflect release) of sham and vagotomized mice in the parietal cortex, hypothalamus, hippocampus and brain stem (Figure 7). Analysis of variance did not indicate any statistically significant effect of subdiaphragmatic vagotomy in any brain region, although a small decrease in the hypothalamus was marginally significant (F(2,28) = 3.69, P = .065) and was consistently observed across all experiments (Table I). However, significant effects of the IL-1β and LPS treatments on MHPG:NE ratios were indicated in all brain regions (parietal cortex: F(2,24) = 9.36, P = 0.001; hypothalamus: F(2,28) = 9.66, p < 0.001; hippocampus: F(2,23) = 5.90, P < 0.01; and brain stem: F(2,28) = 10.66, P < 0.001). When the analyses for the effects of IL-1β and LPS were performed separately, IL-1β increased MHPG:NE ratios significantly in all four regions, whereas LPS increased the ratios significantly in all regions except the hippocampus (Figure 7). These effects were entirely attributable to increases in MHPG, the NE values were not significantly altered by any of the treatments in any brain region. There were no significant interactions between the vagotomy and the IL-1β and LPS treatments.
In sham-operated mice, ip injection of IL-1β increased MHPG:NE ratios in all the brain regions analyzed: parietal cortex, hypothalamus, hippocampus and brainstem (Figure 7). In vagotomized animals, IL-1β also increased MHPG:NE ratios in all these regions, although the effect in the hippocampus was not statistically significant. Injection of LPS in sham-operated animals increased MHPG:NE ratios in all four brain regions, although again the effect in the hippocampus was not statistically significant. In vagotomized mice, LPS also increased MHPG:NE ratios in all four regions, although the effects in the hippocampus and the parietal cortex were not statistically significant. The results obtained in the other three experiments were similar. None of the interactions between the vagotomy and the IL-1β and LPS treatments were statistically significant in this experiment. However, when the results of all four experiments were analyzed together the interactions between the vagotomy and the IL-1β treatment were statistically significant in the hypothalamus, and marginally so in the hippocampus (see Table I).
Dopamine (DA)
IL-1β and LPS did not significantly alter DOPAC:DA ratios (an index of DA metabolism considered to reflect release) in the four brain regions (Figure 8). ANOVA did not reveal any statistically significant effects of the subdiaphragmatic vagotomy, of the IL-1β or LPS treatments, nor any interactions between the vagotomy and the IL-1β and LPS treatments in any of the four brain regions analyzed. However, when the IL-1β and LPS data were analyzed separately, an increase in DOPAC:DA ratios in the brain stem reached statistical significance for IL-1β (F(1,18) = 4.70, P < 0.05). There was also a statistically significant interaction between vagotomy and IL-1β in the hippocampus (F = 8.43, p = 0.01). Small increases in brain stem DOPAC:DA ratios were observed following IL-1β in the other three experiments, along with small increases in the hypothalamus. Thus across all four experiments, increases in hypothalamic and brain stem DOPAC:DA ratios were statistically significant (Table I).
Figure 8.

Effect of IL-1β (100 ng/mouse, ip) or LPS (1 µg/mouse, ip) on brain DOPAC:DA ratios. N = 5-6 for the vagotomized and n = 6 for the sham-operated mice. Mice were decapitated 120 min after injection. The only statistically significant response to IL-1β was in the brain stem (F(1,18) = 4.70, P < 0.05. None of the responses to LPS were statistically significant.
Serotonin (5-HT)
IL-1β and LPS increased 5-HIAA:5-HT ratios (an index of 5-HT metabolism considered to reflect release) in the four brain regions (Figure 9). ANOVA indicated statistically significant effects for the interactions between the vagotomy and the IL-1β /LPS treatments in the hypothalamus (F(2,27) = 3.59. P < 0.05) and the hippocampus (F(2,23) = 3.78 P < 0.05, but not in the parietal cortex (F(2,24) = 0.42) and brain stem (F(2,28) = 0.18). There were no significant effects of the vagotomy in any brain region. However, the effects of IL–1β/LPS were statistically significant in all brain regions (parietal cortex (F(2,24) = 7.95, P = 0.002); hypothalamus (F(2,27) = 3.59, P < 0.05); hippocampus (F(2,23) = 5.80, P < 0.01), and brain stem (F(2,28) = 5.97, P < 0.01). When analyzed separately, IL-1β increased 5-HIAA:5-HT ratios in all brain regions, whereas the effect of LPS reached statistical significance only in the parietal cortex and brain stem, and marginally in the hypothalamus (Figure 9).
Figure 9.

Effect of IL-1β (100 ng/mouse, ip) or LPS (1 µg/mouse, ip) on brain 5-HIAA:5-HT ratios. Mice were decapitated 120 min after the injections. N = 5-6 for the vagotomized, and n = 6 for the sham-operated mice. *Significantly different from saline: *p < 0.05; **p < 0.01. F-values: (IL-1β then LPS) parietal cortex: F(1,15) = 14.6, P < 0.002, F(1,16) = 11.2, P < 0.005; hypothalamus: F(1,17) = 5.67, P < 0.05, F(1,18) = 3.65, P = 0.07; hippocampus: F(1,15) = 16.2, P < 0.02, F(1,16) = 2.80 ns; and brain stem: F(1,18) = 9.59, P < 0.01, F(1,19) = 6.12, P < 0.05).
In sham-operated animals, IL-1β increased the 5-HIAA:5-HT ratios in the parietal cortex (P < 0.005), hippocampus (p < 0.05), and marginally in the brain stem (P < 0.065), whereas the increase in the hypothalamus was not statistically significant. In vagotomized mice, IL-1β increased 5-HIAA:5-HT ratios in the parietal cortex ( P < 0.04), hypothalamus (P < 0.05), hippocampus (P < 0.03), and brain stem (P < 0.01). LPS injections in sham-operated mice increased 5-HIAA:5-HT ratios in the parietal cortex (P < 0.02), and hippocampus (P < 0.01), and marginally in the brain stem (p < 0.08), whereas the increase in the hypothalamus was not statistically significant. In vagotomized mice, LPS increased 5-HIAA:5-HT ratios but the changes in the parietal cortex, hypothalamus and brain stem were not statistically significant. In the hippocampus, vagotomy significantly attenuated the increase in the 5-HIAA:5-HT ratio (F(1,16 = 4.76, P < 0.05). Thus overall IL-1β induced reliable increases in 5-HIAA:5-HT ratios in the parietal cortex, hypothalamus and brain stem, while LPS induced reliable increases only in the brain stem. A reliable attenuation of this response by vagotomy was only observed in parietal cortex (Table I).
Tryptophan
IL-1β and LPS increased tryptophan concentrations in the four brain regions (Figure 10). ANOVA did not indicate any significant interactions between the surgery and the IL-1β /LPS treatments in any of the four brain regions studied. However, the ANOVA indicated significant effects of the vagotomy in the hypothalamus (F(1,28) = 12.0, P < 0.002), and the hippocampus (F(1,23) = 8.05, P < 0.01), but only marginally in the brain stem (F(1,28) = 2.92, P < 0.1), and not in the parietal cortex. There were also significant effects of IL-1β/LPS in the parietal cortex (F(2,24) = 4.44, P < 0.05), hypothalamus (F(2,28) = 5.37, P = 0.01), hippocampus (F(2,23) = 3.78, P < 0.05) and the brain stem (F(2,28) = 3.73, P < 0.05).
In sham-operated animals, ip injections of IL-1β increased tryptophan concentrations in each brain region, but these effects were statistically significant only in the hypothalamus (P < 0.05) and hippocampus (P < 0.05), the effect in the parietal cortex was marginally statistically significant (p <0.07). In vagotomized mice, increased concentrations of tryptophan in response to ip IL-1β were observed in the hypothalamus (P < 0.02), hippocampus (P < 0.03), and parietal cortex (P < 0.01). In the hypothalamus and parietal cortex, vagotomy significantly attenuated the increase in tryptophan concentrations (P < 0.05 and P < 0.01, respectively). In sham-operated mice, ip injections of LPS increased tryptophan concentrations in the parietal cortex (P = 0.05) and brain stem (p < 0.01), but the increases in the hypothalamus and hippocampus were not statistically significant. In vagotomized mice, concentrations of tryptophan were increased in the parietal cortex (P < 0.05) and in the hypothalamus (P < 0.06), while the increases in the hippocampus and brain stem were not statistically significant. In the hypothalamus, vagotomy significantly attenuated the increase in tryptophan concentrations (P < 0.02). Similar effects were observed in the three other experiments. However, the overall analysis of all four experiments showed only a marginal tendency for the vagotomy to attenuate the response to IL-1β in the brain stem (Table 1).
DISCUSSION
The results of the present study confirm the observations of very many studies indicating that ip injection of IL-1β and LPS into mice and rats decrease milk intake, food intake, and body weight [17]. Such injections also decrease activity in an open field [20]. They also activate the HPA axis, elevating plasma concentrations of ACTH and corticosterone [2,6,10]. IL-1β and LPS both increase the activity of noradrenergic and serotonergic neurons, as well as increasing brain concentrations of tryptophan in all brain regions [6-8,10].
There is substantial evidence that the vagus nerve is an important structure for communication of the immune system with the brain. It was first shown in rats that a subdiaphragmatic vagotomy prevented the Fos responses to LPS in the hypothalamus and brain stem [11]. (Total vagotomy results in the loss of critical mechanisms for regulating heart rate and blood pressure, and thus tends to be lethal). Interestingly, a more recent study suggested that the Fos responses in the dorsal vagal complex (specifically the nucleus tractus solitarius and area postrema) were not dependent on the vagus nerve [21]. Subsequently, several research groups showed that subdiaphragmatic vagotomy prevented a variety of behavioral responses to IL-1 and LPS [22,23]. It was also shown that subdiaphragmatic vagotomy prevented the decrease in hypothalamic NE in response to IL-1 injection in the rat [13]. Subdiaphragmatic vagotomy also prevented the reduction by IL-1 of social investigation of a juvenile when the IL-1 was injected intraperitoneally, but not when it was injected intravenously [24], suggesting that the abdominal vagus was a means for communication from the peritoneum (presumably from the gut and the liver), but was less important for systemic IL-1.
In the present experiments, subdiaphragmatic vagotomy slightly attenuated the acute IL-1β -induced decrease in milk intake to IL-1β and to LPS (Figure 2), although ANOVA did not indicate a statistically significant interaction for LPS. This effect appeared to be largely attributable to the reduction of basal milk intake in the vagotomized mice, which may reflect decreased appetite related to the bloated stomachs. Although the magnitude of the effects of IL-1β and LPS on food pellet intake of the following 22 h was substantially smaller, similar results were obtained. The attenuation by IL-1β was statistically significant, while that for LPS was marginal (Figures 3 and 4). Similarly, vagotomy slightly decreased activity in the open field, but there were no statistically significant interactions between the vagotomy and the IL-1β treatments (Figure 5). These small attenuations in sweetened milk and food intake are consistent with earlier studies indicating that the anorexic effects of ip IL-1β and LPS were not prevented by selective vagal rootlet deafferentation [25], nor by subdiaphragmatic vagal deafferentation, celiac superior mesenteric ganglionectomy, or combined vagotomy and ganglionectomy [26]. These results led these authors to speculate that humoral factors were more important than the vagus nerve for this anorectic response. It is also relevant that abdominal vagotomy did not modify the endotoxic shock induced by intravenously injected LPS [27].
The vagus appears to play a significant role in fever responses [28]. In our experiments in rats, subdiaphragmatic vagotomy prevented the fever induced by ip IL-β [14]. The mechanisms may be complex with different mechanisms underlying the various phases of the fever response [29]. Furthermore, the mechanism appears to depend on the dose of IL-1β [30].
Vagotomy clearly attenuated the increases in plasma ACTH and corticosterone in response to both IL-1β and LPS (Figure. 6). This result is consistent with our observations in rats in which subdiaphragmatic vagotomy markedly attenuated the IL-1β -induced increases in plasma ACTH and corticosterone [14]. However, it contrasts with the results of Hansen et al. who found no effect of subdiaphragmatic vagotomy on the ip LPS-induced increases in plasma corticosterone in rats [31].
As in many previous studies, both IL-1β and LPS increased the metabolism of NE, and 5-HT, and to a lesser extent in DA [6,7,10]. Brain concentrations of tryptophan were also increased. Although there were a few statistically significant effects of the subdiaphragmatic vagotomy on the effects of IL-1β and LPS on brain NE and 5-HT metabolism, the interaction terms were for the most part not statistically significant, except for MHPG:NE ratios in the hypothalamus, and 5-HIAA:5-HT ratios in the parietal cortex (Table I). Nevertheless, we consistently observed trends of vagotomy to decrease the responses in brain noradrenergic, dopaminergic and serotonergic systems, and the increases in brain tryptophan. Small decreases in the responses in MHPG:NE ratios were observed in all four experiments, suggesting some attenuation. The changes in NE are consistent with an earlier study of hypothalamic NE in rats, indicating that the IL-1β -induced decrease in hypothalamic NE was prevented by subdiaphragmatic vagotomy [13]. It is also partially consistent with our observation that subdiaphragmatic vagotomy completely prevented the increase in hypothalamic microdialysate NE in rats [14]. Interestingly, vagotomy itself increased basal concentrations of tryptophan in several brain regions. Because this increase in tryptophan is a very common concomitant of stress and is regionally nonspecific [32,33], this response may reflect the stressful nature of the vagal lesion.
It is interesting to compare the current results of subdiaphragmatic vagotomy obtained in mice, with those we obtained previously from rats [14]. In those experiments, vagotomy markedly impaired the behavioral responses to ip IL-1β, and prevented the fever. Vagotomy also markedly attenuated the responses to IL-1β in plasma ACTH and corticosterone. However, in contrast to the results obtained in the present study in mice, subdiaphragmatic vagotomy more or less prevented the increases in the microdialysate concentrations of NE from the medial hypothalamus of the rat. There were some important methodological differences between these experiments. In the rat, we were able to obtain repeated blood samples for determination of ACTH and corticosterone, although it is unlikely that this difference accounts for the more marked effect of subdiaphragmatic vagotomy in the rat. Also in the rat, we assessed hypothalamic noradrenergic secretion by in vivo microdialysis, which would be exceedingly difficult in the mouse. However, it seems unlikely that these methodological differences account for the differences in the effects of the vagotomy in the two species. Thus we conclude that the vagal pathway plays little role in the IL-1β -and LPS-induced activation of NE, DA and 5-HT and tryptophan in the mouse. It is even possible that the small attenuations observed do not indicate a vagal mechanism at all, but rather a nonspecific consequence of the vagotomy-related changes in the abdomen, such as the swollen stomach.
In the rat study, evidence was obtained for multiple mechanisms for behavioral and HPA activation, and so it is likely that the contributions of the various mechanisms differ among the species, and that the vagal route is less important for these responses in the mouse.
These results indicate that signaling via the vagus nerve may contribute to the effects of ip IL-1β and LPS administration to mice on feeding, and the activation of HPA axis and brain noradrenergic and serotonergic systems. However, other mechanisms exist, so that the abdominal vagal pathway does not appear to be essential for these responses. Thus, abdominal vagal afferents appear to be less important for signaling the brain in the mouse than in the rat.
Acknowledgments
We thank Charles Dempsey for technical assistance with the neurochemical assays. We thank Dr. Lisa Goehler (University of Virginia) for the generous gift of FluoroGold and assistance in implementing this procedure. This work was supported by a grant from the National Institutes of Health (NS35370).
References
- 1.Dinarello CA. Interleukin-1. Adv Pharmacol. 1994;25:21–51. doi: 10.1016/s1054-3589(08)60429-9. [DOI] [PubMed] [Google Scholar]
- 2.Besedovsky HO, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 3.Dunn AJ, Scarborough DE. Cytokines and anterior pituitary function. In: Norman AW, editor. Encyclopedia of Hormones. San Diego, CA: Elsevier; 2003. pp. 362–371. [Google Scholar]
- 4.Chang SL, Ren T, Zadina JE. Interleukin-1 activation of Fos protooncogene protein in the rat hypothalamus. Brain Res. 1993;617:123–130. doi: 10.1016/0006-8993(93)90622-t. [DOI] [PubMed] [Google Scholar]
- 5.Ericsson A, Kovács KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn AJ. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 1988;43:429–435. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- 7.Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav Immun. 1988;2:267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 8.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. In: Cohen N, editor. Psychoneuroimmunology. New York: Academic Press; 2001. pp. 649–666. [Google Scholar]
- 9.Dantzer R, Bluthé RM, Castanon N, Chauvet N, Capuron L, Goodall G, Kelley KW, Konsman J-P, Layé S, Parnet P, Pousset F. Cytokine effects on behavior. In: Cohen N, editor. Psychoneuroimmunology. San Diego, CA: Academic Press; 2001. pp. 703–727. [Google Scholar]
- 10.Dunn AJ. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exptl Therapeut. 1992;261:964–969. [PubMed] [Google Scholar]
- 11.Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 12.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Letts. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner M, Goehler LE, Hermann J, Relton JK, Maier SF, Watkins LR. Interleukin-1β induced corticosterone elevation and hypothalamic NE depletion is vagally mediated. Brain Res Bull. 1995;37:605–610. doi: 10.1016/0361-9230(95)00051-f. [DOI] [PubMed] [Google Scholar]
- 14.Wieczorek M, Dunn A. The role of the vagus nerve in the febrile, behavioral, noradrenergic and endocrine responses to interleukin-1 in the rat. submitted for publication. [Google Scholar]
- 15.Wieczorek M, Pournajafi Nazarloo H, Swiergiel AH, Dunn AJ. The role of the vagus nerve in the behavioral, neurochemical and neuroendocrine responses to interleukin-1 and endotoxin in mice. Society for Neuroscience Abstracts. 2004 Program No. 761.27. [Google Scholar]
- 16.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Amer J Physiol. 1987;253:R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- 17.Swiergiel AH, Smagin GN, Dunn AJ. Influenza virus infection of mice induces anorexia: comparison with endotoxin and interleukin-1 and the effects of indomethacin. Pharmacol Biochem Behav. 1997;57:389–396. doi: 10.1016/s0091-3057(96)00335-8. [DOI] [PubMed] [Google Scholar]
- 18.Dunn AJ. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem. 1988;51:406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 19.Dunn AJ. Neurochemical methods for evaluating cerebral biogenic amine responses to cytokines and their involvement in the central actions of interleukin-1. In: De Souza EB, editor. Neurobiology of Cytokines. San Diego: Academic Press, Inc.; 1993. pp. 209–222. [Google Scholar]
- 20.Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005 doi: 10.1016/j.pbb.2005.04.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermann GE, Emch GS, Tovar CA, Rogers RC. c-Fos generation in the dorsal vagal complex after systemic endotoxin is not dependent on the vagus nerve. Amer J Physiol. 2001;280:R289–299. doi: 10.1152/ajpregu.2001.280.1.R289. [DOI] [PubMed] [Google Scholar]
- 22.Bret-Dibat J-L, Bluthé R-M, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- 23.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 24.Bluthé R-M, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. NeuroReport. 1996;7:2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GJ, Plata-Salaman CR, Langhans W. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1 beta in rats. Amer J Physiol. 1997;273:R1193–R1198. doi: 10.1152/ajpregu.1997.273.3.R1193. [DOI] [PubMed] [Google Scholar]
- 26.Porter MH, Hrupka BJ, Langhans W, Schwartz GJ. Vagal and splanchnic afferents are not necessary for the anorexia produced by peripheral IL-1beta, LPS, and MDP. Amer J Physiol. 1998;275:R384–389. doi: 10.1152/ajpregu.1998.275.2.R384. [DOI] [PubMed] [Google Scholar]
- 27.Pitterman AB, Friedlander G, Kelly G, Ropchak TG, Goldstein DJ, Keiser HR. Abdominal vagotomy does not modify endotoxic shock in rats. Life Sci. 1983;33:1033–1037. doi: 10.1016/0024-3205(83)90657-4. [DOI] [PubMed] [Google Scholar]
- 28.Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs. Brain Res. 1996;726:160–166. [PubMed] [Google Scholar]
- 29.Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain. News Physiol Sci. 1997;12:1–9. [Google Scholar]
- 30.Hansen MK, O’Conner KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Amer J Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 31.Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Amer J Physiol. 2000;278:R331–336. doi: 10.1152/ajpregu.2000.278.2.R331. [DOI] [PubMed] [Google Scholar]
- 32.Dunn AJ, Welch J. Stress- and endotoxin-induced increases in brain tryptophan and serotonin metabolism depend on sympathetic nervous system activation. J Neurochem. 1991;57:1615–1622. doi: 10.1111/j.1471-4159.1991.tb06359.x. [DOI] [PubMed] [Google Scholar]
- 33.Dunn AJ, Wang J-P, Ando T. Effects of cytokines on central neurotransmission: Comparison with the effects of stress. Adv Exptl Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]


