Abstract
Animal migrations can affect disease dynamics. One consequence of migration common to marine fish and invertebrates is migratory allopatry—a period of spatial separation between adult and juvenile hosts, which is caused by host migration and which prevents parasite transmission from adult to juvenile hosts. We studied this characteristic for sea lice (Lepeophtheirus salmonis and Caligus clemensi) and pink salmon (Oncorhynchus gorbuscha) from one of the Canada's largest salmon stocks. Migratory allopatry protects juvenile salmon from L. salmonis for two to three months of early marine life (2–3% prevalence). In contrast, host diversity facilitates access for C. clemensi to juvenile salmon (8–20% prevalence) but infections appear ephemeral. Aquaculture can augment host abundance and diversity and increase parasite exposure of wild juvenile fish. An empirically parametrized model shows high sensitivity of salmon populations to increased L. salmonis exposure, predicting population collapse at one to five motile L. salmonis per juvenile pink salmon. These results characterize parasite threats of salmon aquaculture to wild salmon populations and show how host migration and diversity are important factors affecting parasite transmission in the oceans.
Keywords: migration, host diversity, parasite transmission, aquaculture, salmon, sea lice
1. Introduction
Migration is prevalent in the animal world (Dingle 1996), but there are few studies on its interaction with infectious disease (Loehle 1995; Bradley & Altizer 2005; Perez-Tris & Bensch 2005). Migration links habitats with differing physical and ecological characteristics such as temperature and host diversity, which can affect parasite prevalence and evolution—e.g. avian malaria (Perez-Tris & Bensch 2005). Two other effects of migration on infectious disease are migratory escape and migratory culling (Loehle 1995; Bradley & Altizer 2005). Migratory escape occurs when host populations reduce pathogen transmission by migrating away from contaminated habitats (Loehle 1995). Examples include baboon movement among sleeping groves (Papio cynocephalus; Hausfater & Meade 1978) and post-calving reindeer migration (Rangifer tarandus; Folstad et al. 1991). Migratory culling occurs when infected individuals are lost from the migratory population because migratory fitness is compromised (Bradley & Altizer 2005). Examples include loss of diseased herring from migratory populations (Clupea harengus; Holst 1996) and compromised flight of monarch butterflies (Danaus plexippus; Bradley & Altizer 2005). There are other consequences of migration such as the spatial separation of juvenile and adult age classes, which we term migratory allopatry. Such allopatric barriers to parasite transmission have been suggested as selecting for ontogenetic migrations between benthic and pelagic habitats for marine benthic invertebrates and demersal fishes (Strathmann et al. 2002). Also, many anadromous fishes, such as Pacific salmon (Oncorhynchus spp.), link oceanic, coastal and freshwater ecosystems with few periods of sympatry among age classes (Groot & Margolis 1991; Quinn 2004). For these species, parasite transmission from adult to juvenile hosts could be blocked by both a freshwater/marine barrier and allopatry.
The degree to which migration affects disease transmission is also linked to host diversity. For example, the threat of Lyme disease to humans is mediated by vector (tick) infection prevalence that depends on local abundances of competent (e.g. mice) or incompetent (e.g. squirrels) hosts (LoGiudice et al. 2003). Unless explicitly noted, we restrict the terms ‘host’ and ‘host diversity’ to include only competent hosts. Host diversity and migration could interact to affect disease dynamics through the synchrony of migration among host species—there could be periods of high and low abundances of host species in sympatry. Furthermore, humans can influence disease dynamics by augmenting host diversity and abundance along migratory routes through agriculture and aquaculture. Examples include the global spread of H5N1 avian influenza (Kilpatrick et al. 2006) and the spread of sea lice from farm to wild salmon (Costello 2006; Krkošek et al. 2006). Such interactions are important for the conservation of wild fish stocks owing to the rapid growth of industrial aquaculture (Naylor et al. 2000; Duarte et al. 2007) and the continuing decline of ocean fisheries and ecosystems (Jackson et al. 2001; Myers & Worm 2003).
Here, we examine how host migration and diversity influence transmission of sea lice (the salmonid-specific Lepeophtheirus salmonis and the generalist Caligus clemensi) in the early marine life of one of Canada's largest salmon stocks—Skeena River pink salmon (Oncorhynchus gorbuscha). The Skeena River estuary is positioned to become a major salmon aquaculture producer in Canada, but during our surveys there were no salmon farms in operation. Aquaculture can increase parasite exposure of wild juvenile salmon (Costello 2006; Krkošek et al. 2006; Orr 2007). To assess this parasite threat to wild salmon populations, we turn to a simple mathematical model of pink salmon and L. salmonis. The model addresses two opposing ecological scenarios—whether or not disease mortality is compensated by a reduction in other mortality factors. For salmon and sea lice, compensatory mortality could occur if predators (the primary natural mortality factor) selectively preyed on infected fish, thereby reducing parasite abundance and the impact on the host population. The model uses two known parameters—host mortality in the absence of parasites (ϕ), assumed to occur through predation, and parasite-induced host mortality (φ) which allows for selective predation. The results address a parasite threat of salmon farming to pink salmon populations and show how host migration, diversity and aquaculture can affect parasite transmission and host population dynamics.
2. Study system
Skeena River pink salmon are among Canada's largest salmon stocks with escapements of 0.2–4.8 million spawners and returns of up to 25 million. In early spring (April), juvenile pink salmon emerge from the Skeena River and coastal streams into Chatham Sound and adjacent areas (figure 1) where they rear until late summer. During spring, there are few adult salmon hosts in these waters and so juvenile and adult salmon are allopatric until late May or June, when the first adult salmon, in this case chinook (Oncoryhnchus tshawytscha), begin to arrive on their return migration. Abundant returning adult chinook and early runs of coho (Oncoryhnchus kisutch) appear in July. In contrast, the diversity of hosts for C. clemensi includes ubiquitous populations of herring (Clupea harengus pallasi), abundant Pacific sandfish (Trichodon trichodon) and scattered stickleback (Gasterosteus aculeatus), which are sympatric with the juvenile salmon. Juvenile salmon begin marine life without sea lice infections because sea lice rapidly die in fresh water (Pike & Wadsworth 2000). Though sea lice species have similar life histories, they differ in morphology and host range (Kabata 1972; Johnson & Albright 1991; Costello 2006). Lepeophtheirus salmonis is larger and known to complete its life cycle only on salmonids. Caligus clemensi is smaller and has a wider host range (table 1). There are no known resting stages for these species. Transmission to new hosts is via planktonic larvae and motile pre-adults. Once attached to a host fish, sea lice follow a developmental progression from newly attached copepodids through chalimus and then motile stages (Kabata 1972; Johnson & Albright 1991). Parasitic lice feed on the surface tissues of host fish, including muscle and blood, which can cause host stress, osmotic failure, viral or bacterial infection and ultimately host death (Pike & Wadsworth 2000). Lepeophtheirus salmonis frequently infect farm salmon (Costello 2006) that can transmit lice to wild juvenile salmon (Krkošek et al. 2005) and cause epizootic mortality (Krkošek et al. 2006). While there are no reports of L. salmonis infestations of wild juvenile Pacific salmon, in regions without salmon farms (despite many studies of juvenile salmon ecology and the conspicuous nature of L. salmonis infestations), there was one occurrence of C. clemensi noted on juvenile pink salmon before aquaculture began (Parker & Margolis 1964). There is a general paucity of information on juvenile Pacific salmon infections in regions without salmon farms, and this is key missing information for understanding the natural ecology of lice and salmon.
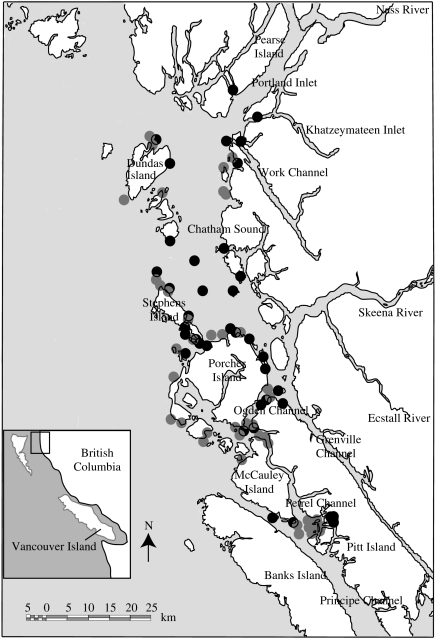
Figure 1.
Study area and sample locations for 2005. The spatial distribution of sample sites was similar in 2004 and 2006. Grey dots, dip net sites; black dots, trawl sites. Chatham Sound and Skeena River are located on the north coast of British Columbia, Canada. There are no salmon farms in this area.
Table 1.
Known host species for L. salmonis (L) and C. clemensi (C) in British Columbia, Canada.
| host species | common name | lice spp | references |
|---|---|---|---|
| Oncorhynchus spp. | Pacific salmon and trout | L, C | Margolis & Kabata (1988) and Jones et al. (2006) |
| Salvelinus malma | dolly varden | L, C | M. Krkošek 2003–2007, personal observation |
| Salmo salar | Atlantic salmon | L, C | Johnson & Kent (1992) |
| Clupea harengus pallasi | Pacific herring | C | Margolis & Kabata (1988) |
| Gasterosteus aculeatus | three-spined stickleback | Ca | Brown & Kodric-Brown (1977) and Margolis & Kabata (1988) |
| Hexagrammos spp. | greenling species | C | Margolis & Kabata (1988) |
| Hydrolagus colliei | spotted ratfish | C | Margolis & Kabata (1988) |
| Sebastes spp. | rockfish species | C | Margolis & Kabata (1988) |
| Theragra chalcogramma | Alaska pollock | C | Margolis & Kabata (1988) |
| Trichodon trichodon | Pacific sandfish | C | A. Gottesfeld 2004–2006, personal observation |
We do not regard stickleback as a competent host for L. salmonis because very few L. salmonis survive to adult stages, and no gravid L. salmonis have been observed on this species (Jones et al. 2006).
3. Data collection and analysis
We studied sea lice infections of juvenile pink salmon in Chatham Sound, Ogden Channel and Petrel Channel, British Columbia (figure 1) during the juvenile salmon ocean migration for the years 2004–2006. We sampled from mid to late April through early August in 2004 and 2005 and from mid-May to mid-July in 2006. In the early season, we collected juvenile salmon using a dip net (45 cm diameter with 5 mm knotless mesh on a 2.45 m pole) from a small skiff (4.17 m flat-bottomed aluminium skiff). In mid-May when the fish reached approximately 55 mm fork length, they moved from very shallow (intertidal) habitats to deeper habitats several tens of metres off the beach. Subsequent to this transition, we collected juvenile salmon using an Ocean Fish Lift trawl (Holst & McDonald 2000) towed behind a fibreglass ex-commercial gill net vessel 11 m in length moving at 2.7–2.9 knots. The trawl net was 5.0 m wide×4.6 m deep and 18.0 m long. The rigid cod-end of the trawl net minimized damage to live samples, in particular the loss of scales and ectoparasites (Holst & McDonald 2000). Collections made during one- to two-week cruises during which most sample sites were fished. Salinity and sea surface temperature were recorded after most collections using a YSI-30 SCT meter. Fish were immediately frozen and labelled for subsequent laboratory analysis. In the laboratory, individual fish were thawed and assayed for sea lice using a dissecting microscope. Motile stages of sea lice were directly determined for species by morphology (Kabata 1972; Johnson & Albright 1991). Copepodid and chalimus lice were removed from the fish, mounted on permanent slides and examined under a compound microscope to make species determinations based on detailed morphology (Kabata 1972; Johnson & Albright 1991). The resulting data were analysed at broad spatial and temporal scales (i.e. per month over the entire study area). This was necessary because sea lice abundance was too low to support detailed spatial analysis and also to accommodate variation in the spatial distribution of sampling effort among cruises. Differences in sea lice abundance over time and between species were tested using generalized linear models with Poisson error.
4. Empirical results
The surveys conducted during 2004–2006 resulted in a total of 21 448 juvenile pink salmon assayed for sea lice infections. Of these fish, 13 139 were collected by dip net and 8309 were collected by the OFL trawl. We did not observe an effect of gear on sea lice abundance when we simultaneously fished both gear types at the same sites. Over the season, the juvenile salmon increased in fork length from 30 mm in early spring up to 130 mm in summer. This corresponds to an increase in weight by two orders of magnitude from approximately 0.2 to 20 g. By July most juvenile salmon had well-developed scales but lacked these scales upon marine emergence and for their first one to two months of marine life. Throughout the study, we periodically collected herring (n=143), Pacific sandfish (n=48) and stickleback (n=47) as incidental by-catch in trawls. All these fish species carried C. clemensi. The 47 stickleback carried 770 lice in total, and of the 345 examined, 223 were L. salmonis and 132 C. clemensi. There was one gravid C. clemensi observed on the herring, no gravid lice on the stickleback and no motile lice on the sandfish. Sea surface temperature ranged from approximately 10°C in April to approximately 15°C in August. Sea surface salinity ranged from approximately 10 ppt near the mouth of the Skeena River to approximately 32 ppt around the western fringe of Chatham Sound and Ogden and Petrel Channels.
The prevalence of L. salmonis was generally approximately 2–3% during the first three months of marine life of pink salmon (figure 2; table 2). During July, there was a marked increase in L. salmonis abundance (p<0.05). Lepeophtheirus salmonis prevalence rose up to 50% in 2004 (figure 2), and this was due to a general rise in the mean abundance of most louse developmental stages (table 2). Interestingly, this includes an abrupt increase in L. salmonis motiles that does not correspond to a preceding developmental progression (table 2). Late June and early July mark the return migration of the first abundant population of adult salmon to these waters, in this case chinook salmon. These adult salmon are known to carry motile and gravid L. salmonis and occur within 10–100 m of abundant populations of juvenile pink salmon (A. Gottesfeld et al. 2004–2006, personal observation). During this time, the juvenile salmon population was predominately distributed among the outer islands of the study area. In these waters, salinity was approximately 28 ppt, which is suitable for sea lice survival and transmission. The abundance of L. salmonis was not significantly related to salinity for any developmental stage (see figure S1 in the electronic supplementary material).
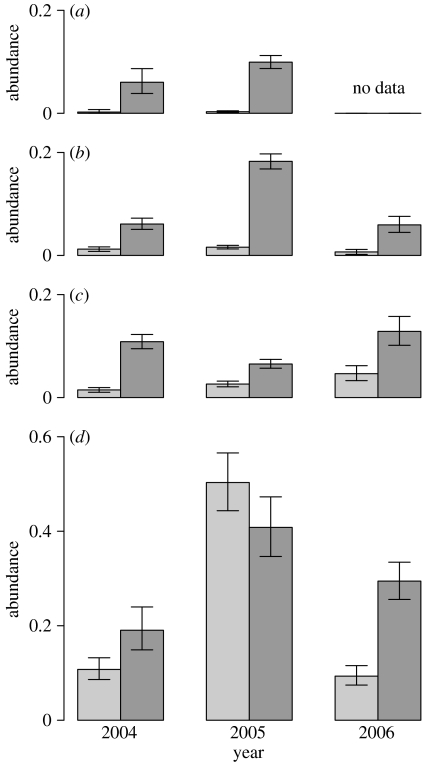
Figure 2.
Sea lice abundance for all developmental stages combined (±95% bootstrap CIs) in (a) April, (b) May, (c) June and (d) July for L. salmonis (light grey bars) and C. clemensi (dark grey bars) over 3 years (2004–2006).
Table 2.
Total sample sizes (n) and mean abundances of sea lice developmental stages (±95% bootstrap CIs) for each sampling period for L. salmonis (Lep) and C. clemensi (Cal) for the years 2004–2006. Sea lice stages are divided into copepodid/chalimus I (C), chalimus II–IV (H) and motiles (M).
| spp/n | year | stage | April | May | June | July |
|---|---|---|---|---|---|---|
| n | 2004 | 415 | 2295 | 2637 | 893 | |
| n | 2005 | 3323 | 4383 | 3549 | 990 | |
| n | 2006 | no data | 1028 | 1035 | 900 | |
| Lep | 2004 | C | 0 | 0 | 0.0008 (0, 0.002) | 0.0011 (0, 0.0034) |
| H | 0.0024 (0,0.0072) | 0.0096 (0.006, 0.014) | 0.004 (0.002, 0.006) | 0.0056 (0, 0.011) | ||
| M | 0 | 0.0026 (0.00087, 0.0050) | 0.0099 (0.0064, 0.014) | 0.10 (0.080, 0.13) | ||
| 2005 | C | 0.0012 (0.00030, 0.0024) | 0.0023 (0.00091, 0.0039) | 0.0019 (0.00056, 0.0036) | 0.20 (0.17, 0.23) | |
| H | 0.0018 (0.00060, 0.0033) | 0.013 (0.0093,0.016) | 0.0045 (0.0023, 0.0067) | 0.098 (0.073, 0.13) | ||
| M | 0 | 0.0011 (0.00023, 0.0023) | 0.020 (0.015, 0.025) | 0.21 (0.17, 0.24) | ||
| 2006 | C | — | 0.0039 (0.00097, 0.0078) | 0.011 (0.0048, 0.018) | 0.02 (0.012, 0.030) | |
| H | — | 0.0029 (0, 0.0068) | 0.00097 (0, 0.0030) | 0.011 (0, 0.033) | ||
| M | — | 0 | 0.035 (0.023, 0.046) | 0.072 (0.056, 0.091) | ||
| Cal | 2004 | C | 0.029 (0.014, 0.046) | 0.013 (0.0087, 0.017) | 0.024 (0.018, 0.030) | 0.0056 (0.0011, 0.010) |
| H | 0.031 (0.014, 0.048) | 0.057 (0.037, 0.058) | 0.071 (0.059, 0.084) | 0.060 (0.034, 0.096) | ||
| M | 0.0013 (0, 0.0031) | 0.013 (0.0087, 0.019) | 0.12 (0.096, 0.15) | |||
| 2005 | C | 0.060 (0.051,0.069) | 0.031 (0.026, 0.037) | 0.0054 (0.0028, 0.0079) | 0.022 (0.013, 0.031) | |
| H | 0.039 (0.032, 0.046) | 0.15 (0.13, 0.16) | 0.046 (0.038, 0.054) | 0.20 (0.15, 0.24) | ||
| M | 0 | 0.0016 (0.00046, 0.0027) | 0.013 (0.0099, 0.017) | 0.19 (0.15, 0.23) | ||
| 2006 | C | — | 0.024 (0.016, 0.034) | 0.019 (0.012, 0.029) | 0.077 (0.059, 0.096) | |
| H | — | 0.032 (0.021, 0.044) | 0.0039 (0.00097, 0.0077) | 0.020 (0.011, 0.031) | ||
| M | — | 0.0029 (0, 0.0068) | 0.11 (0.080, 0.14) | 0.20 (0.17, 0.23) |
Caligus clemensi followed a very different epizootiology. For the first three months of pink salmon marine life, C. clemensi was more abundant than L. salmonis (p<0.001 for April, May and June for the years 2003–2005; figure 3). Note that there were no April data for 2006. Infection prevalence of C. clemensi was 8–20% during this time with a clear developmental progression resulting in many motile stage lice in June and July. The sustained rate of new infections (evidenced by copepodid and chalimus lice presence during April–June) plus the accumulation of motile lice resulted in a marked increase of C. clemensi in July (p<0.001). This contrasts with the more abrupt increase in L. salmonis in July. During July, the dominance of C. clemensi diminished when L. salmonis abundance increased, and in 2005 exceeded C. clemensi (p<0.001). The abundance of C. clemensi copepodids and chalimus lice was significantly related to salinity (figure S1 in the electronic supplementary material).
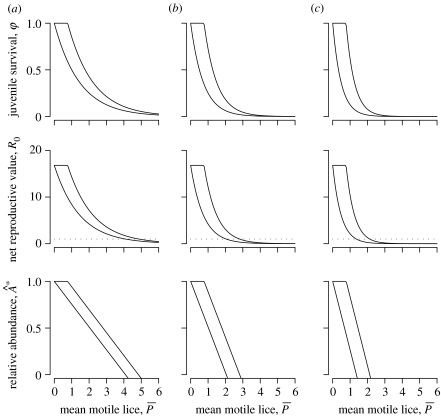
Figure 3.
Effects of increasing motile sea lice infection, , of juvenile pink salmon on juvenile salmon survival from parasites (φ), salmon net reproductive value (R0) and equilibrium abundance relative to abundance at natural sea lice levels for (a) one-month exposure, (b) two-month exposure and (c) three-month exposure to the parasites. Model predictions are bounded by compensatory (right boundary, equation (5.3)) and non-compensatory (left boundary, equation (5.2)) parasite-induced host mortality. The horizontal dotted line shows R0=1, above which salmon populations persist and below which salmon populations collapse.
5. Model and analysis
To explore the disease threat of aquaculture to wild pink salmon populations, we developed an empirically parametrized stage-structured host population model with survival terms determined partly by parasites. The model decomposes juvenile salmon mortality into parasite (φ)- and non-parasite (ϕ)-associated terms that have different formulations depending on the interactions among mortality factors. The effects of aquaculture are captured by increasing early parasitism of wild juvenile fish. For pink salmon, the juvenile stage (Y) corresponds to the first three months of marine life when they are allopatric with large abundances of adult (A) salmonids. The host life cycle can be described by the simple graph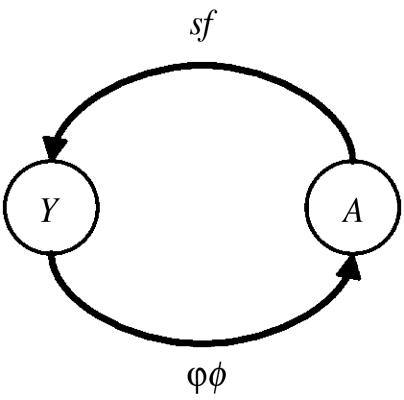
where f is the number of juveniles produced per adult and s is the proportion of adults that survive to spawn. The net reproductive value, R0, of the fish population (not the parasite) can be read directly from the graph as R0=φϕsf. At low population density, it is of interest to know whether R0>1, which means that adults produce on average more than the offspring in their lifetime and the population will grow. As R0 increases, so too does the resilience of the population—the population will recover faster after perturbation. Alternatively, if R0<1, individuals cannot on average replace themselves and the population will decline to extinction. The R0 analysis investigates the effect of lice on pink salmon population persistence and resilience but does not track changes in population abundance.
To track changes in population abundance, we introduce density dependence into reproduction through a modified Ricker equation g=A exp[r(1−A/K)] (Ricker 1954) and replace f with g. The reproduction equation g accounts for competition for spawning habitat where female salmon can damage nests and eggs made by other females, a process known as redd superimposition (Heard 1991; Quinn 2005). Here, K is the carrying capacity of spawners and er is the number of juveniles produced per spawner at low density (the parameter r is r=ln(f)). The equilibrium abundance of adult salmon is A*=[K/r][ln(φϕs)+r], and for juveniles it is Y*=[K/(φϕr)] [ln(*ϕs)+r]. When sea lice abundance is at natural levels, we denote the adult equilibrium abundance as . To gauge the effect of increasing parasite exposure of juvenile salmon on wild salmon population abundance, we define which is the realized abundance relative to population abundance at natural sea lice levels. =1 when there is no population depression and equals 0 when the population is extirpated.
The salmon population dynamics occur in discrete 2-year cycles but parasite- and non-parasite-associated mortality occurs in continuous time within host cohorts. To capture this, we needed continuous time survival models that define φ and ϕ. The proportion of juvenile salmon surviving parasitism can be expressed by the survival model
| (5.1) |
where Φ(t) is the rate of parasite-induced host mortality of juvenile salmon, which can take different forms depending on the interaction between parasitism and predation. T is the duration of the juvenile stage (three months). Let α be the host mortality rate of juvenile salmon induced per parasite and μ be the rate of non-parasite-related mortality of juvenile salmon. If parasite-induced host mortality is not compensatory (i.e. acts independently of other mortality factors), then the rate of parasite-induced host mortality is simply
| (5.2) |
where is the mean parasite abundance on juvenile hosts at time t. Alternatively, one could presume that infection induces compensatory mortality because predators, which are the primary presumed proximate cause of mortality (Groot & Margolis 1991), may selectively prey on infected fish and thereby not change the actual number of fish killed. In this scenario, the only additional mortality caused by infection will be from parasites surviving predation of their immediate host. The rate of host mortality induced by parasites is simply
| (5.3) |
which says that predators consume infected prey, and so the mortality caused by the parasites must exceed that of the predators in order to be felt by the host population. Note that equation (5.3) defines a parasite abundance threshold, , below which there are no population impacts.
We do not include explicit models for sea lice population dynamics on the juvenile salmon because the time scale of the sea lice life cycle is slow (1.5 months) relative to the exposure period (one to three months). This means that the sea lice dynamics on the juvenile salmon can be well approximated by an immigration and death process where sea lice abundance on juvenile salmon is controlled by transmission from natural and/or farm reservoir hosts and subsequent lice survival on juvenile salmon. Explicit inclusion of lice population dynamics on juvenile salmon could result in an increase through time (owing to subsequent generation of lice) but this could also be balanced by declines in transmission from reservoir hosts the juvenile salmon have long passed during their migration. Because we are interested in the general sensitivity of salmon population dynamics to lice exposure, we simply control lice abundance as an exogenous variable. This provides a starting point for linking salmon population dynamics with more fine-scale data and models of sea lice on farm and wild juvenile salmon.
To parametrize the model, we used data on pink salmon fecundity, freshwater and marine survival summarized by Heard (1991). Because there is considerable temporal and spatial variation in these parameters, we tried to be conservative in the parameter estimates. Starting with reproduction, we assume that females carry, on average, 1600 eggs (table 2 in Heard 1991), and that fertilization success is 100%. To estimate egg-to-fry survival, we needed to control for density dependence during spawning. To tease apart density dependence from abiotic factors affecting egg-to-fry survival, we compared egg-to-fry survival estimates from natural rivers (mean=10.8%) and spawning channels (mean=50.3%) in British Columbia (see table 17 in Heard 1991). Assuming that density dependence and abiotic factors are roughly independent, density dependence affects populations spawning in both natural streams and spawning channels and abiotic mortality factors are minimal in artificial channels, we calculated that the average density-independent egg-to-fry survival in natural streams is 10.8/50.3=0.21%. These calculations mean that at low spawner density each female produces 336 fry.
We estimated the subsequent survival from marine emergence through spawning using an established within cohort mortality schedule for pink salmon estimated from detailed long-term observations from a central British Columbia population (fig. 36 in Heard 1991). The estimated mortality schedule must intrinsically account for the natural effects of parasitism. For early marine life of juvenile salmon, the instantaneous rate of mortality at natural parasite levels is υ(t)=0.53 month−1. When mortality in non-compensatory, parasites have an additive effect that must be subtracted to estimate non-parasite-associated mortality . From another study, we have estimated α=0.69 (motile lice·month)−1 (Krkošek et al. 2006), and from the results of this paper, we know that motile lice, and so we can calculate . When mortality is compensatory, μ2(t)=υ(t) (parasites are removed by predators and have no additional effect), and we can calculate . The survival of salmon through the remainder of their life cycle was calculated as , where γ(t) is the instantaneous mortality rate of pink salmon from the third month of marine life through spawning (fig. 36 in Heard 1991). These calculations mean that approximately 5% of pink salmon fry will return as adults under natural parasite abundances. For our purposes, we chose a carrying capacity of 100 000 adults.
With these parameter values, we explored the consequences of compensatory interactions between predation and parasitism and increasing parasite exposure (abundance of motile lice per fish and temporal duration of exposure) on juvenile salmon survival, salmon population persistence (via the salmon net reproductive value R0) and the remaining salmon population abundance relative to abundance at natural sea lice levels. We found that pink salmon populations are highly sensitive to parasitism of juvenile fish, but that compensatory mortality creates a threshold value of motile lice per fish below which there are no effects on salmon population dynamics. This threshold value places a theoretical upper bound on the mean abundance of sea lice per juvenile fish, below which there are no population impacts. The true value would probably occur between zero and , depending on the exact nature of the interaction between parasites and predators. As the parasite abundance increased, there were initial sharp declines in wild salmon abundance and resilience (figure 3). Full population collapse occurred when R0<1, meaning that when lice abundances causing R0<1 are sustained over several salmon generations the salmon populations may be extirpated. In the most conservative case—compensatory mortality and only a one-month period of sea lice exposure—population collapse was predicted at a mean infection abundance of approximately five motile lice per fish. At the other extreme—non-compensatory mortality and three months of exposure—population collapse was predicted to occur at a mean louse abundance of approximately 1.5 motile lice per fish.
6. Discussion
Host migration can affect disease dynamics through migratory culling, migratory escape and pathogen evolution (Loehle 1995; Bradley & Altizer 2005; Perez-Tris & Bensch 2005). Our results provide strong empirical support for another consequence of migration, which we term migratory allopatry, and define as a period of spatial separation between adult and juvenile hosts, which is caused by host migration and which prevents parasite transmission from adult to juvenile hosts. Because salmonids bound the host range of L. salmonis, the parasite's life history is influenced by its host's migration. The bulk of adult salmon populations are offshore and beginning their return migration when juveniles are in coastal waters and beginning their ocean migration (Groot & Margolis 1991; Quinn 2004). This explains the low L. salmonis prevalence (2–3%) during the first two to three months of pink salmon marine life. These lice probably originated from local small populations of cutthroat trout (Oncorhynchus clarki) and dolly varden (Salvelinus malma) and scattered coastal ocean rearing chinook that are orders of magnitude less abundant than other Pacific salmonids with oceanic migrations. Overall, L. salmonis did not reach appreciable numbers until the return migration of adult chinook salmon in July when abundances of all louse developmental stages abruptly increased. This suggests that cross-generational transmission occurs both through infectious larvae and direct transmission of motile lice, which can move among host fish (Ritchie 1997; Hull et al. 1998). It is important to note that juvenile pink salmon are highly vulnerable to L. salmonis infection: they lack scales when young, and at 0.5–2 g they are three or four orders of magnitude smaller than adults. Correspondingly, survival of juvenile pink salmon infected with one or two motile L. salmonis is low (Morton & Routledge 2005; Krkošek et al. 2006). Taken together, these data suggest that migration creates a transmission barrier from adult salmon when juvenile salmon are most vulnerable to L. salmonis infection.
In contrast, C. clemensi followed a different epizootiology explained mostly by host diversity. The host range of C. clemensi includes locally ubiquitous populations of herring, abundant sandfish and scattered stickleback, which are sympatric with juvenile salmon. Correspondingly, juvenile salmon encountered C. clemensi shortly after marine emergence and infections were sustained at a level of 8–20%. Furthermore, we did not detect an increase in C. clemensi infection that could be attributed to the return of adult salmon—the rate of new infections remained the same during this time (table 2), and we observed only one gravid C. clemensi from among thousands of lice on the adult Chinook salmon that we opportunistically sampled. Gravid C. clemensi on returning adult Pacific salmon has also been rare in other field surveys (Beamish et al. 2005). These findings suggest that C. clemensi infections are not sustained over the offshore portion of the salmon life cycle and that the parasite is primarily distributed in coastal ecosystems. Possible explanations include the following: C. clemensi may not be successfully transmitted in pelagic environments; L. salmonis may competitively exclude C. clemensi (Costello 2006); or salmon are generally incompetent hosts sustaining C. clemensi through immigration from source host populations extant in near shore but not pelagic ecosystems (the rescue effect in source–sink dynamics; Brown & Kodric-Brown 1977). It is also important to note that C. clemensi is smaller and apparently less pathogenic than L. salmonis to juvenile salmon. We did not observe mechanical damage to the surface tissues of infected fish or other signs of pathology, which are associated with L. salmonis infection (Morton & Routledge 2005). Taken together, these findings suggest that host diversity maintains C. clemensi infections of juvenile pink salmon but that infection is only an ephemeral feature of pink salmon life history.
Salmon aquaculture creates an abundance of domesticated hosts that are situated in coastal habitats year-round (Costello 2006). Our model presents a quantitative step in understanding the sensitivity of wild salmon populations to parasite exposure in early marine life. Salmon population collapse is predicted to occur at abundances of 1.5–5 motile L. salmonis per juvenile fish. Salmon farms can cause sea lice abundances in this range (Morton & Williams 2003; Morton et al. 2004; Krkošek et al. 2006), and some of these cohorts have collapsed (PFRCC 2002). Nevertheless, the model did not account for other factors such as climate or ocean circulation changes that could dampen or amplify the predictions. Model predictions could be improved with more detailed survival data of infected juvenile salmon over a range of body masses. There is also substantial variation among populations and years in model parameters (Heard 1991), for which our parametrization represents a conservative estimate. For example, the estimated fry-to-spawner survival of 5% is high relative to most other empirical estimates for pink salmon in British Columbia (Heard 1991). We also assumed that 100% of eggs get fertilized and that there are no Allee effects reducing survival or reproductive success of salmon at low abundance. It is likely that the realized threat of sea lice to wild pink salmon populations is more severe than our model predicts. The high sensitivity of salmon populations to parasite exposure in early marine life should inform conservation policy on the spread of salmon aquaculture into the Skeena estuary (Chatham Sound, Ogden Channel and Petrel Channel), which is the rearing ground for some of the largest salmon stocks in Canada.
While allopatry between juvenile and adult hosts can be driven by migration, it may also be a general consequence of ontogenetic niche shifts and spatially dispersed life histories. Ontogenetic niche shifts have been widely appreciated for their ubiquity and effects on species interactions, population dynamics and life-history evolution (Werner & Gilliam 1984), but there has been very little work on the consequences for disease dynamics. Possibly, all marine fishes experience ontogenetic changes in habitat use or spatial distribution owing to active migratory or passive oceanographic processes. Examples include the use of mangrove habitats by juvenile fishes (Laegdsgaard & Johnson 1995), age partitioning of the world's oceans by bluefin tuna (Farley et al. 2007), passive and active processes determining spatial distributions of larval cod (Bradbury et al. 2003) and the use of shallow embayments by juvenile halibut (Fodrie & Mendoza 2006). Many of these fishes are ecologically and economically important and are being developed for industrial aquaculture production. Aquaculture can undermine allopatric barriers to parasite transmission (Costello 2006; Krkošek et al. 2006), and our results suggest that the consequences include reduced abundance, resilience and possible extirpation of wild fish populations.
Acknowledgments
The animal handling in this research was approved by the animal care committee at the University of Alberta.
We thank Marjorie Wonham and Larry Dill for their helpful comments and discussions and Ashley Park for assistance in preparing data and figures. M.K. thanks the School of Environmental Studies at the University of Victoria for their generous hospitality. This work was supported by a Bill Shostak Wildlife Award; a Natural Science and Engineering Research Council Canada Graduate Scholarship; a Canada Research Chair and grants from the National Geographic Society, the David Suzuki Foundation, the Canadian Sablefish Association, the Canadian National Research Council's Mathematics of Information Technology and Complex Systems program, the British Columbia Pacific Salmon Forum and the British Columbia Aquaculture Research and Development Council.
Supplementary Material
Sea lice abundances for (a) C. clemensi and (b) L. samonis plotted against salinity for collections of pink salmon in 2004 when salinity was measured (n=50). Lines are statistically significant linear regressions
References
- Beamish R.J, Neville C.M, Sweeting R.M, Ambers N. Sea lice on adult pacific salmon in the coastal waters of central British Columbia, Canada. Fish. Res. 2005;76:198–208. doi:10.1016/j.fishres.2005.06.007 [Google Scholar]
- Bradbury I.R, Snelgrove P.V.R, Pepin P. Passive and active behavioural contributions to patchiness and spatial pattern during the early life history of marine fishes. Mar. Ecol. Prog. Ser. 2003;257:233–245. doi:10.3354/meps257233 [Google Scholar]
- Bradley C.A, Altizer S. Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol. Lett. 2005;8:290–300. doi:10.1111/j.1461-0248.2005.00722.x [Google Scholar]
- Brown J.H, Kodric-Brown A. Turnover rates in insular biogeography—effect of immigration on extinction. Ecology. 1977;58:445–449. doi:10.2307/1935620 [Google Scholar]
- Costello M.J. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 2006;22:475–483. doi: 10.1016/j.pt.2006.08.006. doi:10.1016/j.pt.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Dingle H. Oxford University Press; Oxford, UK: 1996. Migration: the biology of life on the move. [Google Scholar]
- Duarte C.M, Marbá N, Holmer M. Rapid domestication of marine species. Science. 2007;316:382–383. doi: 10.1126/science.1138042. doi:10.1126/science.1138042 [DOI] [PubMed] [Google Scholar]
- Farley J.H, Davis T.L.O, Gunn J.S, Clear N.P, Preece A.L. Demographic patterns of southern bluefin tuna, Thunnus maccoyii, as inferred from direct age data. Fish. Res. 2007;83:151–161. doi:10.1016/j.fishres.2006.09.006 [Google Scholar]
- Fodrie F.J, Mendoza G. Availability, usage and expected contribution of potential nursery habitats for the California halibut. Estuar. Coast. Shelf. Sci. 2006;68:149–164. doi:10.1016/j.ecss.2006.01.017 [Google Scholar]
- Folstad I, Nilssen A.C, Halvorsen O, Andersen J. Parasite avoidance—the cause of post-calving migrations in Rangifer. Can. J. Zool. 1991;69:2423–2429. [Google Scholar]
- Groot C, Margolis L. UBC Press; Vancouver, Canada: 1991. Pacific salmon life histories. [Google Scholar]
- Hausfater G, Meade B.J. Baboon sleeping grove utilization—strategy for parasite avoidance. Am. J. Phys. Anthropol. 1978;48:404. [Google Scholar]
- Heard W.R. Life history of pink salmon (Oncorhynchus gorbuscha) In: Groot C, Margolis L, editors. Pacific salmon life histories. UBC Press; Vancouver, Canada: 1991. pp. 119–230. [Google Scholar]
- Holst J.C. Estimating the prevalence of Ichthyophonus hoferi (Plehn and Mulsow) in a herring stock (Clupea harengus L.): Observed effects of sampling gear, target school density and migration. Fish. Res. 1996;28:85–97. doi:10.1016/0165-7836(95)00465-3 [Google Scholar]
- Holst J.C, McDonald A. FISH-LIFT: a device for sampling live fish with trawls. Fish. Res. 2000;48:87–91. doi:10.1016/S0165-7836(00)00116-8 [Google Scholar]
- Hull M.Q, Pike A.W, Mordue A.J, Rae G.H. Patterns of pair formation and mating in an ectoparasitic caligid copepod Lepeophtheirus salmonis (Kroyer 1837): implications for its sensory and mating biology. Phil. Trans. R. Soc. B. 1998;353:753–764. doi:10.1098/rstb.1998.0241 [Google Scholar]
- Jackson J.B.C, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. doi:10.1126/science.1059199 [DOI] [PubMed] [Google Scholar]
- Johnson S.C, Albright L.J. The developmental stages of Lepeophtheirus salmonis (Kroyer, 1837) (Copepoda, Caligidae) Can. J. Zool. 1991;69:929–950. [Google Scholar]
- Johnson S.C, Kent M.L. Sea lice. In: Kent M.L, editor. Diseases in seawater netpen-reared salmonid fishes in the Pacific Northwest. Canadian special publication of fisheries and aquatic science. vol. 116. Department of Fisheries and Oceans; Nanaimo, Canada: 1992. pp. 50–55. [Google Scholar]
- Jones S.R.M, Prosperi-Porta G, Kim E, Callow P, Hargreaves N.B. The occurrence of Lepeophtheirus salmonis and Caligus clemensi (Copepoda: Caligidae) on three-spine stickleback Gasterosteus aculeatus in coastal British Columbia. J. Parasitol. 2006;92:473–480. doi: 10.1645/GE-685R1.1. doi:10.1645/GE-685R1.1 [DOI] [PubMed] [Google Scholar]
- Kabata Z. Developmental stages of Caligus clemensi (Copepoda, Caligidae) J. Fish. Res. Bd. Can. 1972;29:1571–1585. [Google Scholar]
- Kilpatrick A.M, Chmura A.A, Gibbons D.W, Fleischer R.C, Marra P.P, Daszak P. Predicting the global spread of H5N1 avian influenza. Proc. Natl Acad. Sci. USA. 2006;103:19 368–19 373. doi: 10.1073/pnas.0609227103. doi:10.1073/pnas.0609227103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M, Lewis M.A, Volpe J.P. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proc. R. Soc. B. 2005;272:689–696. doi: 10.1098/rspb.2004.3027. doi:10.1098/rspb.2004.3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M, Lewis M.A, Morton A, Frazer L.N, Volpe J.P. Epizootics of wild fish induced by farm fish. Proc. Natl Acad. Sci. USA. 2006;103:15 506–15 510. doi: 10.1073/pnas.0603525103. doi:10.1073/pnas.0603525103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laegdsgaard P, Johnson C.R. Mangrove habitats as nurseries: unique assemblages of juvenile fish in subtropical mangroves in eastern Australia. Mar. Ecol. Prog. Ser. 1995;126:67–81. doi:10.3354/meps126067 [Google Scholar]
- Loehle C. Social barriers to pathogen transmission in wild animal populations. Ecology. 1995;76:326–335. doi:10.2307/1941192 [Google Scholar]
- LoGiudice K, Ostfeld R.S, Schmidt K.A, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on lyme disease risk. Proc. Natl Acad. Sci. USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. doi:10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis L, Kabata Z. Part II—Crustacea: Canadian special publication of fisheries and aquatic sciences. vol. 101. Department of Fisheries and Oceans; Nanaimo, Canada: 1988. Guide to the parasites of fishes of Canada. [Google Scholar]
- Morton A, Routledge R. Mortality rates for juvenile pink Oncorhynchus gorbuscha and chum O. keta salmon infested with sea lice Lepeophtheirus salmonis in the Broughton Archipelago. Alask. Fish. Res. Bull. 2005;11:146–152. [Google Scholar]
- Morton A.B, Williams R. First report of a sea louse, Lepeophtheirus salmonis, infestation on juvenile pink salmon, Oncorhynchus gorbuscha, in nearshore habitat. Can. Field-Nat. 2003;117:634–641. [Google Scholar]
- Morton A, Routledge R, Peet C, Ladwig A. Sea lice (Lepeophtheirus salmonis) infection rates on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon in the nearshore marine environment of British Columbia, Canada. Can. J. Fish. Aquat. Sci. 2004;61:147–157. doi:10.1139/f04-016 [Google Scholar]
- Myers R.A, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. doi:10.1038/nature01610 [DOI] [PubMed] [Google Scholar]
- Naylor R.L, et al. Effect of aquaculture on world fish supplies. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. doi:10.1038/35016500 [DOI] [PubMed] [Google Scholar]
- Orr C. Estimated sea louse egg production from Marine Harvest Canada farmed Atlantic salmon in the Broughton Archipelago, British Columbia, 2003–2004. North Am. J. Fish. Manage. 2007;27:187–197. doi:10.1577/M06-043.1 [Google Scholar]
- Parker R.R, Margolis L. A new species of parasitic copepod, Caligus clemensi sp. nov. (Caligoida: Caligidae) from pelagic fishes in the coastal waters of British Columbia. J. Fish. Res. Bd. Can. 1964;21:873–889. [Google Scholar]
- Perez-Tris J, Bensch S. Dispersal increases local transmission of avian malarial parasites. Ecol. Lett. 2005;8:838–845. doi:10.1111/j.1461-0248.2005.00788.x [Google Scholar]
- PFRCC (Pacific Fisheries Resource Conservation Council) Report to the Minister of Fisheries and Oceans and B.C. Minister of Agriculture, Food, and Fisheries. PFRCC; Vancouver, Canada: 2002. Advisory: the protection of Broughton Archipelago pink salmon stocks. [Google Scholar]
- Pike A.W, Wadsworth S.L. Sealice on salmonids: their biology and control. Adv. Parasitol. 2000;44:233–337. doi: 10.1016/s0065-308x(08)60233-x. [DOI] [PubMed] [Google Scholar]
- Quinn T.P. University of Washington Press; Seattle, WA: 2004. The behavior and ecology of Pacific salmon and trout. [Google Scholar]
- Quinn T.P. University of Washington Press; Seattle, WA: 2005. The behavior and ecology of Pacific salmon and trout. [Google Scholar]
- Ricker W.E. Stock and recruitment. J. Fish. Res. Board Can. 1954;11:559–623. [Google Scholar]
- Ritchie G. The host transfer ability of Lepeophtheirus salmonis (Copepoda: Caligidae) from farmed Atlantic salmon, Salmo salar L. J. Fish. Dis. 1997;20:153–157. doi:10.1046/j.1365-2761.1997.00285.x [Google Scholar]
- Strathmann R.R, Hughes T.R, Kuris A.M, Lindeman K.C, Morgan S.G, Pandolfi J.M, Warner R.R. Evolution of local recruitment and its consequences for marine populations. Bull. Mar. Sci. 2002;70:377–396. [Google Scholar]
- Werner E.E, Gilliam J.F. The ontogenetic niche and species interactions in size structured populations. Annu. Rev. Ecol. Syst. 1984;15:393–425. doi:10.1146/annurev.es.15.110184.002141 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sea lice abundances for (a) C. clemensi and (b) L. samonis plotted against salinity for collections of pink salmon in 2004 when salinity was measured (n=50). Lines are statistically significant linear regressions