Abstract
Sicarius and Homalonychus are unrelated, desert-dwelling spiders that independently evolved the ability to cover themselves in fine sand particles, making them cryptic against their background. Observations that particles associate with these spiders' setae inspired us to investigate the role of setal microstructure in particle capture and retention. Here we report that Sicarius and Homalonychus convergently evolved numerous high aspect ratio, flexible fibres that we call ‘hairlettes’ protruding from the setal shaft. We demonstrate that particles attach more densely to regions of Homalonychus with hairlettes than to other regions of the same animal where hairlettes are absent, and document close contact of hairlettes to sand particles that persists after applying force. Mathematical models further suggest that adhesion of hairlettes to sand particles is a sufficient mechanism of particle capture and retention. Together, these data provide the first evidence that hairlettes facilitate sand retention through intermolecular adhesion to particles. Their independent evolutionary origins in Sicarius and Homalonychus suggest that the unique setal structure is adaptive and represents a general biomechanical mechanism for sand capture to cuticle. This discovery has implications for the design of inventions inspired by this system, from camouflage to the management of granular systems.
Keywords: intermolecular adhesion, adaptation, camouflage, convergent evolution, spider
1. Introduction
The incorporation of materials from the environment onto the body, dwellings, egg or larval cases has been observed in groups as diverse as arachnids, crustaceans, insects and echinoderms (Cott 1957; Levi & Levi 1969; Hölldobler & Wilson 1986; Vetter & Cokendolpher 2000; Adams 2001; Brandt & Mashberg 2002; Eberhard 2003; Bruce et al. 2004; Domínguez & Jiménez 2005). The materials attached range from bits of shell and algae to sand grains to the remains of dead prey (Cott 1957; Levi & Levi 1969; Vetter & Cokendolpher 2000; Brandt & Mashberg 2002; Eberhard 2003; Bruce et al. 2004; Domínguez & Jiménez 2005). These materials often provide camouflage that facilitates predator avoidance or stealthy predation (Cott 1957; Hölldobler & Wilson 1986; Eberhard 2003) but can also function to attract prey (Bruce et al. 2004) and protect organisms from UV radiation (Adams 2001).
In spiders, sand or dirt attachment to the cuticle has been documented in six unrelated genera (Sicarius, Homalonychus, Microstigmata, Paratropis, Cryptothele and Bradystichus; Platnick & Raven 1981; Roth 1984; Griswold 1985; Coddington & Levi 1991; Dippenaar-Schoeman & Jocqué 1997) that represent a broad phylogenetic range (Platnick 2007). Most studies mentioning sand/dirt particle attachment are taxonomically focused, and the function of particle coverage is generally assumed to be camouflage. Though some aspects of the system have been investigated in Sicarius and Homalonychus (Reiskind 1965; Levi & Levi 1969; Roth 1984; Domínguez & Jiménez 2005), a detailed description of the mechanism of sand attachment has not been done for any taxon.
Many arthropods that attach materials to their bodies hold them in place with specialized setae (Hölldobler & Wilson 1986; Gorb 2001; Brandt & Mashberg 2002) that sometimes have elaborate microstructures (Hölldobler & Wilson 1986). The same may be true for Sicarius and Homalonychus, which both possess densely distributed setae (Chamberlin 1916; Roth 1984) with which fine particles associate (Levi & Levi 1969; Roth 1984; figure 1). Previous descriptions of Sicarius and Homalonychus lead us to hypothesize that details of setal morphology provide the mechanism of sand capture and retention in these two genera. Fortunately, characteristics of the genera make them well suited for comparative analyses to investigate this hypothesis. These spider genera are unrelated (Coddington 2005), yet both live in sandy microhabitats (Reiskind 1965; Roth 1984) and have specialized behaviours that cover their bodies in fine particles (Reiskind 1965; Domínguez & Jiménez 2005). The sand remains attached for a long time and transforms their body colour to match the background substrate, resulting in remarkable concealment in their native habitats (figure 1a). The striking parallels between these genera in behaviour and natural history, the rarity of sand attachment in spiders and the distant phylogenetic placement of Sicarius (Coddington & Levi 1991) and Homalonychus (Roth 1984) indicate that they independently evolved cuticular sand capture. Under these circumstances, characteristics they uniquely share that are involved in the process of sand capture are candidates for adaptations which originated independently under selection for sand attachment to the cuticle, and are unlikely to be due to retention of a trait that originated in a common ancestor under selection for a different purpose.
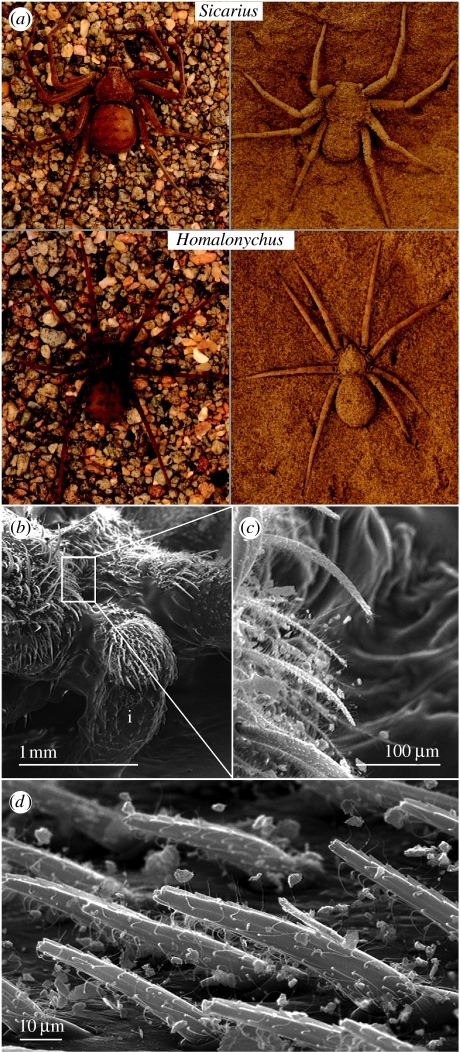
Figure 1.
Sicarius and Homalonychus exoskeletons capture fine sand and are covered in densely distributed setae. (a) Macroscopic views of Sicarius and Homalonychus with (dusted) and without (undusted) cuticular sand coverage. (b–d) Cuticular microstructure of Sicarius viewed by SEM: (b) left anterior side of a Sicarius; (c,d) magnified view of setae; (i) marks the left palp of the spider. The photograph in (a) was taken by Ken Cramer.
Variation within Homalonychus provides further evidence that setal morphology plays a central role in retaining sand particles and also a second level of comparative analysis. In this genus, mature males lack sand adhesion and have setal morphology that differs from mature females and juveniles (Roth 1984). Moreover, within a single individual, setal morphology varies spatially on the carapace providing an ideal framework within which the role of setal structure in sand capture can be tested.
Despite the noted correlation between sand attachment and the morphology of dorsal setae in Homalonychus, few studies to date have carefully analysed the role of setal morphology in cuticular particle capture in terrestrial arthropods and none has proposed a physical mechanism for particle retention. Here, we present evidence that the setae of Sicarius and Homalonychus have convergent high aspect ratio, flexible fibres protruding from them (‘hairlettes’) that adhere to particles. Using a mathematical models, we test the feasibility of particle capture by intermolecular adhesion between sand and hairlettes. This study is the first to propose that hairlettes are required for sand retention in Sicarius and Homalonychus, and that adhesion by intermolecular forces is a sufficient mechanism.
2. Material and methods
(a) Taxon sampling and rationale
For analyses and experiments with Sicarius, we sampled multiple populations from both Africa and South America to ensure that our observations were representative of the entire genus. For Homalonychus, we sampled from multiple populations of both the described species. See electronic supplementary material for details on taxon sampling of haplogyne outgroups.
(b) Specimen collection and care
We collected all Sicarius and Homalonychus individuals from the field of the following locations: Arizona, USA (Homalonychus selenopoides); California, USA (Homalonychus theologus); Namibia (Sicarius sp.); South Africa (Sicarius sp.); Argentina (Sicarius sp.); and Peru (Sicarius sp.). A complete list of species and localities is included in the electronic supplementary material, table 1. We reared spiders in the laboratory and fed them crickets every 1–3 weeks, depending on the population. We kept Sicarius and Homalonychus in coarse-grained sand (electronic supplementary material, figures 3 and 4) to prevent them from dusting themselves after moulting. All specimens that were not destroyed during analysis are either alive and continue to be used for other research or were preserved in 75% ethanol and deposited in the Lewis & Clark College arachnid collection. After completion of the research, vouchers will be deposited in the national museums of the countries of origin and in the American Museum of Natural History. Homalonychus collected from the Organ Pipe Cactus National Monument in Arizona remain in our collection (accession number ORPI-359; catalogue numbers ORPI-15361 to ORPI-15370). See electronic supplementary material for collection localities and specimen deposition information for haplogyne outgroups.
(c) Sample preparation for scanning electron microscopy
We used moults for all analyses of Sicarius and Homalonychus juveniles in order to avoid sacrificing precious specimens needed for other research, or specimens represented in low numbers in our collections. For consistency and since abdominal cuticle is expandable in spiders, we used cuticle from the carapace (either whole carapaces or portions of them) for all analyses and experiments. When using whole carapaces, we affixed them to scanning electron microscopy (SEM) mounting stubs using colloidal silver liquid (Ted Pella) and let the adhesive set overnight. When using portions of carapace cuticle, we attached flat pieces of cuticle to SEM stubs using double sided tape.
We coated all specimens with platinum in a Hummer VI Sputtering System (Technics). Specimens used for morphological descriptions were coated with less than 20 nm of platinum. Owing to difficulties in grounding samples for electron microscopy, we applied a heavier coat (approx. 40 nm) to specimens that we experimentally dusted in fine particles. For most analyses, we visualized samples in an Amray 1810 SEM and captured images digitally in ImageDV (v. 1.3; Evological). For calculating hairlette diameter, we visualized samples in a Serion XL30 SEM (FEI) and captured images digitally.
(d) Seta necessity experiments in Sicarius
Working under a dissecting microscope (Olympus SZ40), we cut each carapace into two flat pieces and removed setae from one piece by carefully brushing an insect pin against them. For each individual, we mounted pieces of cuticle with and without setae on an SEM stub as described above and dusted them with an excess of ceramic microspheres (3M; mean diameter, 40 μm; particles range from approx. 1 to 200 μm diameter; 10th percentile, 12 μm; 50th percentile, 40 μm; 90th percentile, 100 μm; 95th percentile, 200 μm). We used microspheres instead of sand for seta necessity experiments because they completely covered spiders that dusted themselves in them (electronic supplementary material, figure 4) and their uniform shapes facilitated quantitative measurements. Based on the Udden–Wentworth scale used by sedimentologists, the size range of microspheres we used fall into categories of clay, silt and fine sand with a mean of 40 μm in the ‘coarse silt’ category. Despite this distinction we continue to use the term ‘sand’ for simplicity. While we did not measure the particle size distribution of the spiders' native substrate, we are confident that it is in the distribution of the microspheres based on analyses of particle sizes that attach to spiders (electronic supplementary material, figure 3). To remove any loose particles, we subjected samples to an acceleration of 6.5× gravity for 5 s using a minivortexer (VWR) with a custom mount made to hold SEM stubs. We sputter coated samples after dusting them with particles and applying acceleration.
To quantify particle adhesion on bare cuticle and cuticle with setae, we took five to six images (greater than or equal to 3860×) of random, standardized sections of cuticle from each condition for each individual. We captured the first image in an arbitrary corner of the piece of cuticle and then moved the sample along the y-axis to capture the next two images. After the third image, we moved the sample along the x-axis to capture the next two to three images (every third frame captured, where a ‘frame’ fills the SEM monitor and all frames are adjacent to each other). We avoided imaging sections where the cuticle was cracked and in the no setae condition we did not capture images that contained hair sockets. If these occurred in a frame that should have been captured, we moved the sample along the appropriate axis until the cuticle was clear of cracks or hair sockets. If we encountered the edge of the cuticle before obtaining all images, we would move the sample perpendicular to the previous direction of movement to capture the rest.
We quantified the particles manually in ImageJ (NIH), counting only visible particles, and divided the number by the area of the image to estimate the number of particles per square micrometre. For each individual, we averaged particles per square micrometre across all images per condition. We used a paired t-test to statistically compare the mean number of particles that adhered for each condition across all individuals.
(e) Descriptions of setal morphology
We described setal morphology from SEM images. To quantify setal density, we took six images at 550× of each carapace along the anterior–posterior axis starting behind each lateral set of eyes. We counted setae manually and calculated the density in each image using ImageJ (NIH). Hairlette lengths were measured from setae along the A–P axis that we selected using a random number table. For each individual, images of three setae were captured and we measured all clearly visible hairlettes in ImageJ (NIH). To measure hairlette diameter in Sicarius, we imaged hairlettes from setae selected using a random number table, starting just posterior to the left dyad of eyes and counting setae across the left–right axis of the carapace until arriving at the appropriate number. It was difficult to image hairlettes because they frequently moved around in the SEM due to heating effect and some were obstructed by sand grains. We imaged six that were the most motionless and were at an angle from our field of view that was amenable to accurate measurements and visibility of their tips. In ImageJ (NIH), we measured the diameter of the hairlettes at five places that were 50 nm apart, starting 100 nm from the tip or at the base of the globular form present at their tips, whichever was the farthest. We averaged these measurements for each hairlette.
(f) Comparing sand adhesion in regions within the Homalonychus carapace
After mounting carapaces on SEM stubs, we dusted them with fine sand (grade 0; see electronic supplementary material, figure 3) and applied acceleration as described previously before sputter coating. We imaged the samples and used the images to qualitatively compare the overall sand attachment to different parts of the carapace known to vary in setal morphology (Roth 1984).
3. Results
(a) Setae facilitate particle capture in Sicarius
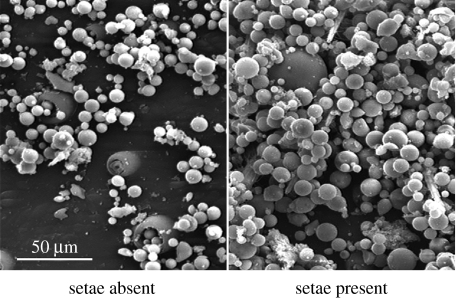
If morphological features of setae are adaptations to confer sand attachment in spiders, then setae should facilitate particle capture on the cuticle. In seta necessity experiments with Sicarius moulted carapaces (four South American and four African Sicarius), unaltered control regions of cuticle retained significantly more particles (49%) than regions of cuticle from which we experimentally removed setae (figure 2; 0.0324±0.0009 particles μm−2 for setae present condition, 0.0217±0.0027 particles μm−2 for setaless cuticle; mean±s.e.; t=−4.202; d.f.=7; p<0.005). These values are underestimates of the actual number of particles that attached to the cuticle when setae were present because many particles were hidden underneath the visible layer of spheres. In addition, particles visually covered a much greater area of the cuticle when setae were present than when they were absent (figure 2). Moreover, while we did not quantify differences in the size of particles that attached to setae-present and -absent conditions, we note anecdotally that much larger spheres tended to attach to the cuticle when setae were present (figure 2). These data demonstrate that setae facilitate particle attachment to the cuticle in Sicarius.
Figure 2.
Setae facilitate particle capture in Sicarius. To test if setae facilitate particle capture in Sicarius, we quantified particle adhesion on cuticle lacking setae and unaltered cuticle and compared the means across all individuals (n=8). Unaltered cuticle retained 49% more particles than cuticle that lacked setae (0.0324±0.00093 particles μm−2 for unaltered, 0.0217±0.0027 particles μm−2 for setaless cuticle; mean±s.e.; t=−4.202; d.f.=7; p<0.005). Particles visually covered much more of the cuticle when setae were present. Images are at same magnification.
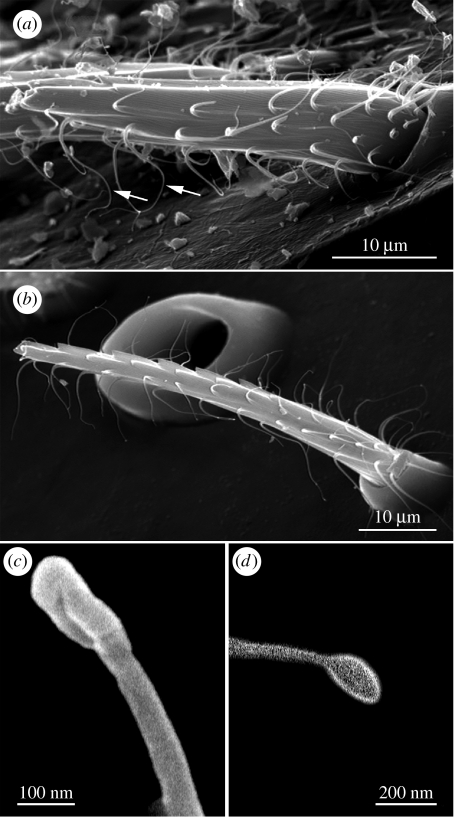
(b) Setal morphology is consistent within and between Sicarius and Homalonychus
If setal morphology has an adaptive role in sand capture, then detailed characteristics important for this function should be consistent across species within Sicarius and Homalonychus and perhaps between these two genera. Indeed, setae covering carapaces of Sicarius (9 South American and 11 African individuals) from all populations observed had long, thin hairlettes (arrows in figure 3a) protruding laterally and anteriorly along the proximal–distal axis of the setal shaft. The posterior side of the setae lacked hairlettes, but had two parallel ridges that were typically pointed. Hairlettes measured 10.17±3.08 μm long in Sicarius and 8.18±2.26 μm long in Homalonychus (mean±s.d.; n=188 hairlettes from 4 Sicarius individuals and 110 hairlettes from 4 Homalonychus individuals), tapered to a diameter of 41.6±6.01 nm in Sicarius (mean±s.d.; n=6 hairlettes each from two setae of one African and one South American individual) and ended in a globular form in both genera (figure 3c,d). Owing to the additional sputtered platinum layer, we estimate hairlettes' diameter to be 10–40 nm.
Figure 3.
Setae of Homalonychus and Sicarius have similar microstructures. SEM images of setae on the carapace of (a,c) Sicarius and (b,d) Homalonychus. Setae project from the cuticle at an angle in (a) Sicarius, have two parallel ridges extending up the posterior side and possess long, thin protruding hairlettes anteriorly and laterally (arrows in (a)). (c,d) Hairlettes taper and end in a globular form. The microstructures of these setae are the same in all Sicarius and Homalonychus individuals and species surveyed (see electronic supplementary material, table 1 for taxon sampling).
Setae were arranged on the carapace at a density of 344.73±166.87 setae mm−2 in Sicarius (mean±s.d., n=4 spiders) and 677.71±148.39 in Homalonychus (mean±s.d.; n=4 spiders). The large standard deviations may be due to a variation in the setal density in different regions of the carapace.
In Sicarius, setae projected from the cuticle at an acute angle (figure 1d) and pointed anteriorly, causing anterior hairlettes to be oriented towards the cuticle (figures 1d and 3a). Homalonychus setae with hairlettes were morphologically indistinguishable from Sicarius setae (Roth 1984; figure 3b,d). Hairlettes thus represent a candidate adaptation for sand particle capture by Sicarius and Homalonychus.
(c) Particles attach more densely to regions of Homalonychus that have hairlettes
The hypothesis that hairlettes are an adaptation conferring sand capture predicts that they should facilitate sand particle capture to the cuticle. We tested this hypothesis by experimentally dusting Homalonychus moulted carapaces with fine sand particles and comparing sand coverage around the eyes and on the posterior slope of the carapace, where setae lack hairlettes (Roth 1984), with sand coverage on the rest of the carapace where hairlettes are present. The carapaces of both Homalonychus (n=5) and Sicarius (n=5) retained a dense cover of particles after dusting them with fine sand and applying acceleration (6.5× gravity) to samples (figure 4a–d). In all Homalonychus individuals, sand attachment was reduced around the eyes (figure 4e). Overall, sand coverage was reduced on the posterior slope of the carapace (figure 4f–h), but sand clumped around setae locally within this region (figure 4h). The posterior slope of the carapace also showed reduced sand coverage in live Homalonychus that dusted themselves (electronic supplementary material, figure 4b).
Figure 4.
Reduced sand coverage occurred where hairlettes were absent in Homalonychus. To test the role of hairlettes in sand adhesion we compared sand adhesion in regions of the carapace where hairlettes are lacking with regions where they were present in Homalonychus. (a,b) Sand coverage on Sicarius carapace. (c,d) Sand coverage on Homalonychus carapace. (e) Sand coverage in eye region of Homalonychus. (f) View of Homalonychus dusted carapace from behind, showing sand coverage on the posterior slope. (g,h) Magnified views of sand coverage on the posterior slope of the Homalonychus carapace. ‘i’ marks the eyes of spiders; arrow in (h) marks localized sand clumping around setae.
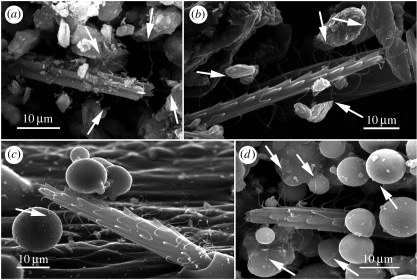
(d) Adhesion occurs between hairlettes and particles
To investigate the potential role of hairlettes in sand retention, we used SEM to observe interactions between hairlettes and particles in Sicarius and Homalonychus. Hairlettes were often observed in contact with sand particles and microspheres (figure 5). In some cases, suspended particles were in close contact with a single hairlette (figure 5c), indicating that adhesion occurs between hairlettes and particles, and that mechanical entrapment of particles between two or more hairlettes is not necessary.
Figure 5.
Hairlettes adhere to sand particles and microspheres. To assess if hairlettes play a role in sand capture we observed seta–particle interactions by SEM. Hairlettes associated with sand grains in (a) Sicarius and (b) Homalonychus, (c,d) as well as with microspheres in Sicarius. (b,c) In some cases, particles suspended in air were only in contact with hairlettes.
(e) Contact mechanics of hairlettes and particles
We assessed the putative role of intermolecular adhesion in the system by using two mathematical models to estimate the force of adhesion between hairlettes and particles and comparing it with the force exerted on particles in our experimental set-up. Sicarius cuticle retained very large particles after undergoing acceleration (e.g. 1×105 μm3, for a spherical particle of radius approx. 28.8 μm). If we consider that a particle that large would have a mass of 280 ng (assuming the density of sand equals the density of glass, 2.8 g cm−3) and that the acceleration applied to particles was 6.5× gravity, using F=ma we can calculate the amount of force required to retain the particle as being at least 18 nN. Assuming a van der Waals (vdW) adhesion mechanism, we model hairlettes as small cylinders that wrap around spherical particles, where the force of adhesion between a hairlette and a particle is given by (Israelachvili 1992; Tian et al. 2006)
| (3.1) |
where A is the Hamaker constant; b is the circumferential contact fraction; R is the radius of the hairlette (5–20 nm); and D is the atomic gap distance. Using conservative vdW adhesion parameters (A=0.4×10−19 J, D=0.3 nm), the circumferential contact fraction required to balance the force of adhesion and the inertial force exerted on the particle is 0.0006 to 0.001 times the circumference of a spherical particle of the volume 1×105 μm3. If the circumferential contact fraction were one-tenth the circumference of the particle, then FvdW would be 1.5–3 μN, two orders of magnitude greater than the force exerted on the particle under an acceleration of 6.5× gravity. Thus, even for a very small contact fraction, an acceleration exceeding 528× gravity would be necessary to detach a very large particle. Smaller and more typical particles in the range of 0.5–7.5 μm (radius) would require 7.8×103–3.5×106× gravity to detach.
To expand our understanding of the contact mechanics in this system, we also modelled hairlettes as flexible strips of tape that detach by peeling (Kendall 1975; Hansen & Autumn 2005). This model describes the force of adhesion between hairlette and particle as
| (3.2) |
where 2Rh is the hairlette width and Wph is the adhesion energy at the interface of the particle and the hairlette. Assuming vdW and using the typical value of 50 mJ m−2 for Wph (Israelachvili 1992), F≈0.5–2.0 nN for a hairlette (diameter, 10–40 nm) attaching to a large particle (radius approx. 28.8 μm). Such a particle would require simultaneous contact with 9–36 hairlettes to balance the force of acceleration applied to it. A single hairlette would be sufficient to hold more typical sized particles (radius, 0.5–7.5 μm), which would require accelerations of 10–1.39×105× gravity to detach.
4. Discussion
(a) Support for an adaptive role of hairlettes in sand covering
Sand or dust covering has evolved in various unrelated spider and insect taxa (Platnick & Raven 1981; Roth 1984; Griswold 1985; Coddington & Levi 1991; Dippenaar-Schoeman & Jocqué 1997; Brandt & Mashberg 2002), and has even been shown to function as camouflage in the larvae of some species of assassin bug (Brandt & Mashberg 2002). The independent evolution of sand/dust covering raises the question of whether similar adaptive morphologies may have arisen convergently in response to selection for particle attachment to the cuticle. Here we have demonstrated that setae facilitate particle capture in Sicarius (figure 2) and have shown that hairlettes, extremely long, flexible barbs protruding from setae, are present in both Sicarius and Homalonychus (figure 3). Reduced sand attachment occurs on regions of the Homalonychus carapace that lack hairlettes (figure 4), suggesting that they play a role in capturing particles. The occurrence of adhesion between hairlettes and particles (figure 5) and model-based evidence that intermolecular adhesion is a sufficient mechanism for particle retention further support a role for hairlettes in sand capture and retention.
The presence of hairlettes on all the Sicarius species we surveyed from both Africa and the Americas means that they were probably present in the most recent common ancestor of extant Sicarius. Comparative surveys of setal microstructure on carapaces of other haplogyne taxa (electronic supplementary material, figure 2) found no setal protrusions that compared with hairlettes in length, flexibility and nanoscale diameter. However, we did observe hairlette-like projections that were similar in dimensions to those of Sicarius and Homalonychus in casual observations of abdominal setae in the African species Loxosceles spinulosa, a member of the sister genus to Sicarius (Platnick et al. 1991). These setal structures have previously been noted in a taxonomic description of L. spinulosa (described as ‘minute barbs’) and were reported to be associated with dirt particles on spiders collected in the field (Newlands 1975). Thus, hairlette-like structures are apparently more taxonomically widespread than is indicated by our systematic survey of carapace setae, and there is variation in hairlette expression on different body regions of at least a few Loxosceles. Nonetheless, current knowledge of the pattern of taxonomic and morphological distribution of hairlettes is consistent with these microstuctures originating either before or concurrently with the origin of cuticular sand capture in this lineage. Our data suggest that this origin was followed in the Sicarius lineage by an increase in the density of expression of hairlettes on the dorsal surface of the animals coincident with the evolutionary origin of dense and long-term sand coverage.
Similar comparative analyses to estimate the timing of origin of hairlettes in Homalonychus are not currently feasible because, while it is clear they are members of the major spider clade entelegynes and thus not related to Sicarius (haplogynes), the exact phylogenetic placement of this genus is unknown (Coddington 2005). Despite this limitation, the convergent presence of hairlettes in Sicarius and Homalonychus (figure 3), along with their absence in mature male Homalonychus that do not retain sand (Roth 1984), further supports the hypothesis that they play an adaptive role in sand adhesion.
Reduction of sand adhesion in body regions that lack hairlettes (figure 4) is consistent with a role of hairlettes in sand capture in Homalonychus. This correlation is corroborated by comparative assessments of sand attachment in close relatives of Sicarius that vary in setal morphologies, but all lack hairlettes on their carapaces. While there was some degree of association between sand grains and setae in all groups, the distribution of particles on setae was less dense in taxa without hairlettes than in taxa with hairlettes (electronic supplementary material, figure 2). While this pattern supports a role of hairlettes in particle attachment, the variation in degree of sand coverage in taxa that lack setal hairlettes suggests that other factors such as alternate morphologies and surface properties could also influence the effectiveness of setae in particle capture. In many of these taxa, particles appeared to be trapped between or under setae that lay close to the carapace. This occurred even in taxa where sand grains associated weakly with individual setae relative to Sicarius and Homalonychus (electronic supplementary material, figure 2). Mechanical entrapment of particles between setae probably also occurs in Sicarius and Homalonychus, especially in regions of more densely distributed setae and for larger particles. In light of the more conservative of our models (equation (3.2)), large particles (such as in figure 2) may rely exclusively on the mechanical entrapment between setal shafts to remain attached to the spiders' cuticle. The density of setae in both genera, however, is not enough to explain the uniform retention of very small particles by mechanical entrapment between setae. Hairlettes may have evolved because they constitute a superior mechanism of long-term sand attachment and retention, particularly for smaller particles.
Adhesion of hairlettes to particles (figure 5) addresses the issue of how their presence may influence differences in the degree of particle attachment to morphologically distinct setae and particular regions of the Homalonychus carapace by introducing a mechanism for their role in particle capture. Based on our models (equations (3.1) and (3.2)), a single hairlette generates more than enough adhesive force to retain most particles (see electronic supplementary material, figure 3), suggesting that hairlettes are a sufficient mechanism for the retention of sand on the cuticle. In addition, reduced sand covering on the posterior slope of the carapace and the area around the eyes in Homalonychus, where setae lacked hairlettes (figure 4), is consistent with our model, as adhesive contacts would be limited to a small region of the large, rigid setal shafts. Indeed, the morphology of hairlette-free setae may deter sand adhesion from these regions of the carapace. Based on these data, we propose that hairlettes facilitate sand capture by adhering to particles via intermolecular forces (Israelachvili 1992; Autumn et al. 2002; Hansen & Autumn 2005), as occurs in gecko setae.
(b) Hairlettes as a design principle for sand retention
Hairs in arthropods have a wide range of functions, including sensory, colour pattern production, thermoregulatory and mechanical (Foelix 1996; Gorb 2001). The morphology of setae that have a mechanical role typically reflects their function, as is observed for the hooked setae of decorator crabs (Gorb 2001), the setal teeth of the calamistrum in cribellate spiders (Griswold et al. 2005) and the knobbed abdominal hairs of female wolf spiders to which spiderlings cling (Rovner et al. 1973). This appears to be true as well of the sand-trapping hairs of Sicarius and Homalonychus, due to the many long, sticky fibres that protrude from them. Though setae are often used for sensory purposes in spiders, the short body hairs are not innervated (Foelix 1996); therefore, it is reasonable that the short, hairlette-bearing setae of Sicarius and Homalonychus are totally mechanical in function. The correlation between the loss of hairlettes and the loss of sand adhesion in adult male Homalonychus (Roth 1984) supports this hypothesis.
The functional role of sand capture in spiders is assumed to be camouflage (Platnick & Raven 1981; Roth 1984; Crews 2005; Domínguez & Jiménez 2005), though a role in thermoregulation has also been proposed (Domínguez & Jiménez 2005). Regardless of its role in the spiders' biology, the underlying mechanism of sand adhesion in Sicarius and Homalonychus beautifully illustrates one of the fundamental principles of evolution; small changes in existing structures, in this case coupled with the evolution of a complex behaviour, confer strikingly different adaptive morphology. In general, hairs are highly variable and ubiquitous among arthropods. Even in a few taxa of haplogyne spiders we saw striking variation in numbers, distribution and morphology of setae on the carapace (electronic supplementary material, figure 2), suggesting that evolving a specialized setal morphology and distribution that maximizes sand capture on the cuticle is relatively easy compared with more elaborate mechanisms like glue-secreting glands. Furthermore, that sand attaches to the cuticle in a uniform, dense layer in the absence of the living spider, strongly suggests that the sand capture mechanism is largely based on structural and perhaps chemical properties of the cuticle, although it is unknown if an active role of the spider is required for long-term sand retention.
Cuticular particle attachment has evolved convergently in various spider and insect taxa (Platnick & Raven 1981; Roth 1984; Griswold 1985; Coddington & Levi 1991; Dippenaar-Schoeman & Jocqué 1997; Brandt & Mashberg 2002). The only other work we are aware of that focuses on the role of setal morphology in particle capture and adhesion in terrestrial arthropods describes a system in basicerotine ants (Hölldobler & Wilson 1986). The setae in this tribe of ants are variable across genera, but the general morphologies are quite different from those in Sicarius and Homolonychus. The ants have larger ‘brush’ setae that are proposed to capture particles, and smaller ‘holding’ setae probably play a role in retaining soil particles. Perhaps the large setae in Sicarius and Homalonychus fill the same functional role as the brush setae in ants for particle capture, and hairlettes are analogous to the holding setae suggesting that any viable system for long-term retention of particles requires that both of these functional roles be filled.
Our study is the first to provide experimental and comparative evidence for an adaptive role for setal morphology and a dominant role for intermolecular forces in particle retention in Sicarius and Homalonychus. Convergent evolution of hairlettes in these two spider genera suggests that they represent a general design principle for particle capture and retention. There is much to be learned about the details of the biophysical mechanisms of particle adhesion in this system and the adhesive properties of hairlettes (e.g. why the hairlettes appear to adhere preferentially to particles rather than other hairlettes or the setal shaft). Such work will pave the way for bio-inspired engineering with potential applications including soil-capturing materials for camouflage, household dusting, air filtration systems and devices for collecting, characterizing, separating and transferring particles.
Acknowledgments
We thank Andrew Merrell, Marjorie G. Weber, Williams Paredes, Lizette Tejada (Peru); Chris Ellison, Facundo Labarque, Jeremy Miller (Argentina); Melissa Callahan, Pablo Berea Nuñez (Namibia) and Marshal Hedin (California) for their help with collecting specimens. We also thank Martín Ramírez (Argentina), Tharina Bird (Namibia), Gerardo Llamas and Williams Paredes (Peru), Organ Pipe Cactus National Monument and Tucson Mountain Park for help with obtaining, collecting and export permits. Sarah Crews provided Homalonychus collection locality information, and Alan Younis, Steve Attinasi and Ed Florence provided technical support. We thank Binford and Autumn laboratory members, and two anonymous reviewers for their helpful comments on the manuscript. This work was funded by the Student Academic Affairs Board at Lewis & Clark College (R.P.D.). Travel for spider collecting was funded by Murdock Charitable Trust (G.J.B.), Silanes Corporation (G.J.B.) and NSF CAREER award IOB-0546858 (G.J.B.).
Supplementary Material
Phylogenetic tree of Haplogynes
Sand associates less with setae lacking hairlettes but still clumps densely in setose regions of the carapace
Particle size distributions of grades 0, 1 and 2 sand
Fine particles attach to Sicarius and Homalonychus
Text
References
- Adams N.L. UV radiation evokes negative phototaxis and covering behavior in the sea urchin Strongylocentrotus droebachiensis. Mar. Ecol. Prog. Ser. 2001;213:87–95. doi:10.3354/meps213087 [Google Scholar]
- Autumn K, et al. Evidence for van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA. 2002;99:12 252–12 256. doi: 10.1073/pnas.192252799. doi:10.1073/pnas.192252799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M, Mashberg D. Bugs with a backpack: the function of nymphal camouflage in the West African assassin bugs Paredocla and Acanthaspis spp. Anim. Behav. 2002;63:277–284. doi:10.1006/anbe.2001.1910 [Google Scholar]
- Bruce M.J, Heiling A.M, Herberstein M.E. Web decorations and foraging success in ‘Araneus’ eburnsus (Araneae: Araneidae) Ann. Zool. Fenn. 2004;41:563–575. [Google Scholar]
- Chamberlin R.V. Results of the yale Peruvian expedition of 1911. The Arachnida. Bull. Mus. Comp. Zool. 1916;LX:214–216. plate 10. [Google Scholar]
- Coddington J.A. Phylogeny and classification of spiders. In: Ubick D, Paquin P, Cushing P.E, Roth V, editors. Spiders of North America: an identification manual. American Arachnological Society; New York, NY: 2005. pp. 18–24. [Google Scholar]
- Coddington J.A, Levi H.W. Systematics and evolution of spiders (Araneae) Annu. Rev. Ecol. Syst. 1991;22:565–592. doi:10.1146/annurev.es.22.110191.003025 [Google Scholar]
- Cott H.B. Methuen & Co. Ltd; London, UK: 1957. Adaptive coloration in animals. [Google Scholar]
- Crews S.C. Homalonychidae. In: Ubick D, Paquin P, Cushing P.E, Roth V, editors. Spiders of North America: an identification manual. American Arachnological Society; New York, NY: 2005. pp. 118–119. [Google Scholar]
- Dippenaar-Schoeman A.S, Jocqué R. ARC–Plant Protection Research Institute; Pretoria, Republic of South Africa: 1997. African spiders: an identification manual. [Google Scholar]
- Domínguez K, Jiménez M.L. Mating and self-burying behavior of Homalonychus theologus Chamberlin (Araneae, Homalonychidae) in Baja California Sur. J. Arachnol. 2005;33:167–174. doi:10.1636/M03-4 [Google Scholar]
- Eberhard W.G. Substitution of silk stabilimenta for egg sacs by Allocyclosa bifurca (Araneae: Araneidae) suggests that silk stabilimenta function as camouflage devices. Behaviour. 2003;140:847–868. doi:10.1163/156853903770238346 [Google Scholar]
- Foelix R.F. 2nd edn. Oxford University Press; New York, NY: 1996. Biology of spiders. [Google Scholar]
- Gorb S. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. Attachment devices of insect cuticle. [Google Scholar]
- Griswold C.E. A revision of the African spiders of the family Microstigmatidae (Araneae: Mygalomorphae) Ann. Natal Mus. 1985;27:1–37. [Google Scholar]
- Griswold C.E, Ramírez M.J, Coddington J.A, Platnick N.I. Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proc. Calif. Acad. Sci. (4th series) 2005;56(suppl. II):1–324. [Google Scholar]
- Hansen W.R, Autumn K. Evidence for self cleaning in gecko setae. Proc. Natl Acad. Sci. USA. 2005;102:385–389. doi: 10.1073/pnas.0408304102. doi:10.1073/pnas.0408304102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B, Wilson E.O. Soil-binding pilosity and camouflage in ants of the tribes Basicerotini and Stegomyrmecini (Hymenoptera, Formicidae) Zoomorphology. 1986;106:12–20. doi:10.1007/BF00311942 [Google Scholar]
- Israelachvili J. 2nd edn. Academic Press; Harcourt Brace & Company, Publishers; London, UK: 1992. Intermolecular & surface forces. [Google Scholar]
- Kendall K. Thin-film peeling—the elastic term. J. Phys. D: Appl. Phys. 1975;8:1449–1452. doi:10.1088/0022-3727/8/13/005 [Google Scholar]
- Levi H.W, Levi L.R. Eggcase construction and further observations on the sexual behavior of the spider Sicarius (Araneae: Sicariidae) Psyche. 1969;76:29–40. [Google Scholar]
- Newlands G. A revision of the spider genus Loxosceles Heinecken & Lowe, 1835 (Araneae: Scytodidae) in southern Africa with notes on the natural history and morphology. J. entomol. Soc. S. Afr. 1975;38:141–154. [Google Scholar]
- Platnick, N. I. 2007 The world spider catalog, version 7.0 New York, NY: American Museum of Natural History. See http://research.amnh.org/entomology/spiders/catalog/index.html
- Platnick N.I, Raven R.J. A revision of the American spiders of the family Microstigmatidae (Araneae, Mygalomorphae) Am. Mus. Novit. 1981;2707:1–20. [Google Scholar]
- Platnick N.I, Coddington J.A, Forster R.R, Griswold C.E. Spinneret morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae) Am. Mus. Novit. 1991;3016:1–73. [Google Scholar]
- Reiskind J. Self-burying behavior in the genus Sicarius (Araneae, Sicariidae) Psyche. 1965;72:218–224. [Google Scholar]
- Roth V.D. The spider family Homalonychidae (Arachnida, Araneae) Am. Mus. Novit. 1984;2790:1–11. [Google Scholar]
- Rovner J.S, Higashi G.A, Foelix R.F. Maternal behavior in wolf spiders: the role of abdominal hairs. Science. 1973;182:1153–1155. doi: 10.1126/science.182.4117.1153. doi:10.1126/science.182.4117.1153 [DOI] [PubMed] [Google Scholar]
- Tian Y, Pesika N, Zeng H.B, Rosenberg K, Zhao B.X, McGuiggan P, Autumn K, Israelachvili J. Adhesion and friction in gecko toe attachment and detachment. Proc. Natl Acad. Sci. USA. 2006;103:19 320–19 325. doi: 10.1073/pnas.0608841103. doi:10.1073/pnas.0608841103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter R.S, Cokendolpher J.C. Short communication: Homalonychus theologus (Araneae, Homalonychidae): description of eggsacs and a possible defensive posture. J. Arachnol. 2000;28:361–363. doi:10.1636/0161-8202(2000)028[0361:HTAHDO]2.0.CO;2 [Google Scholar]
Notice of correction
Section (e) of the Results in now presented in the correct form.
12 October 2007
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of Haplogynes
Sand associates less with setae lacking hairlettes but still clumps densely in setose regions of the carapace
Particle size distributions of grades 0, 1 and 2 sand
Fine particles attach to Sicarius and Homalonychus
Text