Abstract
Some organisms can manipulate the nervous systems of others or alter their physiology in order to obtain benefit. Ants are known to limit alate aphid dispersal by physically removing wings and also through chemical manipulation of the alate developmental pathway. This results in reduced dispersal and higher local densities of aphids, which benefit ants in terms of increased honeydew and prey availability. Here, we show that the walking movement of mutualistic apterous aphids is also reduced by ant semiochemicals. Aphids walk slower and their dispersal from an unsuitable patch is hampered by ants. If aphid walking dispersal has evolved as a means of natural enemy escape, then ant chemicals may act as a signal indicating protection; hence, reduced dispersal could be adaptive for aphids. If, however, dispersal is primarily a means to reduce competition or to maintain persistent metapopulations, then manipulation by ants could be detrimental. Such manipulation strategies, common in host–parasite and predator–prey interactions, may be more common in mutualism than expected.
Keywords: manipulation, mutualism, coevolution, cuticular hydrocarbons, alate aphids
1. Introduction
Dispersal between hosts plays a key role in the outbreak of many pests and diseases (Peltonen et al. 2002). Aphids (Hemiptera: Aphidoidea) are vectors of many plant diseases, in addition to causing considerable losses of agricultural yield themselves (Buckley 1987). Aphid colonies tend to be short lived and transient (Dixon 1998), thus frequent dispersal among host plants is important in maintaining persistent metapopulations. Factors that affect the persistence of aphid colonies or the founding of new colonies via dispersal are likely to alter the population dynamics of pest outbreaks. The presence of mutualistic ants can strongly affect both these parameters. Ants protect aphids from natural enemies (Way 1963; Stadler & Dixon 2005), so ant-attended aphid colonies tend to be more stable and persist for longer (Dixon 1998). Ants are also known to limit aphid dispersal. This can occur through direct physical manipulation, e.g. ants may bite and remove the wings of alate aphids (Kunkel 1973), or it may occur through chemical influence, e.g. the mandibular secretions of ants can inhibit alate development (Kleinjan & Mittler 1975). These ant adaptations limit winged aphid dispersal and probably benefit ants by allowing unusually crowded aphid aggregations, producing more honeydew.
Winged dispersal is not the only means by which aphids colonize new plants. In response to crowded conditions, late instar apterous aphids will also leave colonies and wander to new locations on the same plant or along the ground to a new host (Hodgson 1991). Indeed, this local wandering dispersal can be the primary means of aphid dispersal to neighbouring plants (e.g. Furuta & Aloo 1994). Both aphid dispersal strategies are important in allowing aphids to exploit efficiently resources such as agricultural crops that are homogeneous at a local scale yet patchy at larger spatial scales (Lombaert et al. 2006). Banks & Nixon (1958) and El Ziady (1960) first noted that the presence of ants can produce a ‘tranquillizing’ effect on aphids, limiting their motor functions, although since then, to our knowledge, no further reports or studies have been published on this issue. In the present study, we investigate this phenomenon and determine whether it is attributable to direct contact with ants and whether it can be achieved through interspecific semiochemical communication.
Ants can actively lay semiochemical trails by touching exocrine glands onto a substrate surface. These actively laid chemical marks are often used to recruit nest-mates to profitable food sources (Hölldobler & Wilson 1990). Semiochemicals can also be applied to substrates passively through shedding of cuticular hydrocarbons (Yamoaka & Akino 1994; Depickière et al. 2004). These cuticular hydrocarbons are important in colony nest-mate recognition and, when transferred onto the ground, may also mark out home-range territories (Devigne & Detrain 2002). Other insects, including herbivores (Offenberg 2004) and aphid predators, have recently been found to respond to chemical cues indicating ant presence. In this study, we consider how the movement of apterous mutualistic aphids is affected by ant contact and passively laid ant semiochemicals.
2. Material and methods
(a) Aphids and ants
Aphis fabae Scopoli and Acyrthosiphon pisum Harris collected from Silwood Park, UK and kept in culture for 2 years were cultured on Vicia faba L. plants in netted cages. Aphids were allowed to reach high densities prompting the wandering behaviour of late instar apterae. For each experiment, these aphids were taken directly from the plant using a fine paintbrush and used immediately. Lasius niger L. workers were from a queenless laboratory colony excavated from the field one month previously.
(b) Video recording of aphid movement
Using a camera connected to a PC, aphid walking speeds were recorded under three different treatments: (i) control, (ii) ant semiochemicals only, and (iii) immediately following direct contacts between aphids and ants; the hypothesis being that ant semiochemicals would reduce aphid walking speed. For control treatments, 10 A. fabae were placed onto a filter paper in the lid of a 9 cm Petri dish and the base, with Fluon-coated sides, was placed on top. After 10 min, to allow the aphids to settle, the Petri dish was placed onto the recording platform along with five identical replicates. The recording platform consisted of two semi-transparent sanded glass sheets illuminated underneath by six green LED lights. A sensitive Watec 902 camera with HF9HA-1B Fujinon lens (ALRAD, UK) was suspended on a wooden frame, 50 cm above the viewing platform (figure 1). Images were transmitted to a PC via Video-to-USB converter (Imaging Source, Germany) and recorded with custom-written software (available on request). Each record lasted for approximately 5 min with shots taken at 0.5 s intervals. For the ant semiochemical records, filter papers were contaminated by keeping 10 L. niger on the paper in the Petri dish with Fluon-coated sides for 4 hours prior to the experiment. These L. niger semiochemicals are likely to be passively laid hydrocarbons rather than actively laid trail pheromones, which are only laid when a food source is discovered (Beckers et al. 1992). Ants were then removed and 10 aphids placed into the dish immediately. Recording commenced after 10 min along with five identical replicates. To study the effect of previous direct contact between ants and aphids, 10 L. niger ants were put together with the aphids in the Petri dish onto a clean filter paper. After 10–20 min, ants were removed and the aphids recorded. In this way, there was no need to manually filter the ants’ tracks from the records, which could introduce human error.
Figure 1.
Recording platform and PC.
The recording of six replicates of each of the three treatments comprised one series. Five series were recorded altogether: two with 10 aphids per Petri dish and three with 20 aphids per Petri dish (n=90). Series were recorded on three separate days. The first two days' recordings were complete with all factor levels (three paper contamination levels and two aphid densities). On the third day, however, only the higher densities of aphids were recorded with the three paper contamination levels.
(c) Aphid dispersal
As walking speed is not necessarily equivalent to dispersal (direction change can affect dispersal), an additional experiment was carried out to see whether ant presence affected the time taken for A. fabae aphids to disperse outwards from an unsuitable patch. Also, A. pisum, a non-ant-attended aphid species, was tested to compare differences in the responses of mutualistic and non-mutualistic aphids to ants. A Petri dish with a 3 cm diameter disc cut out of the centre had a V. faba leaf taped to the underside. Five aphids placed on the leaf dispersed outwards. The time taken for each aphid to reach the Petri dish edge was recorded for up to 5 min and the mean time calculated. Another test was carried out with the same aphids on a similar-sized leaf from the same plant, this time including 12 ants in the Petri dish. The experiment was replicated 20 times, each time alternating the order of control and ant treatment.
(i) Data analysis
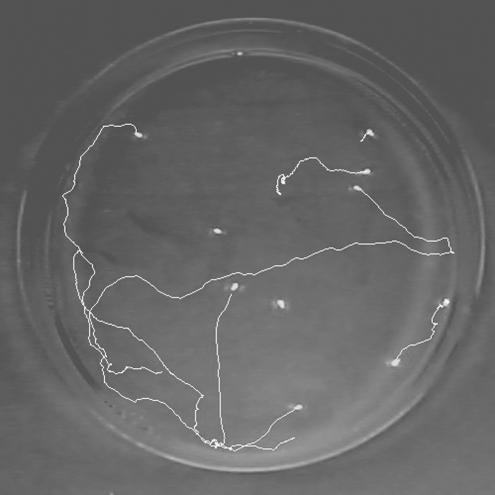
The records were analysed using free software (www.nimr.mrc.ac.uk/GMimPro) designed to track individual objects (Mashanov & Molloy 2007). This software tracks the position of every positively identified object with sub-pixel resolution and stores the coordinates of the object at every time step (figure 2). From the coordinates of individual tracks, the distance moved in 1 s intervals (instant speed) was calculated. On two occasions, there were problems in the recording recognition due to Petri dish layout and the 10 replicates affected were omitted, reducing the total sample size (n=80). Besides this, the mean value of the instant velocities of all the aphids in a Petri dish was used for each individual data point, thereby avoiding the problem of pseudoreplication. Since recordings were taken on different days and day had a significant effect on mean speed (one-way ANOVA: F2,77=36.39, p<0.001), the analysis required a nested structure. Statistical tests were carried out using the program ‘R’ (Ihaka & Gentleman 1996). A mixed effect model (lmer) was used whereby the main effects and interaction between the two fixed effect factors (contamination×aphid density) were nested within day. Non-significant terms were removed in a stepwise fashion to obtain the minimum adequate model for each analysis. Where relevant, the factor levels of the contamination factor were collapsed allowing contrasts between different factor levels. Dispersal times, in the second experiment, were compared using a two-way ANOVA for each aphid species with treatment and treatment order as explanatory variables.
Figure 2.
Aphids and their tracks.
3. Results
(a) Mean speed
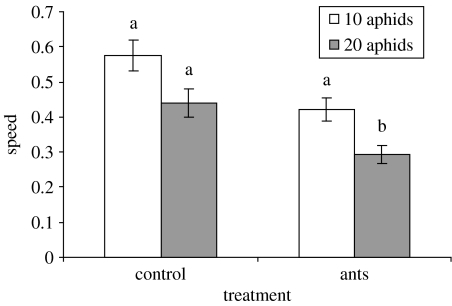
Aphids moved slower in the presence of ant semiochemicals compared with the control. There was no interaction between filter paper treatment and aphid density on the mean speed of wandering aphids (F2,74=0.33, p=0.72), thus a simple additive model can be used to describe the data. There was a significant effect of filter paper treatment on mean speed (F2,77=13.20, p<0.001), but there was no difference between filter papers contaminated with only ant semiochemicals versus those on which the aphids had physically contacted ants previously (Χ12=0.191, p=0.66). Therefore, the two factor levels were combined (mean±s.e.: 0.354±0.0218 mm s−1, n=51). This ant treatment mean was significantly less than the control treatment (mean: 0.496±0.0321 mm s−1, n=29; Χ12=22.93, p<0.001; figure 3). The main effect of aphid density was close to significance (F1,76=3.541, p=0.064).
Figure 3.
Mean walking speed of aphids (mm s−1) on different filter papers at two densities of aphids. Error bars represent the standard error of the means. Different letters above the bars indicate significantly different means (p<0.001, Tukey HSD test).
(b) Dispersal
The dispersal of A. fabae was significantly reduced in the presence of ants. Aphids reached the edge of the Petri dish after 88.3±10.8 s in the control, but only after 117.5±7.5 s when ants were present in the dish (F1,38=4.89, p=0.033). The order of treatment had no effect on mean speed (F1,37=1.16, p=0.29). By contrast, ant presence had no effect on the dispersal times of the non-ant-attended aphid A. pisum (F1,37=0.37, p=0.55), yet treatment order was significant (F1,38=21.9, p<0.001).
4. Discussion
Aphid walking speed was significantly reduced in the presence of ant semiochemicals. Previous direct contact with ants (this treatment includes the effects of ant semiochemicals) did not elicit any different effect on aphid mean speed compared with semiochemicals alone. Thus, ant presence is not required to obtain a tranquillizing effect upon aphids (El Ziady 1960; Way 1963); instead, semiochemical cues are necessary and sufficient. The frequency of tactile contact between aphids has been shown to stimulate the formation of alate dispersers (Lees 1967; Toba et al. 1967; Sutherland 1969). Tactile contact frequency is likely to be affected by the presence of boundaries (e.g. leaf margins) and the density and speed of individuals. If ant semiochemicals limit aphid walking speed, as demonstrated here, then tactile contact and hence alate development may also be affected. Thus, through limiting individual movement, ant semiochemicals may interfere with both local (apterous) and long-distance (alate) dispersal of aphids. Indeed, in three out of four studies on aphid wing induction with different ant/aphid species, ants were found to reduce alate development, with the fourth study finding no effect either way (Müller et al. 2001). Chemicals, such as dendrolasin, that are produced by ant mandibular glands can limit alate development (Kleinjan & Mittler 1975). In addition to this, we propose that there may be indirect effects of ants on alate production, whereby ants limit aphid movement and thus reduce the frequency of tactile contact.
Aphid density had a marginal effect on aphid walking speed, with aphids at higher densities moving more slowly. Other studies have found apterous dispersal to be density independent (e.g. Lombaert et al. 2006), although these are field studies where aphids are able to disperse off plants and are not confined in a Petri dish. Winged dispersal, by contrast, is often found to be positively density dependent (Müller et al. 2001).
Ant presence reduced the dispersal of A. fabae aphids by causing aphids to stop moving when ants contacted them. By contrast, the dispersal of a non-attended aphid, A. pisum, was not affected by such ant contact. We have found that ant semiochemicals also reduce movement speed of mutualistic aphids. Given that ant semiochemicals are similarly effective on leaf surfaces, which is possible if they consist of relatively non-volatile hydrocarbons that are retained on the waxy surface of leaves (E. D. Morgan 2007, personal communication), then aphid colony dispersal from ant-attended plants will be limited, leading to local increases in aphid population density. Thus, we propose ant semiochemicals as an additional explanation for ant-attended aphid colonies found to be larger in size than unattended colonies (Stadler & Dixon 2005). Previous explanations for this phenomenon include ant-mediated protection from predators and direct increases in aphid feeding rate (Way 1963; Stadler & Dixon 1999).
The response of aphids to ant semiochemicals may be adaptive for aphids. By remaining within the foraging territory of mutualistic ants, aphids derive benefits from protection from predators, reduced pathogen contamination, etc. (see Stadler & Dixon (2005) for a comprehensive review). In contrast to these benefits, however, there are costs in the ant–aphid relationship, such as increased parasitism by specialist parasitoids adapted to avoid ant attacks and occasional predation by ants themselves. In addition to these often cited costs, it is possible that reduced dispersal of aphids, maintaining them in close aggregations, leads to a decrease in host plant quality that is reflected in aphid fitness. Indeed, apterous (Johnson 1965; Honek et al. 1998) and possibly alate dispersal (Müller et al. 2001) is often in response to a decline in host plant quality. Additionally, by limiting aphid dispersal, ants affect the ability of aphid clones to colonize new hosts and thus maintain a long-lived metapopulation. An ant-attended colony can be completely destroyed by specialist parasitoids, which are immune to ant attack and tend to remain targeting the same aphid colony over multiple parasitoid generations until it is eliminated (Weisser & Völkl 1997; T. H. Oliver 2006, personal observation). Ants manipulate aphids chemically and physically to limit alate dispersal (Kleinjan & Mittler 1975; Hölldobler & Wilson 1990), so it should not be assumed that reduced apterous dispersal in response to ant semiochemicals is necessarily adaptive for aphids. Ants also delay the timing of dispersal of aphids (on average 0.5–2.5 weeks) and this may be an additional indirect cost of the interaction (Kindlmann et al. 2007).
By contrast, close aggregations of aphids probably benefit ant colonies. They provide dense, highly profitable patches of renewable carbohydrates and proteins. Dispersing aphids could also move into territories of other ant colonies, thus benefitting competitors. This may be the reason why ants appear more likely to prey upon lone, rapidly moving aphids (Way 1963; Cherix 1981, 1987; Rosengren & Sundström 1991). Instead, the movement of aphids to new plants may be closely controlled. Ants transport aphids directly by carrying them to high-quality host plants within the colonies’ foraging range (Collins & Leather 2002).
To summarize, we show how the dispersal of a pest organism can be affected by interspecific chemical communication with a mutualist. The outbreak of such pests is likely to be highly dependent on these interspecific interactions. Whether aphids benefit from these effects may depend on the environmental context (e.g. aphid density, plant quality, natural enemy abundance), and also whether apterous dispersal has evolved primarily through kin selection and to maintain persistent metapopulations or as a means of natural enemy escape. Ants can provide protection against natural enemies, making costly dispersal unnecessary for aphids. By contrast, however, ants may exacerbate intraspecific competition by causing crowded conditions. Also, limited dispersal may result in an inability to form persistent metapopulations. In these cases, reduced dispersal by aphids would be costly and, thus, aphids are ‘manipulated’ by ants. Manipulation being a behavioural or physiological change, induced by another species, that benefits the second species yet is costly to the first. Ants manipulate aphids by physically removing alate wings and using allomones to inhibit alate development. Reduced dispersal by apterous aphids could be a similar behavioural change, costly to aphids, caused by ant semiochemicals. Manipulation is a common strategy in host–parasite interactions (e.g. viruses altering host behaviour to facilitate transmission) and predator–prey interactions (e.g. semiochemical ‘lures’ used by predatory insects), yet it also occurs in mutualisms. Even though the overall interaction is beneficial to both partners, manipulative exploitation by one partner allows derivation of greater benefits than would normally be possible.
Acknowledgments
Thanks to the Royal Entomological Society for providing a useful forum for idea exchange, to G. I. Mashanov for providing PC software and to E. D. Morgan for helpful discussion. Also, to Mick Crawley for statistical advice. T.H.O. and A.M. are grateful in receipt of BBSRC studentships.
References
- Banks C.J, Nixon H.L. Effects of the ant, Lasius niger L., on the feeding and excretion of the bean aphid, Aphis fabae Scop. J. Exp. Biol. 1958;35:703–711. [Google Scholar]
- Beckers R, Deneuborg J.L, Goss S. Trail laying behaviour during food recruitment in the ant Lasius niger (L.) Insect. Soc. 1992;39:59–72. doi:10.1007/BF01240531 [Google Scholar]
- Buckley R.C. Interactions involving plants. Homoptera and ants. Annu. Rev. Ecol. Syst. 1987;18:111–135. doi:10.1146/annurev.es.18.110187.000551 [Google Scholar]
- Cherix D. Universite de Lausanne; Lausanne, Switzerland: 1981. Contribution a la biologie et a l'ecologie de Formica lugubris Zett. Le probleme des supercolonies. [Google Scholar]
- Cherix D. Relation between diet and polyethism in Formica colonies. Exp. Suppl. 1987;54:93–115. [Google Scholar]
- Collins C.M, Leather S.R. Ant-mediated dispersal of the black willow aphid Pterocomma salicis L.;does the ant Lasius niger L. judge aphid–host quality? Ecol. Entomol. 2002;27:238–241. doi:10.1046/j.1365-2311.2002.00390.x [Google Scholar]
- Depickière S, Fresneau D, Detrain C, Deneuborg J.-L. Marking as a decision factor in the choice of a new resting site in Lasius niger. Insect. Soc. 2004;51:243–246. [Google Scholar]
- Devigne C, Detrain C. Collective exploration and area marking in the ant Lasius niger. Insect. Soc. 2002;49:357–362. doi:10.1007/PL00012659 [Google Scholar]
- Dixon A.F.G. Chapman and Hall; London, UK: 1998. Aphid ecology: an optimisation approach. [Google Scholar]
- El Ziady S. Further effects of Lasius niger L. on Aphis fabae Scopoli. Proc. R. Entomol. Soc. A. 1960;35:30–38. [Google Scholar]
- Furuta K, Aloo I.K. Between-tree distance and spread of the sakhalin fir aphid (Cinara todocola Inouye)(Hom-Aphididae) within a plantation. Environ. Entomol. 1994;117:64–71. [Google Scholar]
- Hodgson C. Dispersal of apterous aphids (Homoptera, Aphididae) from their host plant and its significance. Bull. Entomol. Res. 1991;81:417–427. [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Honek A, Jarosik V, Lapchin L, Rabasse J.M. The effect of parasitism by Aphelinus abdominalis and drought on the walking movement of aphids. Entomol. Exp. Appl. 1998;87:191–200. doi:10.1046/j.1570-7458.1998.00320.x [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 1996;5:299–314. doi:10.2307/1390807 [Google Scholar]
- Johnson B. Wing polymorphism in aphids II. Interactions between aphids. Entomol. Exp. Appl. 1965;8:49–64. doi:10.1007/BF00304538 [Google Scholar]
- Kindlmann P, Maurice H, Stadler B. Timing of dispersal: effect of ants on aphids. Oecologia. 2007;152:625–631. doi: 10.1007/s00442-007-0684-4. doi:10.1007/s00442-007-0684-4 [DOI] [PubMed] [Google Scholar]
- Kleinjan J.E, Mittler T.E. A chemical influence of ants in wing development in aphids. Entomol. Exp. Appl. 1975;18:384–388. doi:10.1007/BF00628368 [Google Scholar]
- Kunkel H. Die Kotagabe der Aphiden (Aphidina, Hemiptera) unter Einfluss von Ameisen. Bonn. Zool. Beitr. 1973;24:105–121. [Google Scholar]
- Lees A.D. The production of the apterous and alate forms in the aphid Megoura viciae Buckton, with special reference to the role of crowding. J. Insect Physiol. 1967;13:289–318. doi:10.1016/0022-1910(67)90155-2 [Google Scholar]
- Lombaert E, Boll R, Lapchin L. Dispersal strategies of phytophagous insects at a local scale: adaptive potential of aphids in an agricultural environment. BMC Evol. Biol. 2006;6:75. doi: 10.1186/1471-2148-6-75. doi:10.1186/1471-2148-6-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov G.I, Molloy J.E. Automatic detection of single fluorophores in live cells. Biophys. J. 2007;92:2199–2211. doi: 10.1529/biophysj.106.081117. doi:10.1529/biophysj.106.081117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C.B, Williams I.S, Hardie J. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol. Entomol. 2001;26:330–340. doi:10.1046/j.1365-2311.2001.00321.x [Google Scholar]
- Offenberg J. Evidence that insect herbivores are deterred by ant pheromones. Proc. R. Soc. B. 2004;271:S433–S435. doi: 10.1098/rsbl.2004.0210. doi:10.1098/rsbl.2004.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen M, Leibhold A.M, Bjørnstad O.N, Williams D.W. Spatial synchronicity in forest insect outbreaks: roles of regional stochasticity and dispersal. Ecology. 2002;83:3120–3129. [Google Scholar]
- Rosengren R, Sundström L. The interaction between red wood ants, Cinara aphids, and pines. A ghost of mutualism past? In: Huxley C.R, Cutler D.F, editors. Ant–plant interactions. Oxford University Press; Oxford, UK: 1991. pp. 81–91. [Google Scholar]
- Stadler B, Dixon A.F.G. Ant attendance in aphids: why different degrees of myrmecophily? Ecol. Entomol. 1999;24:363–369. doi:10.1046/j.1365-2311.1999.00195.x [Google Scholar]
- Stadler B, Dixon A.F.G. Ecology and evolution of aphid–ant interactions. Annu. Rev. Ecol. Syst. 2005;36:345–372. doi:10.1146/annurev.ecolsys.36.091704.175531 [Google Scholar]
- Sutherland O.R.W. The role of crowding in the production of winged forms by two strains of the Pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 1969;15:1385–1410. doi:10.1016/0022-1910(69)90199-1 [Google Scholar]
- Toba H.H, Paschke J.D, Friedman S. Crowding as the primary factor in the production of the agamic alate form of Therioaphis maculata (Homoptera: Aphididae) J. Insect Physiol. 1967;13:381–396. doi:10.1016/0022-1910(67)90079-0 [Google Scholar]
- Way M.J. Mutualism between ants and honeydew producing Homoptera. Annu. Rev. Entomol. 1963;8:307–343. doi:10.1146/annurev.en.08.010163.001515 [Google Scholar]
- Weisser W.W, Völkl W. Dispersal in the aphid parasitoid, Lysiphlebus cardui (Marshall) (Hym., Aphidiidae) J. Appl. Entomol. 1997;121:23–28. [Google Scholar]
- Yamoaka R, Akino T. Ecological importance of cuticular hydrocarbons secreted from the tarsus of ants. In: Lenoir A, Arnold G, Lepage M, editors. Les Insectes Sociaux. Université Paris-Nord; Paris, France: 1994. p. 222. [Google Scholar]