Abstract
Cnidarian bleaching results from the breakdown in the symbiosis between the host cnidarian and its dinoflagellate symbiont. Coral bleaching in recent years has increasingly caused degradation and mortality of coral reefs on a global scale. Although much is understood about the environmental causes of bleaching, the underlying cellular mechanisms of symbiont release that drive the process are just beginning to be described. In this study, we investigated the roles of two cellular pathways, host cell apoptosis and autophagy, in the bleaching process of the symbiotic anemone Aiptasia pallida. Host cell apoptosis was experimentally manipulated using gene knockdown of an anemone caspase by RNA interference, chemical inhibition of caspase using ZVAD-fmk and an apoptosis-inducer wortmannin. Autophagy was manipulated by chemical inhibition using wortmannin or induction using rapamycin. The applications of multiple single treatments resulted in some increased bleaching in anemones under control conditions but no significant drop in bleaching in individuals subjected to a hyperthermic stress. These results indicated that no single pathway is responsible for symbiont release during bleaching. However, when multiple inhibitors were applied simultaneously to block both apoptosis and autophagy, there was a significant reduction in bleaching in heat-stressed anemones. Our results allow us to formulate a model for cellular processes involved in the control of cnidarian bleaching where apoptosis and autophagy act together in a see-saw mechanism such that if one is inhibited the other is induced. Similar interconnectivity between apoptosis and autophagy has previously been shown in vertebrates including involvement in an innate immune response to pathogens and parasites. This suggests that the bleaching response could be a modified immune response that recognizes and removes dysfunctional symbionts.
Keywords: apoptosis, autophagy, Aiptasia, coral bleaching, immunity, dinoflagellate
1. Introduction
The global decline of coral reefs continues at an increasing rate despite attempts to abate direct and indirect human impacts. One major cause of this decline is coral bleaching. Bleaching or whitening of the coral tissues is largely due to the loss of the symbiotic dinoflagellate algae from the host. This in turn can lead to reduced growth, calcification and reproduction, and can ultimately result in mortality (Brown 1997; Hoegh-Guldberg 1999). The causes of bleaching are linked to climate change and disease. However, interconnectivity between cellular pathways and physiological responses to environmental stress and pathogenesis remains unresolved (Hughes et al. 2003; Lesser 2004). Increasing our understanding of the stability and complexity of cellular pathways within the symbiosis will offer key insight into the holobiont health, infection and immunity, and the effects of environmental and climate history on acclimatization and susceptibility to bleaching.
Components of multiple pathways are important in the bleaching process (for review, see Lesser 2004). High sea-surface temperature, UV and high light are known to generate potent reactive oxygen species (ROS) in the holobiont as a result of the breakdown in symbiont photosynthesis (for review, see Lesser 2004 and Perez & Weis 2006). ROS cause membrane lipid peroxidation and organelle and DNA damage, all of which lead to cell death (Lesser 1997; Perez & Weis 2006). As an antioxidant defence to ROS, enzymes, such as superoxide dismutase and catalase, scavenge and reduce ROS to less toxic forms thereby reducing damage (Richier et al. 2005, 2006). If antioxidant defences are inhibited or absent, then symbiont loss from the host increases (Merle et al. 2007), presumably from ROS-induced cell death. Alternatively, when there is instability in symbiosis regulation and a symbiosis breakdown due to stress, the host cell may actively control symbiont release through one or more mechanisms (Gates et al. 1992) and symbionts may undergo in situ degradation resulting in bleaching (Dunn 2002).
Identifying the activation of the apoptotic pathway in symbiotic cnidarians during bleaching has offered insight into the cellular initiation of the bleaching process (Dunn et al. 2004). Apoptosis is a form of programmed cell death that occurs in response to cytotoxic agents, cell dysfunction and infection. This intrinsic process can be initiated by either extrinsic or intrinsic stimuli, and is of fundamental importance in the development, growth, health and tissue homeostasis of multicellular organisms (Hengartner & Bryant 2000). Apoptosis occurs in three stages: initiation; execution; and cell deletion. These stages are interconnected by the action of cysteine-dependent aspartate-specific proteases or caspases, which operate in a proteolytic cascade by cleaving and activating one another (Fan et al. 2005). Once activated, caspases cleave other key proteins, cytoskeletal elements and cell adhesion molecules, and conclude with endonuclease cleavage of nuclear material and cell disposal (Woo et al. 2004). Two possible explanations for the observed initiation of host cell apoptosis during bleaching are as follows: (i) apoptosis acts to mitigate tissue damage and maintain homeostasis by removing dysfunctional symbionts (Dunn et al. 2004), and (ii) apoptosis acts as part of an innate immune response that recognizes the once symbiotic algae as non-symbiotic microbes and removes them.
Autophagy is another highly conserved pathway (Boya et al. 2005) that could be active in bleaching. It occurs in a diversity of organisms ranging from yeast to higher vertebrates and is an important cellular process for the removal and degradation of proteins, organelles, cytoplasmic contents and intracellular pathogens (for review, see Gozuacik & Kimchi 2004 and Koul et al. 2004). Induction of autophagy occurs by nutrient deprivation, hypoxia (Cuervo 2004) and infection from invading microbes (Méresse et al. 1999). The autophagic pathway begins with target identification, followed by the formation of vesicles de novo from small membrane structures generated from the endoplasmic reticulum or Golgi. Membrane structures envelop the target as a double membrane autophagosome, which then fuses with a late endosome or autolysosome. The target is released with the inner autophagosome membrane into the lysosome where digestion by hydrolytic enzymes occurs (Cuervo 2004; Gozuacik & Kimchi 2004). In some cases, autophagic digestion results in autophagic cell death (Gozuacik & Kimchi 2004).
The interrelationship between autophagy and apoptosis is diverse and complex. The two processes can be closely linked by specific molecular triggers, either in a positive, regulated manner, such as to balance cell proliferation, or in a negative, unregulated relationship, which results in tumour formation (Gozuacik & Kimchi 2004). Autophagy and apoptosis can operate together as part of a complex cell death pathway, which has characteristics of both processes (González-Polo et al. 2005). Autophagy can also act to rescue apoptosis for cell survival or it can precede apoptosis. Furthermore, apoptosis may be activated to dispose of a cell upon autophagy failure (Boya et al. 2005).
Recent evidence of cell signalling pathways in cnidarian–dinoflagellate symbiosis indicates that there are similarities between the host cnidarian's response to symbionts and an innate immune response to pathogens or parasites. For example, recognition processes during the onset of symbiosis include lectin–glycan interactions (Lin et al. 2001; Wood-Charlson et al. 2006), a common mechanism for detecting harmful microbes. In addition, nitric oxide, a cytotoxic signalling compound used in the innate immune response (Chan et al. 2001), is produced during the early stages of the bleaching process and is hypothesized to trigger downstream cytotoxic events that lead to bleaching (Trapido-Rosenthal et al. 2001; Perez & Weis 2006). Finally, apoptosis occurs both during bleaching (Dunn et al. 2004) and in response to microbial invaders of corals (Ainsworth et al. 2007) during the innate immune response (Fairbairn 2004), as it occurs in other organisms.
Many intracellular parasites such as Leishmania donovani, Legionella pneumophila and Mycobacterium tuberculosis avoid detection by the host immune response by manipulating both apoptotic and autophagic pathways (Dermine et al. 2000; Gutierrez et al. 2004; Koul et al. 2004). Host apoptosis is prevented by parasite manipulation of Ca2+, proapoptotic signals such as Bad and stimulation of immunosuppressive cytokines (for review, see Koul et al. 2004). Prevention of host autophagy is achieved through the manipulation of the phosphoinositide metabolism, the phosphatidylinositol 3-kinase (PI3K)/AKT pathway and Rab GTP-binding proteins (Fratti et al. 2001; Chua et al. 2004; Hilbi 2006).
In this study, we investigated the interconnected roles of apoptosis and autophagy during the onset of hyperthermic stress (HTS)-induced bleaching in the symbiotic sea anemone Aiptasia pallida. We hypothesized that if apoptosis and/or autophagy are active in bleaching, then experimental manipulation of these processes would change the amount of bleaching observed. To test the roles of apoptosis and autophagy during bleaching, we used separate treatments of gene knockdown by RNA interference, biochemical inhibition and induction. Our results show that manipulation of either apoptosis or autophagy alone had no significant effect on bleaching in heat-stressed anemones. However, when both apoptosis and autophagy were inhibited simultaneously, bleaching was significantly reduced in heat-stressed anemones. The apparent interconnectivity of these two processes offers key insight into the complexity of bleaching regulation and suggests that no single process is operating in response to heat stress.
2. Material and methods
(a) Anemone culture conditions and treatments
Aiptasia pallida were placed in individual wells of 48-well plates (Falcon Becton Dickinson) in sterile artificial seawater (ASW) in accordance with Dunn et al. (2007) and incubated at 26±0.5°C, salinity 34‰, photon flux density 50 μmol m−2 s−1 on a 12 hours light/12 hours dark cycle. After one week of acclimation, which included daily water changes, anemones were subjected to one of two temperature treatments. Control plates of anemones were returned to culture conditions described above (termed control temperature, CT). Plates of anemones destined for temperature stress were placed in a separate incubator (Percival) with the same conditions except that the temperature was set at 33–34°C (termed HTS), for 24 hours. These conditions result in bleaching (Dunn et al. 2002, 2004). To inhibit apoptosis, a broad non-reversible caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone (ZVAD-fmk, Enzyme Systems Products, final concentration: 12.5 μM in 0.2% DMSO in sterile ASW) was used. In a separate treatment, specific gene knockdown of an A. pallida caspase, acasp (Dunn et al. 2006) using RNAi was performed according to the methods described in detail by Dunn et al. (2007). Double-stranded RNA was applied to anemones in a vehicle reagent, 1,2-dimyristyloxypropyl-3-dimethyl-hydroxy ethyl ammonium bromide and cholesterol (DMRIE-C; Gibco).
To simultaneously induce apoptosis and inhibit autophagy, two different PI3K pathway inhibitors were used: wortmannin (final concentrations: 250 and 500 nM; Sigma-Aldrich) and 3-methyladenine (3MA, 10 μM; Sigma-Aldrich). PI3K enzymes, known as anti-apoptosis kinases, regulate signal transduction pathways leading to cell proliferation and survival (Chang et al. 2003; Cui et al. 2006). PI3K dysfunction has been implicated in the growth of different types of carcinomas due to uncontrolled cell proliferation (Chang et al. 2003). Wortmannin is an inducer of apoptosis in organisms ranging from cnidarians (David et al. 2005) to vertebrates, and is a known inhibitor of autophagy (Yu et al. 2006). 3MA was used as an alternative promoter of apoptosis and inhibitor of autophagy and inhibits the PI3K membrane trafficking pathway. In another treatment, both wortmannin and 3MA were used in combination because each compound could have partial or separate effects. Using a combination of inhibitors could maximize the inhibition of the PI3K pathway (Seglen & Gordon 1982; Caro et al. 1988; Nakanishi et al. 1995; Blommaart et al. 1997; Pahan et al. 1999; Hirosako et al. 2004).
To induce autophagy, two separate treatments of rapamycin (final concentrations: 5 and 25 μM; Sigma-Aldrich) were applied to anemones. Rapamycin acts to limit protein folding, cell cycle progression and block translation of target of rapamycin (TOR) proteins, resulting in autophagy (Cruz et al. 1999; Hara et al. 2002). Rapamycin inhibits TOR by binding with FK506-binding protein to form a rapamycin–FKBP12 complex, which then inhibits TOR (Cruz et al. 1999). TOR, known as the gatekeeper to autophagy, is constitutively active under normal growth conditions, controlled through the PI3K/AKT pathway, and acts as a master switch of cellular catabolism, anabolism and apoptosis and autophagy activation (Castedo et al. 2002; Neufeld 2003; Gozuacik & Kimchi 2004).
Vehicle control treatments were (i) untreated, (ii) 0.2% DMSO in sterile ASW and (iii) 0.3% DMRIE-C in sterile ASW. Following all treatments, the anemone and the seawater containing expelled symbionts from each well were removed and stored separately at −80°C for later measurement of expulsion and bleaching.
(b) Measurement of bleaching
Bleaching was measured as a proportion of the algal population expelled over 24 hours. Anemones were thawed on ice and centrifuged at 10 000g for 10 min. The supernatant was removed and replaced with 500 μl of sterile ASW. The tissue pellet was homogenized using an Eppendorf tissue homogenizer and centrifuged as described above. The supernatant was removed and the pellet was resuspended with between 100 and 1000 μl of ASW. Cells were counted with a Neubauer-improved bright-line haemacytometer (Hausser Scientific, USA) and density was determined from ten 20 μl subsamples from each resuspended pellet. The well supernatants for individual samples were processed in the same manner and counted. The density of algae in the supernatant and pellet was then combined to calculate the percentage of population expelled from each anemone using the following equation (Perez & Weis 2006):
(c) Statistical analysis
All data were tested for normality and heteroscedasticity. Dinoflagellate expulsion percentages from individual treatments were adjusted using an arcsine transformation. Expulsion percentages between untreated and treated anemones from CT and HTS conditions were tested using one-way ANOVA with post hoc Tukey pairwise multiple comparison. Probability significance was adjusted using Bonferroni conversion in accordance with Sokal & Rohlf (1995). Expulsion percentages from anemones within multiple manipulation treatments were compared using independent Kruskal–Wallis tests and adjusted for multiple comparisons using Bonferroni. All statistical tests were conducted using Minitab (v. 11) software.
3. Results
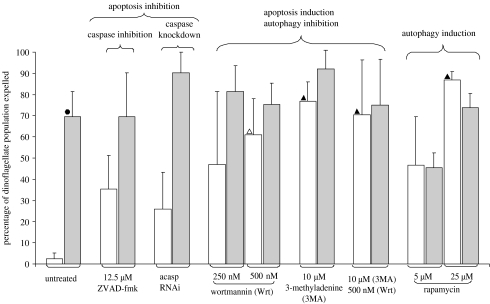
There was a highly significant difference between the percentages of symbionts released from CT compared with HTS animals (figure 1). Only 2.4±2.7% (s.d.) of the algal population was released in CT animals compared with 69.4±12.0% in HTS animals. Both CT and HTS animals incubated in the two vehicle negative controls, 0.2% DMSO only and 0.3% DMRIE-C only, exhibited bleaching percentages not significantly different from untreated controls (electronic supplementary material, figure 1). Animals incubated in ZVAD-fmk, the apoptosis inhibitor, behaved contrary to our predictions. We hypothesized that apoptosis inhibition would result in decreased bleaching under HTS, but the proportion of algae released in HTS individuals did not drop. Instead, bleaching averaged 69.7±20.6% and was not significantly different from untreated HTS animals (figure 1). Furthermore, bleaching in CT anemones trended towards an increase (35.3±21.7%), although the increase was not statistically significant (figure 1). This same pattern was repeated in anemones subjected to caspase knockdown by RNAi. Bleaching in HTS animals had an increasing trend, averaging 90.5±21.7%, but was not significantly different from untreated HTS animals. Bleaching in CT anemones was 25.9±17.4%, trending towards an increase but not significantly elevated from untreated CT animals.
Figure 1.
Expulsion of symbiotic dinoflagellates (error bars, s.d.) in response to separate manipulation of apoptosis or autophagy. Results of multiple independent tests (one-way ANOVA and post hoc Tukey pairwise comparison): between CT (white bars) and HTS (grey bars; filled circles, p≤0.01); between control untreated and treated: open triangle, p≤0.05 and filled triangle, p≤0.01; and between HTS untreated and treated, p≥0.05. N=4.
Simultaneous induction of apoptosis and inhibition of autophagy by the treatment of HTS anemones with either a single or combined PI3K inhibitors did not significantly decrease bleaching when compared with untreated HTS animals (average algal loss 69.4±12.0%; figure 1). HTS anemones treated with 250 and 500 nM wortmannin lost an average of 81.3±21.7 and 75.4±9.9% of their algal population, respectively, while those treated with 10 μM 3MA lost 92.0±8.8%. In HTS anemones treated with both 10 μM 3MA and 500 nM wortmannin, 75.1±21.8% of algae were lost (figure 1). There was, however, a significant increase in bleaching in treated compared with untreated CT anemones. Bleaching rates increased to 61.1±16.8, 76.9±9.0 and 70.2±9.0% in CT animals in 500 nM wortmannin, 10 μM 3MA and the combination of 10 μM 3MA and 500 nM, respectively, corresponding to an average combined 29-fold increase over bleaching rates in untreated CT animals (figure 1). Treatment with 250 nM wortmannin had no significant effect on bleaching in CT or HTS anemones, indicating that the inhibitor effect is concentration dependent (figure 1). There was no significant difference in the amount of bleaching between anemones with a combination of PI3K inhibitors and those individuals treated with a single inhibitor, which suggests that a single form of PI3K inhibitor was as effective as treating with a combination of different types.
To further investigate the role of autophagy in bleaching, anemones were treated with rapamycin, a known inducer of autophagy. There was no significant decrease in algal loss from HTS animals treated with 5 μM (45.5±6.8% of algae lost) and 25 μM (74.0±6.5%) rapamycin compared with untreated HTS controls (69.4±12.0%; figure 1). There was, however, a significant increase in bleaching in CT anemones incubated in 25 μM rapamycin. They lost an average of 86.9±22.5% of their algae, a 43-fold increase over untreated CT animals (figure 1).
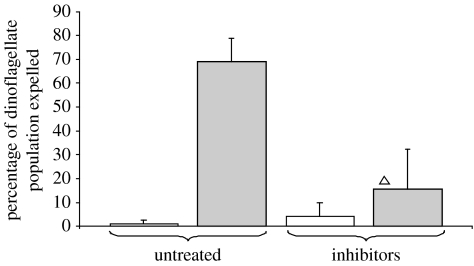
In a separate experiment, CT and HTS anemones were simultaneously treated with both apoptotic and autophagic inhibitors. The percentage of algae lost from HTS anemones treated with the cocktail of inhibitors dropped significantly to an average of 15.6±16.6%, an over fourfold drop compared with values in untreated HTS animals (figure 2). The expulsion of algae from CT anemones was not significantly different between treated and untreated anemones (figure 2).
Figure 2.
Expulsion of symbiotic dinoflagellates (+s.d.) in response to simultaneous manipulation of apoptosis and autophagy. Inhibitors applied were as follows: 10 μM 3MA, 500 nM wortmannin and 12.5 μM ZVAD-fmk in 0.2% DMSO. White bars, CT; grey bars, HTS. (Intra-treatment test: Kruskal–Wallis, adjusted to Bonferroni significance level, open triangle, p>0.05, N=5.)
4. Discussion
This study suggests that no single process operates during HTS-induced bleaching of A. pallida and that there is an interaction of cellular pathways. This study is also the first to implicate a role of autophagy in cnidarian bleaching.
HTS anemones released significantly more symbionts than CT anemones, indicating that experimentally induced bleaching was successful. This is a common and well-described response in symbiotic cnidarians to HTS (for reviews, see Brown 1997, Hoegh-Guldberg 1999 and Lesser 2004). The separate manipulation of apoptosis through gene knockdown, chemical inhibition and chemical induction surprisingly resulted in no significant decrease in bleaching in HTS anemones. The lack of response to apoptosis manipulation during stress could be explained by at least two scenarios. First, it is possible that apoptosis plays no significant role in HTS-induced bleaching. This is unlikely, however, as previous reports have shown an increase in host apoptotic activity during the onset of bleaching (Dunn et al. 2004; Richier et al. 2006). Second, it is possible that additional processes are simultaneously playing a role in stress-induced bleaching. These other processes may be initiated or differentially regulated when apoptosis is manipulated. In this scenario, the role of apoptosis is masked by the change in regulation of another cellular process as bleaching continues.
Autophagy could be one such additional process involved in the heat stress response that either acts simultaneously with apoptosis or is actively induced, as a back-up mechanism to mitigate apoptosis failure. Like the results from apoptosis manipulation, there was no significant decrease in bleaching in HTS animals with autophagy manipulation, either induction or inhibition. This suggests that either autophagy plays no role in stress-induced bleaching or that it is not the sole pathway that is controlling stress-induced bleaching.
There was, however, a dramatic increase in bleaching in CT animals incubated in the autophagy promoter rapamycin. Two possible explanations for bleaching following rapamycin treatment and presumably TOR inhibition are: (i) autophagy is acting at a cellular component level (Kundo & Thompson 2005) to remove and digest organelles including the alga-containing symbiosome, and (ii) host cells undergo autophagic cell death and/or secondary apoptosis induced by rapamycin (Castedo et al. 2002), which results in the release of symbionts. This dramatic response to rapamycin incubation under control conditions suggests that autophagy plays a role in the stability of the symbiosis under non-stressed conditions.
HTS anemones treated with a cocktail of apoptotic and autophagic pathway inhibitors exhibited dramatically decreased bleaching compared with untreated HTS anemones (figure 2). This suggests that the combination of inhibitors counteracted reciprocal activation, thereby preventing a see-saw alternation between the pathways. The expulsion of algae from CT anemones was not significantly different between treated and untreated animals, indicating that any effect induced by single pathway manipulation was counteracted by dual pathway inhibition. One could argue that the concentrations of inducers or inhibitors in the single treatment manipulations are toxic. This would explain continued high bleaching in HTS animals and increased bleaching in CT anemones due to widespread tissue disruption. However, these compounds at the same concentrations, when used in combination, reduced the onset of bleaching in HTS anemones and did not increase bleaching in CT anemones.
Our results suggest that no single process is active in symbiont expulsion during HTS-induced bleaching of A. pallida, an idea that was first suggested by Gates et al. (1992) and expanded by Dunn (2002). Our findings allow us to formulate a model for the interrelated roles of apoptosis and autophagy as part of the cellular mechanisms involved in bleaching. We propose that the two pathways are linked in a see-saw manner such that when apoptosis is inhibited, autophagy is initiated as a back-up mechanism and vice versa. In this way, the two processes act together during HTS-induced bleaching. The interactions between the environmental perturbations that lead to bleaching in nature and underlying cellular and physiological processes remain complex and unresolved. Linkages between our laboratory-generated model and actual bleaching events in the field are areas for future research.
The see-saw relationship between apoptosis and autophagy has been found in vertebrate systems. For example, in mammalian cell lines, when autophagy is inhibited or knocked down by RNAi, apoptosis is triggered (Boya et al. 2005). Furthermore, autophagy is upregulated in mouse cell lines treated with caspase inhibitors or caspase gene knockdown (Yu et al. 2004, 2006). These studies are particularly interesting because cells are destroyed by ROS-induced death due to selective autophagic digestion of catalase. As detailed in §1, ROS plays an important role in the initial stages of HTS in the cnidarian–dinoflagellate symbioses. There is recent evidence of catalase inhibition during bleaching of the anemone Anemonia viridis (Merle et al. 2007). Future studies could explore a link between catalase inhibition and initiation of autophagy.
A recent functional genomic study of cnidarian–dinoflagellate symbiosis (Rodriguez-Lanetty et al. 2006) suggests that manipulation of multiple cellular pathways occurs during symbiosis. One such pathway proposed is the suppression of apoptosis and promotion of cell proliferation through the sphingosine–sphingosine-1-phosphate (S1P) rheostat via downregulation of sphingosine-1-phosphate phosphatase (Rodriguez-Lanetty et al. 2006). The same rheostat regulation controls autophagy via inhibition of the mammalian homologue to TOR (mTOR) in human cell lines (Lavieu et al. 2006). The sphingosine–S1P rheostat is also a control point for autophagy manipulation by invading parasitic Mycobacterium in animals. Mycobacterium tuberculosis arrests autophagosome maturation through the downregulation of sphingosine kinase (Malik et al. 2003; Thompson et al. 2005) and PI3K/AKT signalling (Koul et al. 2004). Control of the rheostat and PI3K/AKT signalling may therefore act as part of one mechanism, common to some microbial parasites and symbionts, which prevent both host apoptosis and autophagy. The end result would be persistence by the microbe within the host cell. In the case of cnidarian bleaching, these regulatory mechanisms could be disrupted during HTS, which in turn could cause the initiation of the autophagic and apoptotic processes leading to symbiont release from the host.
Acknowledgments
We would like to thank the members of the Weis laboratory for their editorial comments and the members of the Department of Statistics at Oregon State University for their valued input. This work was funded by an NSF grant (MCB 0237230).
Supplementary Material
Comparison of symbiotic dinoflagellate expulsion in untreated anemones and vehicle solvent controls. There was no significant difference between samples in both control temperature (open bar) and hyperthermic (grey bar) stress conditions. (One-way ANOVA, post hoc Tukey pairwise comparison adjusted to Bonferonni significance level, p>0.05), N=4
References
- Ainsworth T.D, Kvennefors E.C, Blackall L.L, Fine M, Hoegh-Guldberg O. Disease and cell death in white syndrome of acroporid corals on the Great Barrier Reef. Mar. Biol. 2007;151:19–29. doi:10.1007/s00227-006-0449-3 [Google Scholar]
- Blommaart E.F.C, Krause U, Schellens J.P.M, Vreeling-Sindelárová H, Meijer A.J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. doi:10.1111/j.1432-1033.1997.0240a.x [DOI] [PubMed] [Google Scholar]
- Boya P, et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. doi:10.1128/MCB.25.3.1025-1040.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.E. Coral bleaching: causes and consequences. Coral Reefs. 1997;16:S129–S138. doi:10.1007/s003380050249 [Google Scholar]
- Caro L.H, Plomp P.J, Wolvetang E.J, Kerkhof C, Meijer A.J. 3-Methyadenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur. J. Biochem. 1988;175:325–329. doi: 10.1111/j.1432-1033.1988.tb14200.x. doi:10.1111/j.1432-1033.1988.tb14200.x [DOI] [PubMed] [Google Scholar]
- Castedo M, Ferri K.F, Kroemer G. Mammalian target of rapamycin (mTOR): pro- and anti-apoptotic. Cell Death Differ. 2002;9:99–100. doi: 10.1038/sj.cdd.4400978. doi:10.1038/sj/cdd/4400978 [DOI] [PubMed] [Google Scholar]
- Chan E.D, Morris K.R, Belisle J.T, Hill P, Remigo L.K, Brennan P.J, Riches D.W.H. Induction of inducible nitric oxide synthase-NO by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK-JNK and NF-kB signalling pathways. Infect. Immun. 2001;69:2001–2010. doi: 10.1128/IAI.69.4.2001-2010.2001. doi:10.1128/IAI.69.4.2001-2010.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Lee J.T, Navolanic P.M, Steelman L.S, Shelton J.G, Blalock W.L, Franklin R.A, McCubrey J.A. Involvement of PI3K/AKT pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. doi:10.1038/sj.leu.2402824 [DOI] [PubMed] [Google Scholar]
- Chua J, Vergne I, Master S.S, Deretic V. A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Curr. Opin. Microbiol. 2004;7:71–77. doi: 10.1016/j.mib.2003.12.011. doi:10.1016/j.mib.2003.12.011 [DOI] [PubMed] [Google Scholar]
- Cruz M.C, Cavallo L.M, Görlach J.M, Cox G, Perfect J.R, Cardenas M.E, Heitman J. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol. Cell. Biol. 1999;19:4101–4112. doi: 10.1128/mcb.19.6.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A.M. Autophagy: many paths to the same end. Mol. Biochem. Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. doi:10.1023/B:MCBI.0000041848.57020.57 [DOI] [PubMed] [Google Scholar]
- Cui Q, Tashiro S.-i, Onodera S, Ikejima T. Augmentation of oridonin-induced apoptosis observed with reduced autophagy. J. Pharmacol. Sci. 2006;101:230–239. doi: 10.1254/jphs.fpj06003x. doi:10.1254/jphs.FPJ06003X [DOI] [PubMed] [Google Scholar]
- David C.N, Schmidt N, Schade M, Pauly B, Alexandrova O, Bottger A. Hydra and the evolution of apoptosis. Integr. Comp. Biol. 2005;45:631–638. doi: 10.1093/icb/45.4.631. doi:10.1093/icb/45.4.631 [DOI] [PubMed] [Google Scholar]
- Dermine J.-F, Scianimanico S, Privé C, Descoteaux A, Desjardins M. Leismania promastigotes require lipophosphoglycan to actively modulate the fusion properties of phagosomes at an early step of phagocytosis. Cell. Microbiol. 2000;2:115–126. doi: 10.1046/j.1462-5822.2000.00037.x. doi:10.1046/j.1462-5822.2000.00037.x [DOI] [PubMed] [Google Scholar]
- Dunn, S. R. 2002 Cell death mechanisms during bleaching of the sea anemone Aiptasia sp. In Marine sciences and coastal management, pp. 206. Newcastle upon Tyne, UK: University of Newcastle upon Tyne.
- Dunn S.R, Bythell J.C, Le Tissier M.D.A, Burnett W.J, Thomason J.C. Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. J. Exp. Mar. Biol. Ecol. 2002;272:29–53. doi:10.1016/S0022-0981(02)00036-9 [Google Scholar]
- Dunn S.R, Thomason J.C, Le Tissier M.D.A, Bythell J.C. Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death Differ. 2004;11:1213–1222. doi: 10.1038/sj.cdd.4401484. doi:10.1038/sj.cdd.4401484 [DOI] [PubMed] [Google Scholar]
- Dunn S.R, Phillips W.S, Spatafora J.W, Green D.R, Weis V.M. Highly conserved caspase and Bcl-2 homologues from the sea anemone Aiptasia pallida: lower metazoans as models for the study of apoptosis evolution. J. Mol. Evol. 2006;63:95–107. doi: 10.1007/s00239-005-0236-7. doi:10.1007/s00239-005-0236-7 [DOI] [PubMed] [Google Scholar]
- Dunn S.R, Phillips W.S, Weis V.M. Knockdown of actin and caspase gene expression by RNA interference on the symbiotic sea anemone Aiptasia pallida. Biol. Bull. (Woods Hole) 2007;212:250–258. doi: 10.2307/25066607. [DOI] [PubMed] [Google Scholar]
- Fairbairn I.P. Macrophage apoptosis in host immunity to mycobacterial infections. Biochem. Soc. Trans. 2004;32:496–498. doi: 10.1042/BST0320496. doi:10.1042/BST0320496 [DOI] [PubMed] [Google Scholar]
- Fan T.-J, Han L.-H, Cong R.-S, Liang J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. doi:10.1111/j.1745-7270.2005.00108.x [DOI] [PubMed] [Google Scholar]
- Fratti R.A, Backer J.M, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 2001;154:631–643. doi: 10.1083/jcb.200106049. doi:10.1083/jcb.200106049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates R.D, Baghdasarian G, Muscatine L. Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Biol. Bull. 1992;182:324–332. doi: 10.2307/1542252. doi:10.2307/1542252 [DOI] [PubMed] [Google Scholar]
- González-Polo R.-A, Boya P, Pauleau A.-L, Jalil A, Larochette N, Souquère S, Pierron G, Saftig P, Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J. Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. doi:10.1242/jcs.02447 [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. doi:10.1038/sj.onc.1207521 [DOI] [PubMed] [Google Scholar]
- Gutierrez M.G, Master S.S, Singh S.B, Taylor G.A, Colombo M.I, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. doi:10.1016/j.cell.2004.11.038 [DOI] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K.-i, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Kazuyoshi Y. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:179–189. doi: 10.1016/s0092-8674(02)00833-4. doi:10.1016/S0092-8674(02)00833-4 [DOI] [PubMed] [Google Scholar]
- Hengartner M.O, Bryant J.A. Apoptotic cell death: from worms to wombats…but what about the weeds? In: Bryant J.A, Hughes S.G, Garland J.M, editors. Programmed cell death in animals and plants. BIOS Scientific Publishers Ltd; Oxford, UK: 2000. pp. 1–9. [PubMed] [Google Scholar]
- Hilbi H. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell. Microbiol. 2006;8:1697–1706. doi: 10.1111/j.1462-5822.2006.00793.x. doi:10.1111/j.1462-5822.2006.00793.x [DOI] [PubMed] [Google Scholar]
- Hirosako K, et al. 3-Methyladenine specifically inhibits retrograde transport of cation-independent mannose 6-phosphate/insulin-like growth factor II receptor from the early endosome to the TGN. Biochem. Biophys. Res. Commun. 2004;316:845–852. doi: 10.1016/j.bbrc.2004.02.119. doi:10.1016/j.bbrc.2004.02.119 [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. Climate change, coral bleaching anf the future of the world's coral reefs. Mar. Freshw. Res. 1999;50:839–866. doi:10.1071/MF99078 [Google Scholar]
- Hughes T.P, et al. Climate change, human impacts, and the resilence of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. doi:10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- Koul A, Herget T, Klebl B, Ullrich A. Interplay between mycobacteria and host signalling pathways. Nat. Rev. Microbiol. 2004;2:189–202. doi: 10.1038/nrmicro840. doi:10.1038/nrmicro840 [DOI] [PubMed] [Google Scholar]
- Kundo M, Thompson C.B. Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ. 2005;12:1484–1485. doi: 10.1038/sj.cdd.4401780. doi:10.1038/sj.cdd.4401780 [DOI] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase1 and its role in cell survival during nutrient starvation. J. Biol. Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. doi:10.1074/jbc.M506182200 [DOI] [PubMed] [Google Scholar]
- Lesser M.P. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs. 1997;16:187–192. doi:10.1007/s003380050073 [Google Scholar]
- Lesser M.P. Experimental biology in coral reef ecosystems. J. Exp. Mar. Biol. Ecol. 2004;300:217–252. doi:10.1016/j.jembe.2003.12.027 [Google Scholar]
- Lin K.L, Wang J.T, Fang L.S. Participation of glycoproteins on zooxanthellal cell walls in the establishment of a symbiotic relationship with the sea anemone, Aiptasia pulchella. Zool. Stud. 2001;39:172–178. [Google Scholar]
- Malik Z.A, Thompson C.B, Hashimi S, Porter B, Iyer S.S, Kusner D.J. Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibiton of sphingosine kinase. J. Immunol. 2003;170:2811–2815. doi: 10.4049/jimmunol.170.6.2811. [DOI] [PubMed] [Google Scholar]
- Méresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel J.-P. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1999;1:E183–E187. doi: 10.1038/15620. doi:10.1038/15620 [DOI] [PubMed] [Google Scholar]
- Merle P.-L, Sabourault C, Richier S, Allemand D, Furla P. Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic. Biol. Med. 2007;42:236–246. doi: 10.1016/j.freeradbiomed.2006.10.038. doi:10.1016/j.freeradbiomed.2006.10.038 [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Catt K.J, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl Acad. Sci. USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. doi:10.1073/pnas.92.12.5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T.P. Body building: regulation of shape and size by PI3k/TORsignalling during development. Mech. Dev. 2003;120:1283–1296. doi: 10.1016/j.mod.2003.07.003. doi:10.1016/j.mod.2003.07.003 [DOI] [PubMed] [Google Scholar]
- Pahan K, Raymond J.R, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J. Biol. Chem. 1999;274:7528–7536. doi: 10.1074/jbc.274.11.7528. doi:10.1074/jbc.274.11.7528 [DOI] [PubMed] [Google Scholar]
- Perez S, Weis V.M. Nitric oxide and cnidarian bleaching: an eviction notice mediates breakdown of a symbiosis. J. Exp. Biol. 2006;209:2804–2810. doi: 10.1242/jeb.02309. doi:10.1242/jeb.02309 [DOI] [PubMed] [Google Scholar]
- Richier S, Furla P, Plantivaux A, Merle P.-L, Allemand D. Symbiosis-induced adaptation to oxidative stress. J. Exp. Biol. 2005;208:277–285. doi: 10.1242/jeb.01368. doi:10.1242/jeb.01368 [DOI] [PubMed] [Google Scholar]
- Richier S, Sabourault C, Courtiade J, Zucchini N, Allemand D, Furla P. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. FEBS J. 2006;273:4186–4198. doi: 10.1111/j.1742-4658.2006.05414.x. doi:10.1111/j.1742-4658.2006.05414.x [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lanetty M, Phillips W.S, Weis V.M. Transcriptome analysis of a cnidarian–dinoflagellate mutualism reveals complex modulation of host gene expression. BMC Genom. 2006;7:23. doi: 10.1186/1471-2164-7-23. doi:10.1186/1471-2164-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P.O, Gordon P.B. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl Acad. Sci. USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. doi:10.1073/pnas.79.6.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W. H. Freeman and Company; New York, NY: 1995. Biometry: the principles and practice of the statistics in biological research. [Google Scholar]
- Thompson C.B, Iyer S.S, Melrose N, VanOosten R, Johnson K, Pitson S.M, Obeid L.M, Kusner D.J. Sphingosine kinase 1 (Sk1) is recruited to nascent phagosomes in human macrophages: inhibtion of SK1 translocation by Mycobacterium turberculosis. J. Immunol. 2005;174:3557–3561. doi: 10.4049/jimmunol.174.6.3551. [DOI] [PubMed] [Google Scholar]
- Trapido-Rosenthal H.G, Sharp K.H, Galloway T.S, Morrall C.E. Nitric oxide and cnidarian–dinoflagellate symbiosis: pieces of a puzzle. Am. Zool. 2001;41:247–257. doi:10.1668/0003-1569(2001)041[0247:NOACDS]2.0.CO;2 [Google Scholar]
- Woo E.J, Kim Y.G, Kim M.S, Han W.D, Shin S, Robinson H, Park S.Y, Oh B.H. Structural mechanism for inactivation and activation of CAD/DFF40 in the apoptotic pathway. Mol. Cell. 2004;14:531–539. doi: 10.1016/s1097-2765(04)00258-8. doi:10.1016/S1097-2765(04)00258-8 [DOI] [PubMed] [Google Scholar]
- Wood-Charlson E.M, Hollingsworth L.L, Krupp D.A, Weis V.M. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 2006;8:1985–1993. doi: 10.1111/j.1462-5822.2006.00765.x. doi:10.1111/j.1462-5822.2006.00765.x [DOI] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke E.H, Lenardo M.J. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. doi:10.1126/science.1096645 [DOI] [PubMed] [Google Scholar]
- Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke E.H, Lenardo M.J. Autophagic programmed cell death by selective catalase degradation. Proc. Natl Acad. Sci. USA. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. doi:10.1073/pnas.0511288103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of symbiotic dinoflagellate expulsion in untreated anemones and vehicle solvent controls. There was no significant difference between samples in both control temperature (open bar) and hyperthermic (grey bar) stress conditions. (One-way ANOVA, post hoc Tukey pairwise comparison adjusted to Bonferonni significance level, p>0.05), N=4