Abstract
The cestode Schistocephalus solidus is a frequent parasite of three-spined sticklebacks and has a large impact on its host's fitness. Selection pressure should therefore be high on stickleback defence mechanisms, like an efficient immune system, and also on parasite strategies to overcome these. Even though there are indications for manipulation of the immune system of its specific second intermediate host by the cestode, nothing is yet known about the chronology of specific interactions of S. solidus with the stickleback immune system. We here expected sticklebacks to first mount an innate immune response directly post-exposure to the parasite to clear the infection at an early stage and after an initial lag phase to upregulate adaptive immunity. Most interestingly, we did not find any upregulation of the specific lymphocyte-mediated immune response. Also, the pattern of activation of the innate immune system did not match our expectations: the proliferation of monocytes followed fluctuating kinetics suggesting that the parasite repeatedly installs a new surface coat not immunogenic to the host. Furthermore, the respiratory burst activity, which has the potential to clear an early S. solidus infection, was upregulated very late during infection, when the parasite was too big to be cleared but ready for transmission to its final host. We here suggest that the late activation of the innate immune system interferes with the neuroendocrine system, which mediates reduced predation avoidance behaviour and so facilitates the transmission to the final host.
Keywords: Gasterosteus aculeatus, Schistocephalus solidus, host–parasite interaction, host manipulation, head kidney leucocytes, respiratory burst activity
1. Introduction
For survival, parasites strongly depend on their hosts (MacInnes 1976) and often seriously impair their fitness. Thus, selection pressure is high in hosts to increase parasite resistance and in parasites to maximize host exploitation, often resulting in an arms race of host–parasite counter-adaptations (Hamilton & Zuk 1982; Hamilton et al. 1990). The outcome of host–parasite interaction is difficult to predict since it strongly depends on a series of (specific and non-specific) defence barriers of the host and at the same time the ability of the parasite to overcome these (Schmid-Hempel & Ebert 2003).
Aspects of host–parasite interactions have been studied for several years in the well-established host–parasite model system Schistocephalus solidus and its three consecutive hosts, a cyclopoid copepod, the three-spined stickleback and any fish-eating bird (Smyth 1946; Dubinina 1966; Wedekind 1997). The tapeworm takes up all its resources from the two intermediate hosts and has a huge impact on their fitness: during infection both hosts suffer from reduced or no reproduction (Tierney et al. 1996; Wedekind 1997; Bagamian et al. 2004). When the parasite is infective for the next host, both intermediate hosts are used as vessels to get there and die as prey of the next host (Arme & Owen 1967). Selection pressure on host defence mechanisms should therefore be high. Specifically, the defence barriers the tapeworm has to overcome when interacting with the three-spined stickleback have been of great interest. It is known that there is no behavioural defence barrier against S. solidus since sticklebacks are unable to avoid infected copepods (Wedekind & Milinski 1996). The next step, the time between ingestion and establishment in the body cavity of the sticklebacks, is of crucial importance for infection success (Hammerschmidt & Kurtz 2007): between 50 and 75% of the parasites fail to infect the fish. Still, the proportion of infected fish can be quite high, but strongly depends on the population and year (Arme & Owen 1967). Once infected, the only chance of defence is the host's immune system. There have been already a few studies indicating that there are indeed interactions between S. solidus and stickleback immune system.
The tapeworm on one side seems to evade the innate host immune system by adjusting its surface carbohydrate composition (Hammerschmidt & Kurtz 2005b). It also interferes with cell-mediated immunity, since leucocytes isolated from infected sticklebacks failed to respond to S. solidus antigens in vitro (Scharsack et al. 2004). The stickleback immune system on the other side should eliminate the tapeworm as early in infection as possible, since this will be potentially impossible later during infection due to the dramatic increase in parasite size. Whether large helminth parasites of fish are killed by cellular responses in vivo is not clear (Secombes & Chappell 1996), a potential defence against S. solidus could be through activation of the innate immune system. A typical pattern in the innate immune response of fish to helminth parasites is the mobilization and activation of granulocytes (Hoole & Arme 1983; Sharp et al. 1992; Taylor & Hoole 1993, 1995; Nie & Hoole 2000), which then produce oxygen intermediates like nitric oxide (NO) and reactive oxygen species (ROS; Whyte et al. 1990; Secombes & Chappell 1996). However, despite freeing the host from its parasite burden, such an activation of innate immunity also poses immunopathological costs on the host (Lochmiller & Deerenberg 2000), which is also suggested to play a role in sticklebacks infected with S. solidus (Hammerschmidt & Kurtz 2005a). This stresses the importance of a fast and efficient response at the beginning of an infection with the tapeworm.
In addition to the innate cellular line of defence (Jones 2001), fish hosts possess adaptive immunity that produces specific antibodies against parasite antigens (Roberts et al. 2005; Wiegertjes et al. 2005). Clonal expansion (proliferation) of lymphocytes is a fundamental part of the specific immune response of fish (Rijkers et al. 1980; Le Morvan-Rocher et al. 1995), and is used as a measure of activation of the specific immune system against parasites in fish hosts (Hamers & Goerlich 1996; Nie et al. 1996; Scharsack et al. 2000). In sticklebacks, nobody has so far directly measured activation of adaptive immunity, but results from Kurtz et al. (2004) strongly suggest that parasite growth is restricted by adaptive immunity, since worm size depended on stickleback major histocompatibility complex (MHC) genetics.
Despite all indications that interactions between S. solidus and the stickleback immune system are relevant for both host and parasite, to date no study has monitored the chronology of S. solidus infections from an immunological perspective. For the first time, we simultaneously access parameters of innate and adaptive lines of stickleback immunity during ongoing S. solidus infection. Specifically, we expected the stickleback's cell-dependent innate immunity (granulocyte mobilization and increased production of ROS, proliferation of monocytes) to become activated immediately after parasite exposure to clear the infection at an early stage. Adaptive immunity of the stickleback, after an initial lag phase, should be upregulated and reflected in elevated proliferation of lymphocytes. Additionally, we monitored parasite and host weights to link the strength of the host immune activation to the fitness of both host and parasite.
2. Material and methods
(a) Parasites and the infection of the two intermediate hosts
Sticklebacks infected with S. solidus were caught from a brackish lagoon of the Baltic Sea in northern Germany (Neustädter Binnenwasser) and screened for S. solidus. Schistocephalus solidus (plerocercoids) were matched pairwise with regard to body weight to ensure that the worms reproduced mainly by outcrossing (Lüscher & Milinski 2003). Three S. solidus pairs were bred for 6 days in vitro (Smyth 1946; Wedekind 1997). After removal of culture medium, all eggs from each tapeworm pair were collected and stored in tap water at 4°C in the dark until use. The offspring from each pair will be referred to as ‘parasite sibship’. Three weeks before exposure of parasites to copepods (first intermediate hosts), eggs were placed at 20°C in the dark. One day before infection of copepods, eggs were transferred in Petri dishes with tap water and exposed to a 3 hour light stimulus in the evening, followed by an 8 hour dark period overnight so that eggs were hatched the next morning, when exposed to light again (after Dubinina 1966).
Copepods (Macrocyclops albidus) were kept in laboratory cultures as described by van der Veen & Kurtz (2002). The culture used here was initiated with M. albidus from a brook (Kremper Au, Neustadt, Germany) that flows to the brackish lagoon (Neustädter Binnenwasser), the source of the parasite population. Copepods were maintained individually and exposed to a single tapeworm larva (i.e. coracidium) of S. solidus as in Hammerschmidt & Kurtz (2005a). Six days post-exposure (dpe), infection status of each copepod was determined microscopically. Infected copepods were fed to fish 21 dpe.
Experimental sticklebacks were laboratory hatched and raised offspring from adults originating from the same brackish lagoon (Neustädter Binnenwasser) as the parasites. Offspring from nine pairs of parental sticklebacks were used for infection. The siblings will be referred to as fish families. Fourteen weeks before infection, all experimental sticklebacks were individually marked by spine clipping within the respective family group and housed in groups of 20 animals per 16 l tank. Sticklebacks (n=537) were transferred to individual tanks (2 l water) and starved for one week to enhance consumption of infected/uninfected copepods. Parasite sibships were assigned randomly to individual stickleback from the different families in a balanced design. One copepod infected with one 21-day-old procercoid was added to each of the 423 fish. For controls, uninfected copepods were offered to 114 sticklebacks from the same families. Three dpe, sticklebacks were returned to 16 l tanks in family groups of seven individuals per tank. The tanks were distributed randomly across the shelves in two aquaria rooms (18°C and 16 : 8 light/dark cycles). Sticklebacks were fed ad libitum three times a week with frozen chironomids.
(b) Dissection and fitness parameters of sticklebacks
Starting at 7 dpe to the parasite, fish from each group were dissected every 10 days until day 67 post-exposure. Stickleback weight (to the nearest 0.1 mg) and length (to the nearest mm, from the snout to the base of the tail) were determined. After fish were killed by cutting the vertebral column, the head kidney was removed for immunological assays. The body cavity was rinsed with 0.67% NaCl and screened for tapeworms. The liver was removed and weighed (to the nearest 0.1 mg). The hepatosomatic index exclusive parasite weight of sticklebacks was calculated as 100×liver weight/(fish weight−parasite weight).
(c) Measurement of parasite fitness
Owing to their small size, no reliable weight measurements could be made for procercoids. Therefore, weights (volumes) of small parasite stages were calculated based on size (i.e. area) measurements with the formula, volume (mm3)=e0.279×area (μm2)1.385×10−9, as given by Wedekind et al. (2000). Procercoids were dissected from infected copepods 21 dpe, killed and relaxed by transfer to a final concentration of 4% (v/v) acetic acid in tap water. To stop the relaxation process and preserve the material, formalin was added to a final concentration of 4% (v/v) after 5 min. Plerocercoids collected from sticklebacks 7–37 dpe were relaxed with 17% (v/v) acetic acid and formalin (4%) was added after 15 min. From relaxed procercoids and plerocercoids (7–17 dpe), images were taken with a video camera and size (i.e. area) was measured with the image analysis program Image J v. 1.31 (Wayne Rasband, National Institutes of Health, USA; Hammerschmidt & Kurtz 2005a). From plerocercoids collected 7–37 dpe, formalin weight was measured with a micro fine balance (Sartorius, SE MA 2.1 g). For comparison, formalin weight and fresh weights were measured 27 and 37 dpe. Starting at 47 dpe, plerocercoids were weighed alive (fresh weight) and transferred to the artificial breeding system to produce eggs (used in another experiment). Each parasite was dried and weighed thrice (to the nearest 0. 1 μg) to calculate the mean weight. To be able to use one fitness parameter for the parasite throughout the whole experiment, a standard parasite weight was calculated.
For all parasites which had been in the fish for less than 17 days, we used the formula: standard parasite weight=parasite volume×0.7048202+0.000013. This formula was obtained by the regression of volume and formalin weight of plerocercoids at 7 and 17 dpe.
For all parasites which had been in the fish for more than 17 days, we used the formula: standard parasite weight=fresh parasite weight×0.8793691+(−0.000175). This formula was obtained by the regression of fresh and formalin weights of plerocercoids at 27 and 37 dpe.
For parasites collected at 17–37 dpe, formalin weight was used as measured.
(d) Immune parameters
For immunological assays, head kidney leucocytes (HKL) were isolated from sticklebacks and analysed for proliferation and respiratory burst activity as described by Scharsack et al. (2007). Briefly, total cell numbers in individual HKL isolates were determined by means of flow cytometry with the standard cell dilution assay (Pechhold et al. 1994) as modified by Scharsack et al. (2004). Suspensions of HKL were adjusted to 1.2×105 viable cells per millilitre with RPMI 1640 diluted with 10% (v/v) distilled water (R-90).
As one of the most important effector mechanisms of the cellular innate immune system, the respiratory burst activity of HKL was quantified in a lucigenin-enhanced chemiluminescence assay modified after Scott & Klesius (1981), as described by Kurtz et al. (2004). In 96-well flat bottom microtitre plates, 80 μl of HKL suspension (1×105 HKL per well) was added to 80 μl R-90 and 20 μl lucigenin solution (2.5 g l−1 PBS). Plates were incubated for 30 min at 18°C to allow uptake of lucigenin by the cells. Phagocytosis and production of ROS were initiated by the addition of 20 μl zymosan suspension (7.5 g l−1 PBS) and measured for 3 hour at 20°C.
As a parameter for leucocyte activation, we determined the relative number of proliferating lymphocytes and monocytes (precursors of granulocytes and macrophages) among HKL. Isolated HKL were fixed with ethanol (200 μl cell suspension as described above in 800 μl of 98% ice-cold ethanol) and stored at 4°C. After DNA labelling with propidium iodide (7.5 mg l−1), HKL in the S and G2–M phases of the cell cycle were determined by means of flow cytometry. For analysis, doublet cells were subtracted from single cells as described by Wersto et al. (2001). Leucocyte subsets were identified according to their characteristic FSC/SSC profile (monocytes, FSC/SSChigh; lymphocytes, FSC/SSClow). Frequencies of cells in the monocyte and lymphocyte gates in G0–1, S and G2–M phases were acquired by DNA content analysis of red fluorescence intensity (propidium iodide labelling) of single cells.
(e) Statistical analyses
Using a chi-squared test, we checked for differences in infectivity in sticklebacks between the time points (7–67 dpe). To investigate the differences between the time points, we used nested ANOVAs with the time points and treatments (sham-exposed control, exposed but not infected and infected) nested within the time points as independent variables and hepatosomatic index, respiratory burst, lymphocyte and monocyte proliferation as the response variables. Afterwards, data were tested with a Tukey–Kramer post hoc test in pairwise comparisons within the three treatments and the time points. We tested all parameters with the Kolmogorov–Smirnov test for normality and log transformed the respiratory burst activity and the monocyte proliferation. Significant differences between the three parasite sibships were not observed in any of the tested parameters and effects of fish family were only detected in the proliferation of lymphocytes. As equal numbers of control, exposed and infected fish per fish family were dissected at each time point (so that the design was balanced) and differences between fish families were not the focus of this study, we pooled the data from the three parasite sibships and all fish families in the statistical analysis. All statistical analyses were performed using JMP v. 4.0.4.
3. Results
(a) Stickleback infection and survival
From the 537 sticklebacks exposed to experimental conditions, in total 532 were analysed (two fish died, one was infected with two S. solidus parasites and two had an above-average spleen weight). The numbers of sticklebacks (n) from the different groups as analysed at the respective time points are given in table 1. Prevalence of infection was high (>60%) at 7 and 17 dpe for infective copepods, which then decreased to values approximately 50% (table 1); however, prevalence of infection did not differ significantly between the time points (likelihood ratio (LR) Χ22=8.26, p=0.22).
Table 1.
Number of sticklebacks (n) analysed at time points post-exposure and prevalence of infection (%) after exposure to infective parasites
| days post-exposure (dpe) | sham-exposed control (n) | exposed not infected (n) | infected (n) | prevalence of infection (%) |
|---|---|---|---|---|
| 7 | 17 | 22 | 40 | 64.5 |
| 17 | 16 | 22 | 38 | 63.3 |
| 27 | 14 | 33 | 28 | 45.9 |
| 37 | 16 | 28 | 28 | 50.0 |
| 47 | 16 | 33 | 28 | 45.9 |
| 57 | 16 | 29 | 31 | 51.7 |
| 67 | 16 | 33 | 28 | 45.9 |
| total | 111 | 200 | 221 | 52.5 |
(b) Growth/fitness of parasites
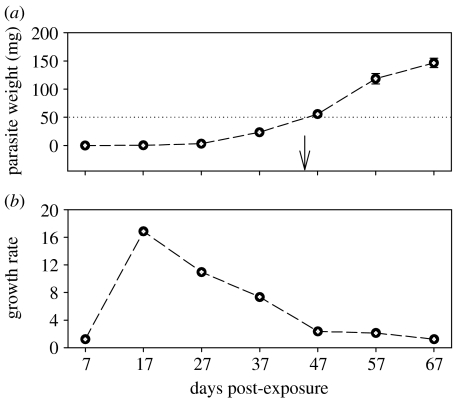
The parasite weight in the copepods used for exposure was on average 0.014 mg. During the 67-day period in the stickleback host, parasites increased weight approximately 10 000-fold (figure 1a). The highest growth rates of S. solidus (fold weight increase in previous time point) were observed from 7 to 17 dpe (16.8-fold, figure 1b). Growth rates decreased from 11.0 (days 17–27) to 1.2 (days 57–67, figure 1b). The threshold weight for infectivity of the definitive bird host of 50 mg (Tierney & Crompton 1992) was reached at 45 dpe (arrow, figure 1a).
Figure 1.
Growth of Schistocephalus solidus plerocercoids in Gasterosteus aculeatus. (a) Parasite weight (mean±s.e.) and (b) growth rate given as fold increase in average weight of parasites relative to previous time point. Dotted line indicates 50 mg standard parasite weight, the potential threshold for infectivity of definitive bird host, which was reached after 45 days (arrow). Growth rate at day 7 post-exposure was calculated based on weight measurements of procercoids isolated from copepods at the day of exposure (n=20 at day 0).
(c) Fitness and immune parameters of sticklebacks
Stickleback weight generally increased during the experiment. Significant changes over time (within treatment groups) and differences between control, exposed but not-infected and infected stickleback within the time points were detected in hepatosomatic index (figure 2), respiratory burst activity (figure 3), lymphocyte proliferation (figure 4a) and monocyte proliferation (figure 4b; ANOVA results are given in table 2).
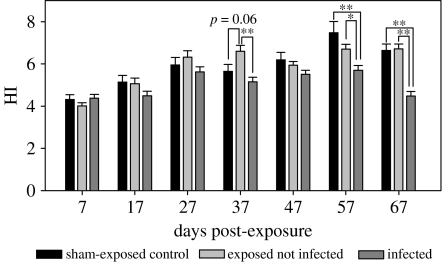
Figure 2.
Hepatosomatic index (HI) of experimental sticklebacks (mean+s.e.). The hepatosomatic index, calculated without parasite weight, was lower in infected stickleback compared with sham-exposed controls at days 57 and 67. *p<0.05; **p<0.01.
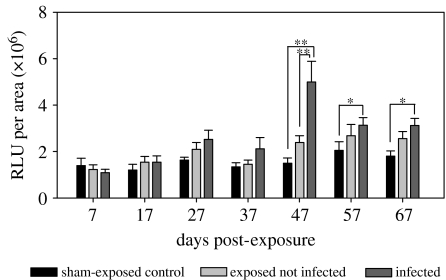
Figure 3.
Respiratory burst activity of HKL measured in chemiluminescence assays (mean+s.e.). In 96-well flat bottom microtitre plates, from each fish 1×105 HKL per well were loaded with lucigenin. Respiratory burst activity was induced by phagocytosis of zymosan particles, recorded for 3 hours and expressed as total relative luminescence (RLU per area). *p<0.05; **p<0.01.
Figure 4.
Proliferation of (a) lymphocytes and (b) monocytes. In individual head kidney samples, proportion of lymphocytes and monocytes in the S+G2−M phase of the cell cycle was determined by means of flow cytometry. Values (mean±s.e.) recorded at the respective time points are given as percentage of mean values of corresponding controls. Note that lymphocyte proliferation is responding to infection to a minor extent only in the initial phase of infection. Monocyte proliferation shows a fluctuating kinetic up to 37 dpe. *Significantly different from corresponding control p<0.05, *) significantly different from previous or (*next time point p<0.01.
Table 2.
Results of nested ANOVAs with the time points (tp; 7–67 dpe) and treatments nested within the time points (ttp) as independent variables and the hepatosomatic index, respiratory burst, lymphocyte proliferation and monocyte proliferation as the response variables
| hepatosomatic index | respiratory burst | lymphocyte proliferation | monocyte proliferation | |||||
|---|---|---|---|---|---|---|---|---|
| source | tp | ttp | tp | ttp | tp | ttp | tp | ttp |
| d.f. | 6 | 14 | 6 | 14 | 6 | 14 | 6 | 14 |
| F | 27.6 | 8.9 | 15.4 | 2.9 | 4.8 | 2.0 | 17.7 | 2.4 |
| p | <0.0001 | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.0133 | <0.0001 | 0.0031 |
In detail, the hepatosomatic index of the infected fish decreased and was significantly lower as the control and exposed treatment quite late during infection, at 57 and 67 dpe (figure 2). The respiratory burst activity did not show significant differences between treatment groups in the initial phase of the infection until 37 dpe, but starting at 47 dpe the respiratory burst activity from infected sticklebacks increased dramatically and remained significantly higher than in controls until 67 dpe (figure 3).
Lymphocyte proliferation only showed significant differences in exposed but not in infected stickleback between the time points in the initial phase of infection (7–17 dpe) and dropped below controls at 17 dpe. No significant difference in lymphocyte proliferation between S. solidus-infected and non-infected sticklebacks was observed throughout the observation period (figure 4a). Monocyte proliferation was significantly higher in infected sticklebacks than controls at 7 dpe and then dropped at 17 dpe below the values of controls, increased again at 27 dpe followed by another decrease at 37 dpe and remained in the range of controls until 67 dpe (figure 4b).
4. Discussion
The chronology of the infection by S. solidus in its intermediate host, the three-spined stickleback, was studied under an immunological approach. Contrary to our expectation, sticklebacks did not show an upregulated respiratory burst activity of leucocytes as an early response to cell-mediated innate immunity in the beginning but in a later phase of infection (47–67 dpe). During the whole experiment, lymphocyte proliferation in infected sticklebacks did not exceed the range of sham-treated controls, suggesting that antibody-mediated adaptive immunity is not upregulated in an infection with S. solidus. This is in line with recent theory stating that tissue injury is needed to evoke a lymphocyte-mediated immune response (Matzinger 2007), which was not observed after S. solidus establishment in the stickleback's body cavity (Arme & Owen 1967).
Most strikingly, a key function of cell-mediated innate immunity to eliminate an invading macro-parasite, the respiratory burst activity, was not accelerated in the initial phase of infection. One possible reason could be that individual parasites hide themselves from the host's immune system (Aeschlimann et al. 2000), for example by adapting their surface carbohydrate composition to the vertebrate host (Hammerschmidt & Kurtz 2005b). However, the second parameter of cell-mediated innate immunity, the proliferation of monocytes (precursors of macrophages and granulocytes), clearly indicates that immune cells of the infected sticklebacks are mobilized at the beginning of the infection, suggesting recognition of the invader before 7 dpe. This peak of monocyte proliferation was followed by a decrease only 10 days later, which was then followed by an increase and decrease at the later time points. These fluctuating kinetics suggest that even though the cestode is probably recognized by the host immune system at first, it is either able to manipulate monocyte proliferation by the induction of an anti-inflammatory response, as shown in mammals (Hartmann & Lucius 2003) or it sheds its surface antigens several times during infection, as known for Schistosoma mansoni schistosomula (Pearce et al. 1986) and so evades the host responses. Generally, S. solidus only seems to be at risk of being cleared by the stickleback immune system early during infection, which is supported by two observations from the present study: (i) prevalence of infection was higher (>60%) early in infection (7–17 dpe) than at all the later time points (45–52% at 27–67 dpe) and (ii) dead parasites (n=4) were only found until day 17 post-exposure in the body cavity of infected fish. Apparently, S. solidus is more easily eliminated early in infection (until day 7), potentially due to its relatively small size. Between days 7 and 17, the parasites grew approximately 17-fold, which could be a life-history adaptation of the parasites to simply outgrow the size range of elimination by the immune system. If in principle S. solidus is able to hide from the immune system, it is even more surprising that infected sticklebacks show a significantly elevated respiratory burst activity starting at 47 days post-infection. Although the parasite can probably no longer be destroyed, this strong reaction nevertheless might harm the cestode. For the host, mounting such a strong immune response that late during infection, with no chance of eliminating the parasite, only causes costs, namely for mounting the immune response, but probably more importantly immunopathological costs (Lochmiller & Deerenberg 2000). This is reflected in the hepatosomatic index, a measure of the short-term energy reserves (Chellappa et al. 1995), which was significantly reduced in infected compared with control sticklebacks at 57 and 67 dpe. Such energetic costs of the elevated respiratory burst activity were also shown in a previous study on the same system (Hammerschmidt & Kurtz 2005a). Thus, upregulation of respiratory burst activity that late during infection with S. solidus does not seem to make sense from an immunological point of view and is additionally very costly for the host, but most interestingly, it occurs shortly after the parasites reached 50 mg. This is the threshold weight in S. solidus for a successful infection and production of fertile eggs in its final bird host (Tierney & Crompton 1992). It is well established in this host–parasite system that the tapeworm is able to interfere with the host's behaviour in such a way that the likelihood for the parasite increases to reach its final host. Sticklebacks show reduced predator avoidance behaviour, when they harbour a high burden (parasite index is more than 25%) of S. solidus (Milinski 1984, 1985; Øverli et al. 2001; Barber et al. 2004) or when the parasite weight is more than 50 mg (Tierney et al. 1993; Barber et al. 2004), but not in early infection with parasite weight being less than 50 mg (Aeschlimann et al. 2000). These behavioural changes are thought to be caused by an increase in concentrations of monoamine neurotransmitters in neuronal tissues of the brain in S. solidus-infected sticklebacks (Øverli et al. 2001). Whether the neurotransmitters are produced directly by the parasite or their increase reflects a chronic stress reaction in infected fish, which could well be the result of an immune response (Øverli et al. 2001), still has to be investigated. It is generally difficult to distinguish whether a parasite directly or indirectly manipulates its host behaviour due to the complex interactions between immunity and nervous systems (Adamo 2002; Thomas et al. 2005). Only a few studies on invertebrates suggest parasite secretions to activate components of the immune system and so manipulate their host's nervous system (Adamo 2002). Also, in vertebrates, it is well established that behavioural changes associated with acute infections (known as ‘acute sickness behaviour’) are typically immunologically mediated (Vollmer-Conna 2001), even though the mechanism is still unclear (Engelsma et al. 2002; Dantzer 2004). In our system, the upregulation of respiratory burst activity of leucocytes late during infection with S. solidus could thus lead to the neuronal changes that induce behavioural modifications of the stickleback, and so elegantly enhance parasite transmission to the final host.
We show here that S. solidus is able to interfere with the immune system of its specific host, the three-spined stickleback, at various levels and time points during its infection period. Generally, the specific lymphocyte-mediated immune response was not activated throughout the infection. A cellular response of the innate immune system, the proliferation of monocytes, after fluctuating kinetics early in infection, is downregulated during ongoing infection, potentially due to repeated installation of surface coats by S. solidus that are not immunogenic to the host. Only late during infection, when the parasite is ready for transmission to its final host, is the respiratory burst activity upregulated, most probably mediating reduced predation avoidance behaviour, facilitating transmission to the final host. Future investigations may elucidate whether S. solidus uses its ability to change the carbohydrate surface during ongoing infection of sticklebacks for immune evasion, thus explaining the fluctuating kinetics of monocyte proliferation. Furthermore, the question of whether the correlation between increased respiratory burst activity and behavioural change is causal or coincidental needs careful investigation.
Acknowledgments
All experiments described were approved by the Ministry of Nature, Environment and Country Development, Schleswig-Holstein, Germany.
We thank M. Kalbe for breeding sticklebacks, A. Busekow, W. Derner and V. Büscher for dissecting sticklebacks; G. Augustin, P. Deines, L. Janke, R. Leipnitz, D. Lemke and D. Martens for their help during the experiment and M. Milinski and the people of the Department of Evolutionary Ecology for discussion and comments on earlier versions of the manuscript.
References
- Adamo S.A. Modulating the modulators: parasites, neuromodulators and host behavioural change. Brain Behav. Evol. 2002;60:370–377. doi: 10.1159/000067790. doi:10.1159/000067790 [DOI] [PubMed] [Google Scholar]
- Aeschlimann P, Häberli M, Milinski M. Threat-sensitive feeding strategy of immature sticklebacks (Gasterosteus aculeatus) in response to recent experimental infection with the cestode Schistocephalus solidus. Behav. Ecol. Sociobiol. 2000;49:1–7. doi:10.1007/s002650000273 [Google Scholar]
- Arme C, Owen R.W. Infections of the three-spined stickleback, Gasterosteus aculeatus L., with the plerocercoid larvae of Schistocephalus solidus (Müller, 1776), with special reference to pathological effects. Parasitology. 1967;57:301–314. doi: 10.1017/s0031182000072103. [DOI] [PubMed] [Google Scholar]
- Bagamian K.H, Heins D.C, Baker J.A. Body condition and reproductive capacity of three-spined stickleback infected with the cestode Schistocephalus solidus. J. Fish Biol. 2004;64:1568–1576. doi:10.1111/j.0022-1112.2004.00411.x [Google Scholar]
- Barber I, Walker P.A, Svensson P. Behavioural responses to simulated avian predation in female three spined sticklebacks: the effect of experimental Schistocephalus solidus infections. Behaviour. 2004;141:1425–1440. doi:10.1163/1568539042948231 [Google Scholar]
- Chellappa S, Huntingford F.A, Strang R.H.C, Thomson R.Y. Condition factor and hepatosomatic index as estimates of energy status in male three-spined stickleback. J. Fish Biol. 1995;47:775–787. doi:10.1111/j.1095-8649.1995.tb06002.x [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. doi:10.1016/j.ejphar.2004.07.040 [DOI] [PubMed] [Google Scholar]
- Dubinina M.N. Nauka Publishers; Moscow, Russia: 1966. Tapeworms (Cestoda, Ligulidae) of the fauna of the UDSSR. (Remnetsy (Cestoda, Ligulidae) Fauny SSSR) [Google Scholar]
- Engelsma M.Y, Huising M.O, van Muiswinkel W.B, Flik G, Kwang J, Savelkoul H.F, Verburg-van Kemenade B.M. Neuroendocrine-immune interactions in fish: a role for interleukin-1. Vet. Immunol. Immunopathol. 2002;87:467–479. doi: 10.1016/s0165-2427(02)00077-6. doi:10.1016/S0165-2427(02)00077-6 [DOI] [PubMed] [Google Scholar]
- Hamers R, Goerlich R. Flow cytometric DNA analysis of the haematopoietic tissue of carp Cyprinus carpio during experimental infection with the haemoflagellate Trypanoplasma borreli. Dis. Aquat. Org. 1996;24:119–134. doi:10.3354/dao024129 [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc. Natl Acad. Sci. USA. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. doi:10.1073/pnas.87.9.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Kurtz J. Evolutionary implications of the adaptation to different immune systems in a parasite with a complex life cycle. Proc. R. Soc. B. 2005a;272:2511–2518. doi: 10.1098/rspb.2005.3241. doi:10.1098/rspb.2005.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Kurtz J. Surface carbohydrate composition of a tapeworm in its consecutive intermediate hosts: individual variation and fitness consequences. Int. J. Parasitol. 2005b;35:1499–1507. doi: 10.1016/j.ijpara.2005.08.011. doi:10.1016/j.ijpara.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Kurtz J. Schistocephalus solidus: establishment of tapeworms in sticklebacks—fast food or fast lane? Exp. Parasitol. 2007;116:142–149. doi: 10.1016/j.exppara.2006.12.013. doi:10.1016/j.exppara.2006.12.013 [DOI] [PubMed] [Google Scholar]
- Hartmann S, Lucius R. Modulation of host immune responses by nematode cystatins. Int. J. Parasitol. 2003;33:1291–1302. doi: 10.1016/s0020-7519(03)00163-2. doi:10.1016/S0020-7519(03)00163-2 [DOI] [PubMed] [Google Scholar]
- Hoole D, Arme C. Ultrastructural studies on the cellular response of fish hosts following experimental infection with the plerocercoid of Ligula intestinalis (Cestoda: Pseudophyllidae) Parasitology. 1983;87:139–149. [Google Scholar]
- Jones S.R.M. The occurrence and mechanisms of innate immunity against parasites in fish. Dev. Comp. Immunol. 2001;25:841–852. doi: 10.1016/s0145-305x(01)00039-8. doi:10.1016/S0145-305X(01)00039-8 [DOI] [PubMed] [Google Scholar]
- Kurtz J, Kalbe M, Aeschlimann P.B, Haberli M.A, Wegner K.M, Reusch T.B, Milinski M. Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc. R. Soc. B. 2004;271:197–204. doi: 10.1098/rspb.2003.2567. doi:10.1098/rspb.2003.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Morvan-Rocher C, Troutaud D, Deschaux P. Effects of temperature on carp leucocyte mitogen-induced proliferation and nonspecific cytotoxic activity. Dev. Comp. Immunol. 1995;19:87–95. doi: 10.1016/0145-305x(94)00057-m. doi:10.1016/0145-305X(94)00057-M [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Lüscher A, Milinski M. Simultaneous hermaphrodites reproducing in pairs self-fertilize some of their eggs: an experimental test of predictions of mixed-mating and Hermaphrodite's Dilemma theory. J. Evol. Biol. 2003;16:1030–1037. doi: 10.1046/j.1420-9101.2003.00552.x. doi:10.1046/j.1420-9101.2003.00552.x [DOI] [PubMed] [Google Scholar]
- MacInnes A.J. How parasites find their hosts: some thoughts on the inception of host–parasite integration. In: Kennedy C.R, editor. Ecological aspects of parasitism. North Holland Publishing Company; Amsterdam, The Netherlands: 1976. pp. 3–20. [Google Scholar]
- Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. doi:10.1038/ni0107-11 [DOI] [PubMed] [Google Scholar]
- Milinski M. Parasites determine a predators optimal feeding strategy. Behav. Ecol. Sociobiol. 1984;15:35–37. doi:10.1007/BF00310212 [Google Scholar]
- Milinski M. Risk of predation of parasitized sticklebacks (Gasterosteus aculeatus L.) under competition for food. Behaviour. 1985;93:203–216. [Google Scholar]
- Nie P, Hoole D. Effects of Bothriocephalus acheilognathi on the polarization response of pronephric leucocytes of carp, Cyprinus carpio. J. Helminthol. 2000;74:253–257. [PubMed] [Google Scholar]
- Nie P, Hoole D, Arme C. Proliferation of pronephric lymphocytes of carp, Cyprinus carpio induced by extracts of Bothriocephalus acheilognathi. J. Helminthol. 1996;70:127–131. doi: 10.1017/s0022149x00015273. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Pall M, Borg B, Jobling M, Winberg S. Effects of Schistocephalus solidus infection on brain monoaminergic activity in female three-spined sticklebacks Gasterosteus aculeatus. Proc. R. Soc. B. 2001;268:1411–1415. doi: 10.1098/rspb.2001.1668. doi:10.1098/rspb.2001.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.J, Basch P.F, Sher A. Evidence that the reduced surface antigenicity of developing Schistosoma mansoni schistosomula is due to antigen shedding rather than host molecule acquisition. Parasite Immunol. 1986;8:79–94. doi: 10.1111/j.1365-3024.1986.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Pechhold K, Pohl T, Kabelitz D. Rapid quantification of lymphocyte subsets in heterogeneous cell populations by flow-cytometry. Cytometry. 1994;16:152–159. doi: 10.1002/cyto.990160209. doi:10.1002/cyto.990160209 [DOI] [PubMed] [Google Scholar]
- Roberts M.L, Lewis J.W, Wiegertjes G.F, Hoole D. Interaction between the blood fluke, Sanguinicola inermis and humoral components of the immune response of carp, Cyprinus carpio. Parasitology. 2005;131:261–271. doi: 10.1017/s0031182005007651. doi:10.1017/S0031182005007651 [DOI] [PubMed] [Google Scholar]
- Rijkers G.T, Frederix-Wolters E.M, van Muiswinkel W.B. The immune system of cyprinid fish. Kinetics and temperature dependence of antibody-producing cells in carp (Cyprinus carpio) Immunology. 1980;41:91–97. [PMC free article] [PubMed] [Google Scholar]
- Scharsack J.P, Steinhagen D, Korting W, Leibold W, Schuberth H.J. Flow cytometric analysis of proliferative responses of carp Cyprinus carpio peripheral blood leucocytes to mitogens and to the hemoflagellate Trypanoplasma borreli. Dis. Aquat. Org. 2000;41:203–210. doi: 10.3354/dao041203. doi:10.3354/dao041203 [DOI] [PubMed] [Google Scholar]
- Scharsack J.P, Kalbe M, Derner R, Kurtz J, Milinski M. Modulation of granulocyte responses in three-spined sticklebacks (Gasterosteus aculeatus L.) infected with the tapeworm (Schistocephalus solidus, Muller 1776) Dis. Aquat. Org. 2004;59:141–150. doi: 10.3354/dao059141. [DOI] [PubMed] [Google Scholar]
- Scharsack J.P, Kalbe M, Harrod C, Rauch G. Habitat-specific adaptation of immune responses of stickleback (Gasterosteus aculeatus) lake and river ecotypes. Proc. R. Soc. B. 2007;274:1523–1532. doi: 10.1098/rspb.2007.0210. doi:10.1098/rspb.2007.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. doi:10.1016/S0169-5347(02)00013-7 [Google Scholar]
- Scott A.L, Klesius P.H. Chemiluminescence: a novel analysis of phagocytosis in fish. Dev. Biol. Stand. 1981;49:243–254. [Google Scholar]
- Secombes C.J, Chappell L.H. Fish immune responses to experimental and natural infection with helminth parasites. Annu. Rev. Fish Dis. 1996;6:167–177. doi:10.1016/S0959-8030(96)90012-5 [Google Scholar]
- Sharp G.J, Pike A.W, Secombes C.J. Sequential development of the immune response in rainbow trout [Oncorhynchus mykiss (Walbaum, 1792)] to experimental plerocercoid infections of Diphyllobothrium dendriticum (Nitzsch, 1824) Parasitology. 1992;104:169–178. doi: 10.1017/s0031182000060911. [DOI] [PubMed] [Google Scholar]
- Smyth J.D. Studies on tapeworm physiology. I. The cultivation of Schistocephalus solidus in vitro. J. Exp. Biol. 1946;23:47–70. doi: 10.1242/jeb.23.1.47. [DOI] [PubMed] [Google Scholar]
- Taylor M.J, Hoole D. Ligula intestinalis (L.) (Cestoda: Pseudophyllidea): polarization of cyprinid leucocytes as an indicator of host- and parasite-derived chemoattractants. Parasitology. 1993;107:433–440. doi: 10.1017/s0031182000067792. [DOI] [PubMed] [Google Scholar]
- Taylor M.J, Hoole D. The chemiluminescence of cyprinid leucocytes in response to zymosan and extracts of Ligula intestinalis (Cestoda) Fish Shellfish Immunol. 1995;5:191–198. doi:10.1016/S1050-4648(05)80013-0 [Google Scholar]
- Thomas F, Adamo S, Moore J. Parasitic manipulation: where are we and where should we go? Behav. Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. doi:10.1016/j.beproc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Tierney J.F, Crompton D.W. Infectivity of plerocercoids of Schistocephalus solidus (Cestoda: Ligulidae) and fecundity of the adults in an experimental definitive host, Gallus gallus. J. Parasitol. 1992;78:1049–1054. doi:10.2307/3283228 [PubMed] [Google Scholar]
- Tierney J.F, Huntingford F.A, Crompton D.W.T. The relationship between infectivity of Schistocephalus solidus (Cestoda) and anti-predator behaviour of its host, the three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 1993;46:603–605. doi:10.1006/anbe.1993.1229 [Google Scholar]
- Tierney J.F, Huntingford F.A, Crompton D.W.T. Body condition and reproductive status in sticklebacks exposed to a single wave of Schistocephalus solidus infection. J. Fish Biol. 1996;49:483–493. [Google Scholar]
- van der Veen I.T, Kurtz J. To avoid or eliminate: cestode infections in copepods. Parasitology. 2002;124:465–474. doi: 10.1017/s0031182001001275. doi:10.1017/S0031182001001275 [DOI] [PubMed] [Google Scholar]
- Vollmer-Conna U. Acute sickness behaviour: an immune system-to-brain communication? Psychol. Med. 2001;31:761–767. doi: 10.1017/s0033291701003841. doi:10.1017/S0033291701003841 [DOI] [PubMed] [Google Scholar]
- Wedekind C. The infectivity, growth, and virulence of the cestode Schistocephalus solidus in its first intermediate host, the copepod Macrocyclops albidus. Parasitology. 1997;115:317–324. doi: 10.1017/s0031182097001406. doi:10.1017/S0031182097001406 [DOI] [PubMed] [Google Scholar]
- Wedekind C, Milinski M. Do three-spined sticklebacks avoid consuming copepods, the first intermediate host of Schistocephalus solidus?—An experimental analysis of behavioural resistance. Parasitology. 1996;112:371–383. [Google Scholar]
- Wedekind C, Christen M, Schärer L, Treichel N. Relative helminth size in crustacean hosts: in vivo determination, and effects of host gender and within-host competition in a copepod infected by a cestode. Aquat. Ecol. 2000;34:279–285. doi:10.1023/A:1009976420423 [Google Scholar]
- Wersto R.P, Chrest F.J, Leary J.F, Morris C, Stetler-Stevenson M.A, Gabrielson E. Doublet discrimination in DNA cell-cycle analysis. Cytometry. 2001;46:296–306. doi: 10.1002/cyto.1171. doi:10.1002/cyto.1171 [DOI] [PubMed] [Google Scholar]
- Whyte S.K, Chappell L.H, Secombes C.J. Protection of the rainbow-trout, Oncorhynchus mykiss (Richardson), against Diplostomum spathaceum (Digena)- the role of specific antibody and activated macrophages. J. Fish Dis. 1990;13:281–291. doi:10.1111/j.1365-2761.1990.tb00784.x [Google Scholar]
- Wiegertjes G.F, Forlenza M, Joerink M, Scharsack J.P. Parasite infections revisited. Dev. Comp. Immunol. 2005;29:49–58. doi: 10.1016/j.dci.2005.01.005. [DOI] [PubMed] [Google Scholar]