Abstract
In many long-lived vertebrates (including humans), adult males have shorter lifespans than adult females, partly as a result of higher annual rates of mortality in males and partly owing to sex differences in the rate of ageing. A probable explanation of the evolution of sex differences in ageing is that, in polygynous species, intense intrasexual competition between males restricts the number of seasons for which individual males are able to breed successfully, weakening selection pressures favouring adult longevity in males relative to females. If this is the case, sex differences in adult longevity and in the onset and rate of senescence should be greater in polygynous species than in monogamous ones and their magnitude should be related to the duration of effective breeding males compared with females. Here, we use data from longitudinal studies of vertebrates to show that reduced longevity in adult males (relative to females) is commonly associated with a more rapid decline in male than female survival with increasing age and is largely confined to polygynous species. The magnitude of sex differences in adult longevity in different species is consistently related to the magnitude of sex differences in the duration of effective breeding, calculated across surviving adults. Our results are consistent with the suggestion that sex differences in senescence in polygynous species are a consequence of weaker selection for longevity in males than females.
Keywords: sex differences, longevity, ageing, life histories, breeding systems
1. Introduction
In many polygynous vertebrates (including humans), intense reproductive competition between males and associated adaptations of growth and behaviour generate higher annual rates of mortality in males than females throughout much or all of their lifespan (Trivers 1972). In addition, as the end of the lifespan approaches, males commonly show accelerating levels of annual mortality relative to those in females and, in many mammalian populations, there are very few males left alive in the oldest age groups (Promislow 1992; Owen-Smith 1993; Low 1998; Loison et al. 1999; Mysterud et al. 2002; Toigo & Gaillard 2003). For example, in red deer (Cervus elaphus), sex differences in mortality begin before birth (Kruuk et al. 1999), increase in the first 2 years of life, especially when resources are scarce (Clutton-Brock et al. 1985), decline among young adults that have not yet started to breed but then increase rapidly towards the end of the lifespan until no males are left alive (Clutton-Brock et al. 1982; Catchpole et al. 2003; Carranza et al. 2004).
Reduced longevity in adult males compared with females is commonly associated with an earlier onset and more rapid progression of senescence in males than females. For example, in western human societies where populations have access to abundant resources, adult males typically show earlier senescence and lower life expectancies than females (Mealey 2000). Earlier senescence is also common in male mammals maintained in captivity (Ralls et al. 1980) while, in red deer, tooth wear progresses more rapidly and indices of body condition begin to decline at an earlier age in males than females (Clutton-Brock et al. 1982; Catchpole et al. 2003; Carranza et al. 2004). An extreme case of sex differences in senescence occurs in the marsupial mouse, Antechinus stuartii, where males show accelerated ageing after the end of the mating season and die before the next breeding season while females can live for more than one season (Cockburn 1985, 1989).
A probable explanation for the evolution of earlier senescence in males than females is that intense intrasexual competition for breeding opportunities between males in polygynous societies, combined with the costs of traits or strategies that enhance competitive success, shorten the period for which adult males are able to attract or defend females against their competitors, so that selection pressures favouring longevity are weaker than in females (Williams 1957; Kirkwood & Rose 1991; Stearns 1992). If so, sex differences in ageing and adult longevity should be more pronounced in polygynous than in monogamous species and their magnitude should be related to sex differences in the duration of the period over which individuals are likely to breed successfully (the Duration of Effective Breeding, DEB). However, until recently, few studies have been able to measure age-specific survival and breeding success in both sexes and, as yet, there has been no systematic attempt to compare the magnitude of sex differences in longevity between polygynous and monogamous species or to determine whether sex differences in longevity are consistently related to sex differences in the DEB.
Improvements in our ability to measure variation in reproductive success in males (Pemberton et al. 1992) and an increase in the number of long-term studies that have monitored age-related changes in breeding success and survival in both sexes (Clutton-Brock 1988; Newton 1989) now make it possible to investigate whether sex differences in adult longevity are confined to polygynous species and whether or not their magnitude is related to the magnitude of sex differences in the DEB. To investigate these relationships, we collected estimates of age-specific survival and reproductive success from detailed long-term studies of 35 vertebrates with average adult lifespans exceeding a year, and investigated relationships between sex differences in survival and in the DEB.
To ensure the reliability of our estimates, we restricted our data to studies that had been able to monitor the life histories of large samples of recognizable individuals. In most cases, measures of reproductive success in both sexes were based on observational data validated by genetic techniques. Estimates of age-specific survival are available for at least 30 species, out of which 9 are socially monogamous while 21 are polygynous, and estimates of age-specific breeding success are available for 15 species, out of which 5 are socially monogamous and 10 polygynous (see electronic supplementary material 1). It is an inevitable consequence of the distribution of breeding systems that the majority of polygynous species in our sample were mammals while the majority of monogamous ones were birds, so that it is difficult to allow for phylogenetic biases. Wherever possible, we repeat analyses for our entire sample of vertebrates within mammals and birds separately and, in one case, we have been able to use a phylogenetic analysis.
2. Material and methods
For each species in our sample, we extracted measures of age-specific survival from life-table data and used these to calculate the life expectancy of males and females reaching adulthood (which is equal to the average age at first breeding in females). Adult life expectancy, the number of years that an individual expects to live, on an average, after reaching adulthood, was calculated as
where ex is the adult life expectancy; ly is the survivorship at age y (proportion of original population surviving to beginning of age y); x is the age at reaching adulthood; and lx is the survivorship at age x (Pianka 1974). This is equal to the mean lifespan remaining to those individuals reaching adulthood. For 28 out of the 30 species in our sample, age-specific probabilities of survival, calculated from life tables, were used to calculate survivorship values. For two species, black grouse, Tetrao tetrix, and spotted hyenas, Crocuta crocuta, studies reported average annual survival of adults rather than age-specific survival, and these were used. The life tables used were typically based on data generated by long-term studies, which monitored the life histories of large samples of marked or recognizable individuals. For a few species (Rangifer tarandus, Cervus elaphus canadensis, Castor canadensis, Syncerus caffer, Tursiops truncatus), life tables reported were based on age distributions of animals found dead or shot at random. Sex differences in lifespan were calculated as the ratio of male to female estimates and were loge-transformed to conform to normality assumptions. To compare the rate at which survival declined in males and females, we fitted straight lines to age-specific estimates of survival over the last third of the maximum lifespan for each sex (maximum lifespan=birth to maximum recorded age) and used the difference in slope between the two sexes as an estimate of the relative rate at which survival declined in the two sexes.
While in most natural populations of vertebrates, both sexes are usually fertile throughout their lives, in polygynous animals the capacity of males to gain access to females and breed is commonly limited by competition to a relatively short number of years. For the purposes of this study, we defined the DEB as the length of time during which the average individual of one sex can expect a substantial amount of reproductive success. This was calculated from data on mean reproductive success achieved at different ages. At every age, mean reproductive success was calculated using only data from individuals alive at that age. A quadratic function was fitted to data on mean number of offspring produced at different ages. The DEB for individuals of one sex was then calculated as the time period in their lifespan (length of the x-axis) during which the quadratic curve was at least one-quarter the maximum (see electronic supplementary material 2). We tried setting this boundary at different levels and found that, although this inevitably affected the absolute duration of DEB, it had little influence on relationships between relative values of DEB in the two sexes and other parameters. The duration of DEB ranged from 5 to 20 years for females and 4 to 14 years for males. Like sex differences in lifespan, sex differences in DEB were calculated as the ratio of male to female measures and were loge-transformed.
Records of the average number of adult females in breeding groups were used as an index of the potential for polygyny. We used parametric t-tests to compare sex differences in lifespan between species allocated to different mating system categories and used correlations to evaluate the relationship between relative male lifespan and the indices of polygyny. All summary statistics shown are back-transformed from estimates obtained after loge-transformation and standard errors are therefore asymmetrical. Parametric t-tests and correlations were supplemented by randomization tests in which the null hypothesis was simulated by repeatedly shuffling the data (Manly 1997). Hypothesis tests based on these randomizations matched those based on parametric tests in all cases. All p values reported are two-tailed. All analyses were carried out in the statistical language R, v. 2.4.1 (R Development Core Team 2006).
In one case, the data were sufficient to perform a phylogenetic analysis using phylogenetic generalized least-squares methods (Martins & Hansen 1997) with the APE package (Paradis et al. 2006) in R and a composite tree based on Beck et al. (2006) for relationships at the level of the family and smaller-scale phylogenies for relationships among genera and species within families (Purvis 1995; Bininda-Emonds et al. 1999; Huchon et al. 2002). Branch lengths were set to 1.
3. Results
(a) Sex differences in survival
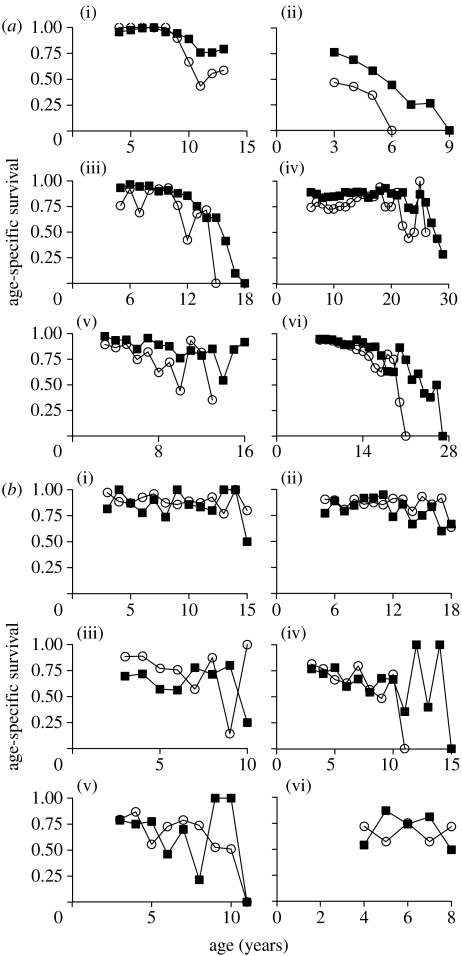
In 16 out of the 19 polygynous species with age-specific measures, adult males showed lower annual survival than adult females. In most (but not all) of these species, older males also showed a more rapid decline in survival with increasing age (figure 1a). In contrast, in our sample of monogamous species, there was no consistent tendency for adult males to show lower average survival than adult females or an earlier decline in survival with increasing age (figure 1b). In some monogamous species, there was little difference in average survival between adults of the two sexes, while in others average survival was generally lower in adult females than in adult males. A comparison of the rate at which survival declined during the last third of the lifespan showed that male survival declined more rapidly than female survival in 14 out of the 18 polygynous species (binomial test; n1=14, n2=4, p=0.031) but only in 5 out of the 9 monogamous ones.
Figure 1.
Age-specific survival for females (filled squares) and males (open circles) in (a) six socially polygynous ((i) red deer, Cervus elaphus; (ii) black-tailed prairie dog, Cynomys ludovicianus; (iii) African lion, Panthera leo; (iv) Japanese macaque, Macaca fuscata; (v) roe deer, Capreolus capreolus; and (vi) savannah baboon, Papio cynocephalus) and (b) six socially monogamous ((i) barnacle goose, Branta leucopsis; (ii) Bewicks's swan, Cygnus columbianus; (iii) Arabian babbler, Turdoides squamiceps; (iv) dwarf mongoose, Helogale parvula; (v) African wild dog, Lycaon pictus; and (vi) American beaver, Castor canadensis), long-lived vertebrates from studies of marked or recognizable individuals. Survival values are shown from the beginning of adulthood (see §2 for details; data sources are given in the electronic supplementary material 1).
Sex differences in average survival and in the timing of the final, age-specific decline in survival both contributed to sex differences in adult life expectancy (figure 2). In 16 out of the 21 polygynous species, adult males had life expectancies at least 20% shorter than adult females while, in 4 species, male life expectancies were either shorter than those of females or within 10% of female life expectancy. In contrast, in eight out of the nine monogamous species, life expectancies of adult males were within 10% of those of adult females or males had higher life expectancies than females. Formal comparisons of indices of relative survival (see §2) in the two groups show that sex differences in adult life expectancy are significantly larger in polygynous species than in monogamous ones (ratio of male to female adult life expectancy in polygynous species: mean=0.72; mean−1 s.e.m., mean+1 s.e.m.=0.68,0.76; n=21; monogamous species: mean=1.05; mean−1 s.e.m., mean+1 s.e.m.=0.99,1.12; n=9; t-test comparing polygynous and monogamous species: t2,28=4.031, p=0.0004; see table 1 and electronic supplementary material 1). A similar trend occurs within mammals: male life expectancy is either as long (within 10%) or longer (greater than 10%) than female lifespans in all three monogamous mammals but in only 2 out of 19 polygynous mammals.
Figure 2.
Survivorship curves (proportion of original population remaining) for females (filled squares) and males (open circles) in (a) six socially polygynous ((i) black-tailed prairie dog, Cynomys ludovicianus; (ii) red deer, Cervus elaphus; (iii) African lion, Panthera leo; (iv) Soay sheep, Ovis aries; (v) southern elephant seal, Mirounga leonina; and (vi) savannah baboon, Papio cynocephalus) and (b) six socially monogamous ((i) barnacle goose, Branta leucopsis; (ii) Bewicks's swan, Cygnus columbianus; (iii) Arabian babbler, Turdoides squamiceps; (iv) dwarf mongoose, Helogale parvula; (v) African wild dog, Lycaon pictus and (vi) American beaver, Castor canadensis), long-lived vertebrates from studies of marked or recognizable individuals. Survivorship values are shown from the beginning of adulthood (see §2 for details; data sources are given in the electronic supplementary material 1).
Table 1.
Relative adult life expectancy of males (male/female adult life expectancy) in 30 long-lived vertebrates, and relative DEB of males (male/female DEB) in 15 long-lived vertebrates. (Data sources are given in the electronic supplementary material 1. SM, socially monogamous; SP, socially polygynous.)
| species | breeding system | male/female adult life expectancy | male/female DEB |
|---|---|---|---|
| Cynomys ludovicianus | SP | 0.62 | 0.69 |
| Castor canadensis | SM | 0.94 | |
| Propithecus verreauxi verreauxi | SP | 1.03 | |
| Mandrillus sphinx | SP | 0.57 | 0.59 |
| Macaca mulatta | SP | 0.58 | |
| Macaca fuscata | SP | 0.59 | |
| Macaca sylvanus | SP | 0.66 | |
| Papio cynocephalus | SP | 0.85 | 0.62 |
| Theropithecus gelada | SP | 0.79 | |
| Gorilla beringei | SP | 0.80 | |
| Crocuta crocuta | SP | 0.87 | |
| Panthera leo | SP | 0.60 | 0.67 |
| Helogale parvula | SM | 1.03 | 1.09 |
| Suricata suricatta | SM | 0.99 | |
| Lycaon pictus | SM | 1.11 | |
| Mirounga angustirostris | SP | 0.33 | |
| Mirounga leonina | SP | 0.75 | |
| Capreolus capreolus | SP | 0.63 | |
| Rangifer tarandus | SP | 0.47 | |
| Cervus elaphus | SP | 0.75 | 0.48 |
| Cervus elaphus (elk) | SP | 0.48 | |
| Tursiops truncatus | SP | 0.79 | |
| Equus caballus | SP | 0.65 | |
| Syncerus caffer | SP | 1.00 | |
| Ovis aries | SP | 0.61 | 0.68 |
| Oreamnos americanus | SP | 0.71 | |
| Tetrao tetrix | SP | 0.66 | |
| Agelaius phoeniceus | SP | 0.93 | |
| Turdoides squamiceps | SM | 1.46 | |
| Aphelocoma caerulescens | SM | 0.94 | |
| Melanerpes formicivorus | SP | 1.39 | |
| Pandion haliaetus | SM | 0.98 | |
| Rissa tridactyla | SM | 0.80 | 1.00 |
| Cygnus columbianus | SM | 1.24 | 1.00 |
| Branta leucopsis | SM | 1.11 | 0.93 |
(b) Sex differences in the DEB
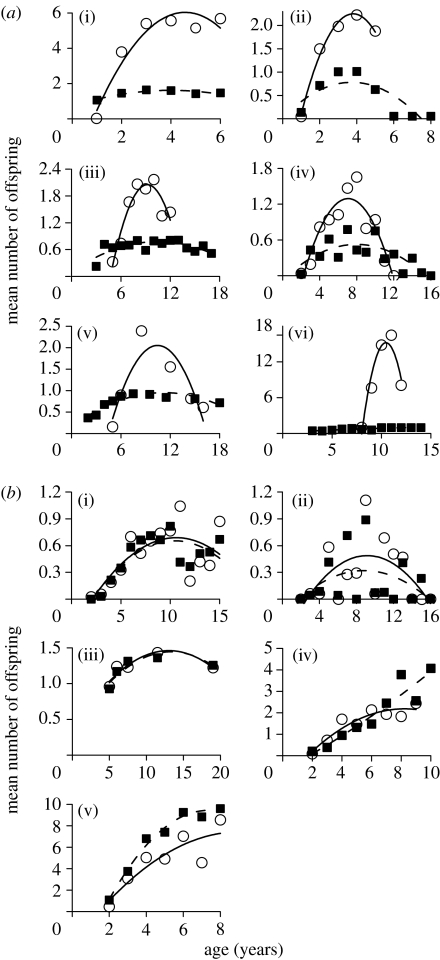
Age-specific reproductive success among surviving individuals also declines at an earlier age in males than in females in most polygynous vertebrates (figure 3a). In contrast, in monogamous species, sex differences in age-specific reproductive success are slight, absent or reversed (figure 3b). In 15 species for which estimates of age-specific breeding success are available for both the sexes, sex differences in the DEB are larger in polygynous than monogamous species (ratio of male DEB to female DEB in polygynous species: mean=0.61; mean−1 s.e.m., mean+1 s.e.m.=0.56,0.67; n=10; monogamous species: mean=1.00; mean−1 s.e.m., mean+1 s.e.m.=0.98,1.03; n=5; t-test comparing polygynous and monogamous species: t2,13=3.967, p=0.0016). Data on DEB are available for two monogamous and nine polygynous mammals (table 1). In both monogamous species, male DEB is within 10% of female DEB, whereas in all polygynous mammals, male DEB is at least 30% shorter than female DEB.
Figure 3.
Mean annual reproductive success (number of offspring produced) for females (filled squares, dashed line) and males (open circles, solid line) at different ages in (a) six socially polygynous ((i) red-winged blackbird, Agelaius phoeniceus; (ii) black-tailed prairie dog, Cynomys ludovicianus; (iii) red deer, Cervus elaphus; (iv) African lion, Panthera leo; (v) feral horse, Equus caballus; and (vi) northern elephant seal, Mirounga angustirostris) and (b) five socially monogamous, long-lived vertebrates ((i) Bewicks's swan, Cygnus columbianus; (ii) barnacle goose, Branta leucopsis; (iii) kittiwake gull, Rissa tridactyla; (iv) dwarf mongoose, Helogale parvula; and (v) meerkat, Suricata suricatta), based on estimates of breeding success in recognizable individuals (data sources are given in the electronic supplementary material 1).
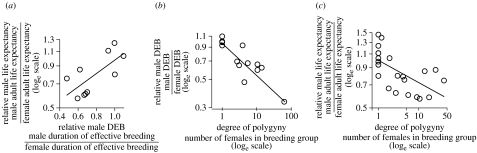
If sex differences in life expectancy are a consequence of weaker selection for longevity in males as a result of reductions in the DEB, they should be consistently related to sex differences in the DEB. Comparisons of species for which estimates of age-specific reproductive success and survival are available in both sexes show that, as predicted, the magnitude of sex differences in life expectancy is closely associated with the magnitude of sex differences in DEB (figure 4a; Pearson's correlation R=0.70, n=10, p=0.0231). In addition, sex differences in both measures increase as the number of females per breeding group (an index of the degree of polygyny) rises (sex differences in DEB: Pearson's R=−0.88, n=14, p=0.00003; sex differences in life expectancy: Pearson's R=−0.62, n=23, p=0.0015; figure 4b,c). For the second of these two relationships, the data for mammals were adequate for a phylogenetic analysis. This showed that, as predicted, relative life expectancy in males was negatively related to our index of polygyny when the effects of phylogeny were allowed for (PGLS on loge-transformed data: slope (standard error)=−0.103(0.044), t=−2.333, n=15 species, p=0.036).
Figure 4.
The relationships between (a) relative male life expectancy and relative DEB of males for different species (N=10), (b) relative DEB of males and the degree of polygyny (average number of females in breeding groups, N=14) and (c) relative male life expectancy and the average number of females in breeding groups (N=23).
4. Discussion
Our results confirm that, in long-lived polygynous vertebrates, adult males commonly show higher rates of annual mortality and an earlier onset of age-related increases in mortality as the end of the lifespan approaches in adult females. Together, these effects generate substantial differences in adult life expectancy between the sexes. In contrast, sex differences in mortality and adult life expectancy are smaller and less consistent in monogamous species (figures 1 and 2) and are sometimes reversed (Liker & Székely 2005). Among surviving individuals, the DEB is also usually shorter in males than females in polygynous species, partly because males commonly begin to breed later and partly because they cease breeding at a younger age. In contrast the DEB is usually similar in the two sexes in monogamous species (figure 3). Across species, the magnitude of sex differences in life expectancy is consistently related to the magnitude of sex differences in the DEB (figure 4).
It is unfortunate (but inevitable) that estimates of age-specific survival and reproductive success are available for very few monogamous mammals and polygynous birds, so that our sample of polygynous species is strongly biased towards mammals while our sample of monogamous species is biased towards birds. All three studies of monogamous mammals that we have included (and other studies that do not provide comparable measures of age-specific breeding success and survival for both sexes) suggest that the annual survival rates of mature males are not consistently lower than that of mature females—as is the case for long-lived monogamous birds. Whether sex differences in the life histories of polygynous birds differ from those in mammals remains to be seen. Red-winged blackbirds (Agelaius phoeniceus) do not appear to show such a pronounced reduction in the DEB in males (figure 3) but this could be because they are relatively short-lived. There is some indication that the DEB is shorter in males than in females in longer-lived, polygynous birds, like the tetraonids (Kruijt & de Vos 1988).
The higher mortality of males throughout the lifespan in polygynous mammals probably occurs because the potential reproductive benefits of winning competitive encounters are greater for males than females and behavioural traits that enhance competitive success commonly trade-off against survival, generating increased mortality in males relative to females in species where there is a strong selection for competitive success in males (Trivers 1972). For example, in many ungulates, males expend most of their fat reserves in the autumn rut, and their condition at the onset of winter is inferior to that of females, making them more susceptible to adverse weather or to density-dependent resource shortages (Clutton-Brock & Albon 1989). The effects of testosterone on immune responses may also affect the parasite loads of males, with downstream effects on their energy balance and susceptibility to food shortages (Moore & Wilson 2002). Secondary sexual characters (including weaponry and increased body size) of males may also have energetic costs to males and render them more susceptible to adverse environmental conditions. However, systematic interspecific analyses of sexual dimorphism have found no consistent relationship between sex differences in body size and sex differences in survival (Toigo & Gaillard 2003), and sex differences in longevity occur in some polygynous species where the sexes are the same size (Berger 1986) as well as in some where males are smaller than females (Hofer & East 1995).
The immediate cause of age-related reductions in male breeding success towards the end of the lifespan in polygynous mammals is that the ability of males to win fights declines with increasing age so that they are excluded from access to females (Clutton-Brock 1988; Le Boeuf & Reiter 1988). Several different mechanisms may underlie these changes and may help to explain why they are more pronounced in males. First, frequent contests may expose the effects of increasing age on physical performance, raising the chance that males will be displaced without affecting their condition or performance directly (Clutton-Brock et al. 1987a,b). Second, fighting may lead to cumulative phenotypic damage, with immediate phenotypic effects on the ability of males to win subsequent contests, so restricting both their DEB and their survival (Clutton-Brock 1988; Partridge 1988). Third, reduced life expectancy may weaken selection for deferring senescence in males, leading to heritable changes in competitive ability with increasing age (Stearns 1992). And finally, older males may invest more heavily in reproductive competition as a result of reduction in their reproductive value and this may reduce their condition and ability to invest in subsequent years (Pianka 1976; Clutton-Brock 1984). Empirical evidence that distinguishes between these mechanisms in natural populations is scarce. However intraspecific comparisons suggest that intense competition between males commonly has direct effects on the reproductive tenure of males in polygynous species. For example, the tenure of coalitions of male lions Panthera leo is reduced for male groups that defend large prides of females that attract many competitors (Packer et al. 1988). Similarly, in Thomas' langurs Presbytis thomasi, the frequency of male takeovers increases with female group size (Steenbeek 2000). And, in several polygynous antelopes, males holding territories that attract substantial numbers of females have shorter tenures than males defending less attractive territories (Gosling 1986; Bro-Jorgensen & Durant 2003).
Like changes in the reproductive performance of males with increasing age, the earlier and more rapid decline in annual rates of survival in males compared with females in polygynous species probably has multiple causes. In some cases, the energetic expenditure of successful males in the annual rut is so high that, once males reach an age where they can breed effectively, their annual rates of survival rapidly decline. For example, when male greater kudu Tragelaphus strepsiceros reach full size and begin to rut successfully, they compete so intensely in the annual rut that they commonly either die from starvation or are killed by predators and rarely survive for more than 1 or 2 years (Owen-Smith 1993). Cumulative phenotypic damage from fighting or from repeated periods of starvation may also contribute to earlier increases in mortality in males than females (Clutton-Brock 1994). Alternatively, the earlier increase in mortality in males than females may be caused by earlier ageing in males as a result of increased impact of antagonistic pleiotropy or mutation accumulation in their soma caused by the earlier decline in their reproductive value. Finally, increased investment in reproductive competition by old males may generate progressively higher rates of annual mortality towards the end of the lifespan (Pianka 1976; Clutton-Brock 1984).
Irrespective of the causes, reductions in the DEB and longevity in adult males in polygynous species have important consequences for population structure and reproductive strategies. They are likely to reduce variance in lifetime reproductive success among males relative to females, which may weaken selection for characteristics associated with competitive success in males (Clutton-Brock 1983, 2004; Gowaty 2004). By reducing the breeding tenure of individual males, they are also likely to lower coefficients of relatedness between successive cohorts (Shields 1987). Finally, reductions in the reproductive tenure of males in polygynous species may help to explain why female philopatry and male reproductive dispersal are characteristic of group-living mammals, while male philopatry and female dispersal are characteristic of group-living birds (Greenwood 1980; Clarke et al. 1997). Studies of social mammals show that, where females commonly remain and breed in their natal groups, the age of females at first breeding usually exceeds the average tenure of breeding males (so that a male's daughters rarely reach sexual maturity during his period of tenure) while, in species where the average duration of male breeding lifespans exceeds the age at which females reach sexual maturity, females typically disperse from their natal group to avoid close inbreeding (Clutton-Brock 1989). The prevalence of polygyny and associated reductions in male tenure may help to explain why female philopatry is common in social mammals. In contrast, the prevalence of monogamous breeding, long male tenure and early age at first breeding in females in birds may explain why, in group-living birds, females typically disperse from their ‘natal’ groups to breed elsewhere (Clarke et al. 1997; Koenig & Haydock 2004).
Acknowledgments
For access to unpublished data, we are grateful to J. Altmann, S. Alberts, J. Pemberton and S. Hodge. We are grateful to R. Seyfarth, D. Cheney, A. Young, S. Hodge, S. Quader, P. Gowaty, R. L. Trivers and an anonymous reviewer for their comments. K.I. was funded by the John Stanley Gardiner Fund and Madgalene College, Cambridge.
Supplementary Material
Estimates of adult life expectancy of males and females from thirty long-lived vertebrates and the duration of effective breeding for males and females from 15 long-lived vertebrates
A description of the methods used to calculate the duration of effective breeding
References
- Beck R.M.D, Bininda-Emonds O.R.P, Cardillo M, Liu F.R, Purvis A. A higher-level mrp supertree of placental mammals. BMC Evol. Biol. 2006;6:93. doi: 10.1186/1471-2148-6-93. doi:10.1186/1471-2148-6-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. The University of Chicago Press; Chicago, IL: 1986. Wild horses of the great basin. [Google Scholar]
- Bininda-Emonds O.R.P, Gittleman J.L, Purvis A. Building large trees by combining phylogenetic information: a complete phylogeny of the extant carnivora (mammalia) Biol. Rev. 1999;74:143–175. doi: 10.1017/s0006323199005307. doi:10.1017/S0006323199005307 [DOI] [PubMed] [Google Scholar]
- Bro-Jorgensen J, Durant S.M. Mating strategies of topi bulls: getting in the centre of attention. Anim. Behav. 2003;65:585–594. doi:10.1006/anbe.2003.2077 [Google Scholar]
- Carranza J, Alarcos S, Sanchez-Prieto C.B, Valencia J, Mateos C. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature. 2004;432:215–218. doi: 10.1038/nature03004. doi:10.1038/nature03004 [DOI] [PubMed] [Google Scholar]
- Catchpole E.A, Fan Y, Morgan B.J.T, Clutton-Brock T.H, Coulson T. Sexual dimorphism, survival and dispersal in red deer. J. Agric. Biol. Environ. Stat. 2003;9:1–26. [Google Scholar]
- Clarke A.L, Saether S.A, Roskaft E. Sex biases in avian dispersal: a reappraisal. Oikos. 1997;79:429–438. doi:10.2307/3546885 [Google Scholar]
- Clutton-Brock T.H. Selection in relation to sex. In: Bendall B.J, editor. Evolution from molecules to men. Cambridge University Press; Cambridge, UK: 1983. [Google Scholar]
- Clutton-Brock T.H. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 1984;123:212–229. doi:10.1086/284198 [Google Scholar]
- Clutton-Brock, T. H. 1988 Reproductive success: studies of individual variation in contrasting breeding systems. In Reproductive success Chicago, IL: The University of Chicago Press.
- Clutton-Brock T.H. Female transfer and inbreeding avoidance in social mammals. Nature. 1989;337:70–71. doi: 10.1038/337070a0. doi:10.1038/337070a0 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. The costs of sex. In: Short R.V, Balaben E, editors. The differences between the sexes. Cambridge University Press; Cambridge, UK: 1994. pp. 347–362. [Google Scholar]
- Clutton-Brock T.H. What is sexual selection? In: Kappeler P.M, van Schaik C.P, editors. Sexual selection in primates. Cambridge University Press; Cambridge, UK: 2004. pp. 24–36. [Google Scholar]
- Clutton-Brock T.H, Albon S.D. Blackwell Scientific Publications; Oxford, UK: 1989. Red deer in the highlands. [Google Scholar]
- Clutton-Brock T.H, Guinness F.E, Albon S.D. Edinburgh University Press; Edinburgh, UK: 1982. Red deer: behavior and ecology of two sexes. [Google Scholar]
- Clutton-Brock T.H, Albon S.D, Guinness F.E. Parental investment and sex differences in juvenile mortality in birds and mammals. Nature. 1985;313:131–133. doi:10.1038/313131a0 [Google Scholar]
- Clutton-Brock T.H, Albon S.D, Guinness F.E. Interactions between population density and maternal characteristics affecting fecundity and survival in red deer. J. Anim. Ecol. 1987a;56:53–67. doi:10.2307/4799 [Google Scholar]
- Clutton-Brock T.H, Major M, Albon S.D, Guinness F.E. Early development and population dynamics in red deer. 1: density-dependent effects on juvenile survival. J. Anim. Ecol. 1987b;56:53–67. doi:10.2307/4799 [Google Scholar]
- Cockburn A. Antechinus as a paradigm in evolutionary ecology. In: Lee A.K, Cockburn A, editors. Evolutionary ecology of marsupials. Cambridge University Press; Cambridge, UK: 1985. pp. 162–185. [Google Scholar]
- Cockburn A. Adaptive patterns in marsupial reproduction. Trends Ecol. Evol. 1989;4:126–130. doi:10.1016/0169-5347(89)90210-3 [Google Scholar]
- Gosling L.M. The evolution of mating strategies in male antelopes. In: Rubenstein D.I, Wrangham R.W, editors. Ecological aspects of social evolution. Princeton University Press; Princeton, NJ: 1986. pp. 244–281. [Google Scholar]
- Gowaty P.A. Sex roles, contests for the control of reproduction and sexual selection. In: Kappeler P.M, editor. Sexual selection in primates. Cambridge University Press; Cambridge, UK: 2004. pp. 37–54. [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. doi:10.1016/S0003-3472(80)80103-5 [Google Scholar]
- Hofer H, East M. Population dynamics, population size, and the commuting system of Serengeti spotted hyenas. In: Sinclair A.R.E, Arcese P, editors. Serengeti II: dynamics, management, and conservation of an ecosystem. The University of Chicago Press; Chicago, IL: 1995. pp. 332–363. [Google Scholar]
- Huchon D, Madsen O, Sibbald M.J.B.B, Ament K, Stanhope M.J, Catzeflis F, de Jong W.W, Douzery E.J.P. Rodent phylogeny and a timescale for the evolution of glires: evidence from an extensive taxon sampling using three nuclear genes. Mol. Biol. Evol. 2002;19:1053–1065. doi: 10.1093/oxfordjournals.molbev.a004164. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L, Rose M.R. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. doi:10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- Koenig W.D, Haydock J. Incest and incest avoidance. In: Koenig W.D, Dickinson J, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 142–156. [Google Scholar]
- Kruijt J.P, de Vos G.J. Individual variation in reproductive success in male black grouse, Tetrao tetrix l. In: Clutton-Brock T.H, editor. Reproductive success. The University of Chicago Press; Chicago, IL: 1988. pp. 279–290. [Google Scholar]
- Kruuk L.E.B, Clutton-Brock T.H, Albon S.D, Pemberton J.M, Guinness F.E. Population density affects sex ratio variation in red deer. Nature. 1999;399:459–461. doi: 10.1038/20917. doi:10.1038/20917 [DOI] [PubMed] [Google Scholar]
- Le Boeuf B.J, Reiter J. Lifetime reproductive success in northern elephant seals. In: Clutton-Brock T.H, editor. Reproductive success. The University of Chicago Press; Chicago, IL: 1988. pp. 344–362. [Google Scholar]
- Liker A, Székely T. Mortality costs of sexual selection and parental care in natural populations of birds. Evolution. 2005;59:890–897. [PubMed] [Google Scholar]
- Loison A, Festa-Bianchet M, Gaillard J.M, Jorgenson J.T, Jullien J.M. Age-specific survival in five populations of ungulates: evidence of senescence. Ecology. 1999;80:2539–2554. [Google Scholar]
- Low B.S. The evolution of human life-histories. In: Crawford C.B, Krebs D.L, editors. Handbook of evolutionary psychology: ideas, issues, and applications. Lawrence Erlbaum; Mahwah, NJ: 1998. pp. 131–161. [Google Scholar]
- Manly B.F.J. Chapman & Hall; Boca Raton, FL: 1997. Randomization, bootstrap and Monte Carlo methods in biology. [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. doi:10.1086/286013 [Google Scholar]
- Mealey L. Academic Press; New York, NY: 2000. Sex differences: developmental and evolutionary strategies. [Google Scholar]
- Moore S.L, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. doi:10.1126/science.1074196 [DOI] [PubMed] [Google Scholar]
- Mysterud A, Coulson T.N, Stenseth N.C. The role of males in the dynamics of ungulate populations. J. Anim. Ecol. 2002;71:907–915. doi:10.1046/j.1365-2656.2002.00655.x [Google Scholar]
- Newton I. Academic Press; London, UK: 1989. Lifetime reproduction in birds. [Google Scholar]
- Owen-Smith N. Comparative mortality rates of male and female kudus: the costs of sexual size dimorphism. J. Anim. Ecol. 1993;62:428–440. doi:10.2307/5192 [Google Scholar]
- Packer C, Herbst L, Pusey A.E, Bygott J.D, Hanky J.B, Cairns S.J, Borgerhoff-Mulder M. Reproductive success of lions. In: Clutton-Brock T.H, editor. Reproductive success. The University of Chicago Press; Chicago, IL: 1988. pp. 403–418. [Google Scholar]
- Paradis, E. et al 2006 APE: analyses of phylogenetics and evolution. R package, version 1.8-5.
- Partridge L. Lifetime reproductive success in Drosophila. In: Clutton-Brock T.H, editor. Reproductive success. The University of Chicago Press; Chicago, IL: 1988. pp. 11–23. [Google Scholar]
- Pemberton J.M, Albon S.D, Guinness F.E, Clutton-Brock T.H, Dover G.A. Behavioral estimates of male mating success tested by DNA fingerprinting in a polygynous mammal. Behav. Ecol. 1992;3:66–75. doi:10.1093/beheco/3.1.66 [Google Scholar]
- Pianka E.R. Harper & Row; New York, NY: 1974. Evolutionary ecology. [Google Scholar]
- Pianka E.R. Natural selection of optimal reproduction tactics. Am. Zool. 1976;16:775–784. [Google Scholar]
- Promislow D.E.L. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. B. 1992;247:203–210. doi:10.1098/rspb.1992.0030 [Google Scholar]
- Purvis A. A composite estimate of primate phylogeny. Phil. Trans. R. Soc. B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. doi:10.1098/rstb.1995.0078 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2006 R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-900051-07-0. See. http://www.R-project.org.
- Ralls K, Brownell R.L, Jr, Ballou F. Differential mortality by sex and age in mammals, with special reference to the sperm whale. Rep. Int. Whaling Comm. 1980;2:233–243. [Google Scholar]
- Shields W.M. Dispersal and mating systems: investigating their causal connections. In: Chepko-Sade B.D, Halpin Z.T, editors. Mammalian dispersal patterns: the effects of social structure on population genetics. The University of Chicago Press; Chicago, IL: 1987. pp. 9–24. [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Steenbeek R. Infanticide by males and female choice in Thomas' langurs. In: van Schaik C.P, Janson C.H, editors. Infanticide by males and its implications. Cambridge University; Cambridge, UK: 2000. pp. 153–197. [Google Scholar]
- Toigo C, Gaillard J.M. Causes of sex-biased adult survival in ungulates: sexual size dimorphism, mating tactic or environment harshness? Oikos. 2003;101:376–384. doi:10.1034/j.1600-0706.2003.12073.x [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine-Atherton; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Williams G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi:10.2307/2406060 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimates of adult life expectancy of males and females from thirty long-lived vertebrates and the duration of effective breeding for males and females from 15 long-lived vertebrates
A description of the methods used to calculate the duration of effective breeding