Abstract
A cross-sectional survey of Oncomelania quadrasi, the intermediate host for Schistosoma japonicum, was conducted between 2004 and 2005 in 50 villages of the Province of Samar, the Philippines. The villages were classified as rain-fed (25) or with some man-made irrigation system (25). The primary objective was to identify all snail colony sites in the 50 villages and to compare snail population density and S. japonicum infection prevalence between the two types of villages. The presence of snail colonies was surveyed along streams, springs, various canals and swampy areas or grass land. A total of 198 colony sites were identified out of the 845 sites surveyed. Of these, a sufficient number of O. quadrasi snails were identified to measure density and infection in 147 sites. Density of O. quadrasi was remarkably uniform across habitats and there were no significant differences across habitats and between village type. The prevalence of infected snails showed more variability among habitats. Indeed, there was an interaction between the type of habitat and the type of village with irrigated villages being associated with a prevalence proportion ratio of 5.76 (1.31, 25.42) as compared to rain-fed villages among streams and springs. No such association was found among other habitats. The results suggest that once a suitable habitat exists, O. quadrasi populations establish and reach a plateau density. These results are discussed in light of possible ecological measures of control.
Keywords: Oncomelania, schistosomiasis, irrigation, the Philippines
Introduction
Schistosomiasis due to Schistosoma japonicum remains a public health concern in parts of the Philippines (Leonardo et al., 2005). Despite regular and ongoing attempts to keep the prevalence of S. japonicum infection at a low level, the infection persists in some areas of the Philippines, such as Samar. This is believed to be partly due to the zoonotic nature of transmission (McGarvey et al., 2006). The prevalence of infection in animals and humans is very heterogeneous across villages and infection has been found in cats, cattle, dogs, goats, pigs, rats and water buffaloes (Carabin et al., 2005; McGarvey et al., 2006; Fernandez et al., 2007). It has been reported that intensified water usage through creation of water distribution systems may result in creation of new habitats for the intermediate hosts and subsequently increase transmission (Yasuraoka 1979).
The intermediate host, Oncomelania quadrasi was considered as a subspecies of O. hupensis but allozyme variation suggests that it should be recognized as a full species (Viyanant et al. 1987, Woodruff et al. 1988). Oncomelania quadrasi are small, amphibious and dioecious snails (Pesigan et al., 1958). Females tend to be larger than males, and eggs are laid singly on solid objects mostly above the water line (Pesigan et al., 1958). Hatching occurs after 10–25 days, depending on the temperature, and the newly-hatched snails pass through an aquatic stage of 1–2 weeks. Snails reach sexual maturity after 10–16 weeks and may live for 24–35 weeks (Pesigan et al., 1958). The pristine habitat of O. quadrasi is flood plains, forests and swamps (Pesigan et al., 1958; Yasuraoka, 1979). Man-made habitats resulting from agricultural development, such as drainage channels, roadside ditches and small canals and drainage canals of irrigation works, are thought to be especially important habitats. Snails are found primarily on the banks but some are also found in very shallow water (i.e. depth less than 20 cm). Habitats preferred by O. quadrasi are shaded by vegetation, where the temperature is relatively constant and cool. Rice fields do not constitute an optimal habitat for O. quadrasi because they are rather unstable (Pesigan et al., 1958; Lipayon et al., 2002).
In Samar Province, a great variability in agricultural practices exists ranging from entirely rain fed to primarily irrigated culture with well developed canal system. The prevalence of human infection was recently estimated to range from village-to-village between 0% (95% Bayesian credible interval (BCI): 0% to 3.1%) and 45.2% (36.5% to 53.9%) and between 0% and 23.0% (16.4% to 31.2%) for lightly and at least moderate intensity of infection, respectively (Tarafder et al., 2006). The area provided an opportunity to compare the density of the intermediate host snail, O. quadrasi and the prevalence of infection in the colonies between rain-fed and irritated villages.
Material and Methods
Study area
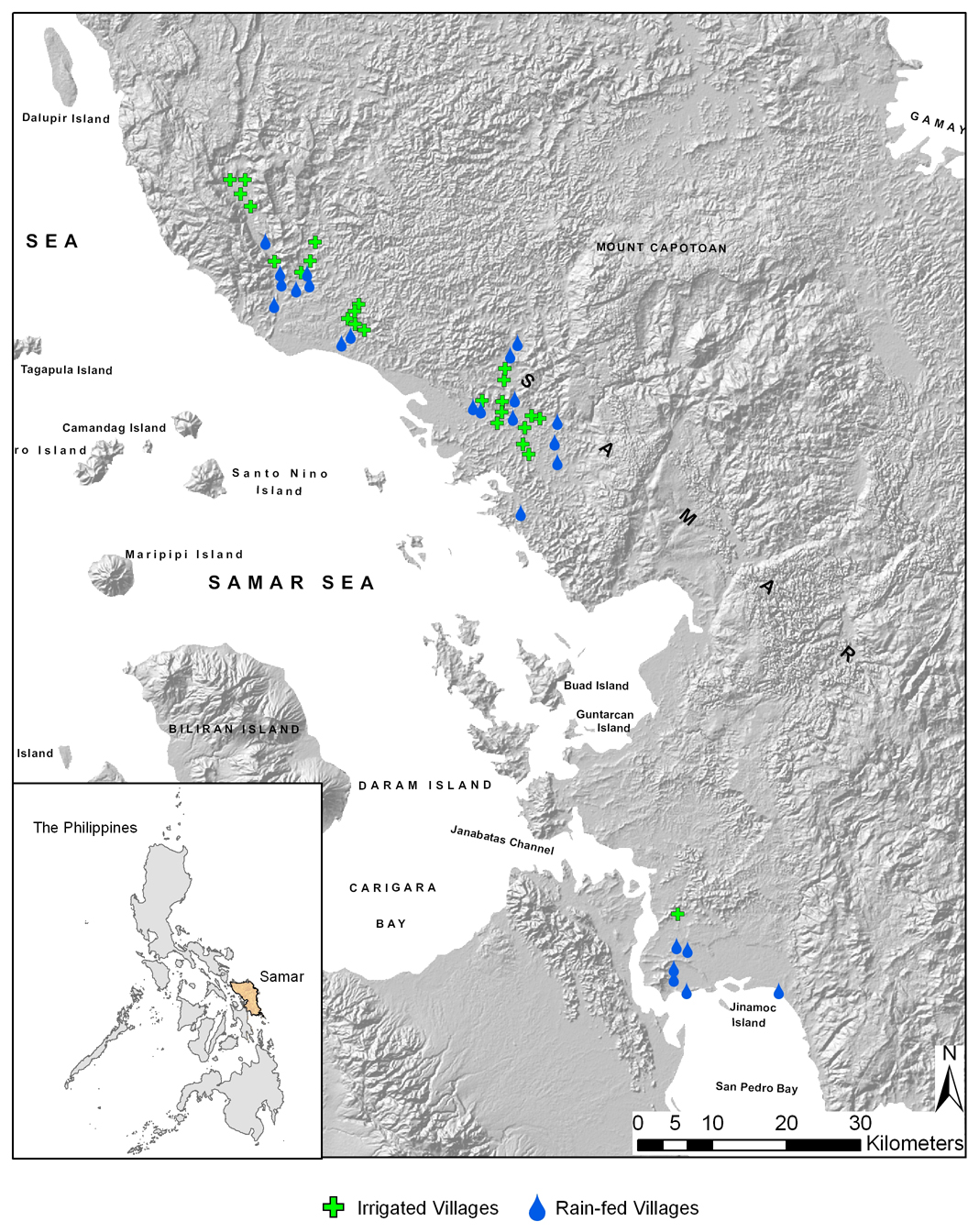
A total of 50 villages, 25 with some man-made irrigation systems and 25 with mostly rain-fed culture, in Samar Province (Fig. 1) were selected on basis of irrigation infrastructure or absence of such, accessibility and location. More details on the selection of villages can be found elsewhere (McGarvey et al., 2006; Tarafder et al., 2006).
Figure 1.
Map of the location of the 50 villages included in the snail survey in Western Samar Province, the Philippines.
Legend: green crosses represent rain-fed villages and blue dots irrigated villages.
Mapping of the villages
Selected villages were carefully mapped before snail surveys were initiated. All water courses (natural streams, springs, irrigation canals of various types and drainage canals), ponds and swamps were traced by walking the entire distance along these and regularly taking GPS records. Maps were printed and brought to the field.
Snail sampling
Based on our preliminary surveys and literature (Pesigan et al., 1958), we established a list of criteria to where snail colonies should be searched. These criteria included the presence of well-shaded areas along streams, springs or various canals (drainage canals and others) and swampy areas or grass land (often currently unplanted rice fields). Areas of seepage and affluents and swampy sections next to streams wider than 1–2 meters were also included. The following sites were excluded: streams wider than 1–2 meters with steep banks which are very unlikely habitats (and usually very difficult to access) and rice fields which do not constitute an optimal habitat for O. quadrasi. Data collection took place between September 2003 and September 2004.
A team of three technicians walked the entire perimeters of all mapped major water bodies and systematically checked for potential snail sites meeting the above-mentioned criteria. These sites were then inspected for the presence of snails. For each snail site identified, a sample of 10 voucher specimens of O. quadrasi (if possible) was preserved in 70 % ethanol (stored at Research Institute for Tropical Medicine). Quantitative sampling (see below) was done in sites where the 10 voucher specimens could be collected within 10 minutes. Sites where less than 10 voucher specimens were found were recorded as snail positive sites but were considered as newly established sites and no quantitative estimates of snail density and infection were conducted. Such sites with few snails were considered as temporary due to small habitat patches and the likely interference from agricultural activities leading to snail habitat destruction. Each site inspected was positioned on the map. For habitat sites with snails, the extension (length and width) of the snail infested area was estimated.
Quantitative snail sampling
To get a reliable estimate of density within a sampling site, 30 ring samples were taken. The ring was 13.5 cm in diameter and all snails found within this area were collected. The sampling techniques were used according to the type of habitat and on the extension of the snail site. For streams, canals or other water courses where snails were found in a relatively narrow zone along which a 100-m stretch could be chosen, three ring samples 1 m apart were taken every 10 m along every transect. If a 100-m stretch could not be found, samples were spaced more closely. In more extensive habitats, such as swampy area, seepage or grass land, a 100-m stretch was selected and three ring samples 1 m apart (across transect line) were taken. The 100 m stretch usually covered the entire length of the snail colony (only 2 sites exceeded 100 m in length and in those cases the most accessible stretch was sampled). If a 100 m stretch could not be chosen, ring samples were placed more closely. In irregular habitats such as swamp or grassland, ring samples were placed so as to cover the entire area of the snail colony. For more extensive swamps, however, sampling was done along the periphery because it could be difficult to access the interior part. Snails collected within one ring sample were transferred to a pre-labelled envelope. A note indicating which plants provided shading was taken at the location of each ring sample.
In the laboratory, snails were grouped into small (<3.0 mm in shell height) and large snails (≥3.0 mm) and assessed for infection by the crushing method (Frandsen & Christensen 1984); each snail was crushed between two microscope slides and tissues checked under a microscope for sporocysts or cercariae of S. japonicum.
Measurement of environmental factors
Several environmental factors were measured at each site where a quantitative survey was conducted. Shade was measured as the presence of tree cover, grass, palm trees and other vegetation at the site of each ring. The proportion of ring location where such vegetation was found per site was then calculated for the analysis. Some habitats were very rarely reported and hence we categorised habitat into three categories: 1) stream or spring; 2) canals and 3) other (i.e. pond, swamp, seepage, grassland and others). The substratum was dichotomised as with plants or organic debris as compared to other types of substratum (i.e. sand, stones, clay).
Statistical analysis
Descriptive statistics were first conducted to explore the association between infected sites and environmental factors. For the quantitative snail sampling, the snail density was calculated per site as the arithmetic mean of counts from individual ring samples. The association between habitat characteristics and the log(10) of the per site snail density was estimated using a linear regression. The appropriateness of the linear regression was verified by looking at the distribution of errors and at the Cook’s distances (Kleinbaum et al., 1988). The association between habitat characteristics and the presence of at least one infected snail per site was estimated using a logistic regression. We have explored the possibility of clustering of effect by village using random-effect versions of the models described above but did not find any significant clustering. Therefore, simple logistic and linear regressions were used. All statistical analyses were conducted using STATA 9.2 for windows (StataCorp 2006).
Results
A total of 845 sites were inspected and 198 sites (23.4%) were found with O. quadrasi. Most snail colonies were of rather limited extension, thus 90.8% of the sites identified with O. quadrasi covered areas of less than 200 m2, 81.7% were less than 100 m2 and 63.5% covered an area less than 50 m2. The area of the snail sites ranged from 6 to 750 m2 in the rain-fed villages (n=113) and from 1 to 1500 m2 in irrigated villages. The median areas were 37.5 m2 and 30 m2 in the rainfed and irrigated villages, respectively. The quantitative survey was conducted in 147 (74.2%) of the 198 sites. The reasons for not conducting a quantitative survey included that a site was very small so that 10 voucher specimens of O. quadrasi could not be collected (45 sites) or that the areas was too difficult or dangerous to access (2 sites). No reason was provided for not conducting a quantitative survey in four sites.
Among the 147 sites where quantitative sampling was conducted, the number of snails found in a single ring sample was in the range 0–65 in rain fed villages and 0–32 in irrigated villages. The number of infected snails in a single ring sample ranged from 0–3 in both types of villages. Table 1 shows the characteristics of the snails according to the village’s irrigation type. These characteristics were very similar in the two types of villages. Density of O. quadrasi on average was around 200 snails m−2 but two sites had densities beyond 600 snails m−2 (one each in rain-fed and irrigated villages). Overall, 30 (33.0%) and 25 (44.6%) sites located in rain-fed and irrigated villages had infected snails, respectively. Among those sites with infected snails, the median prevalence of infection was 1.8% (Inter Quartile Range (IQR) = 1.1% to 4.5%) and 2.6% (IQR = 1.4% to 3.9%) in rain-fed and irrigated villages, respectively.
Table 1.
Descriptive statistics of 147 sites where quantitative snail sampling was conducted in villages mostly rain-fed and in villages using some man-made irrigation systems, Samar Province, the Philippines 2002–2004.
| Rain fed | Irrigated | Difference of the means (95% CI for difference) | |
|---|---|---|---|
| No. of sites | 91 | 56 | |
| No. of snails collected | 7723 | 5080 | |
| Mean number of snails m−2 (range) | 197.69 (23.29 to 619.45) | 209.59 (25.62 to 654.38) | −0.02 (−0.10 to 0.07)** |
| Proportion of young snails (range) | 0.31 ( 0.00 to 0.71) | 0.28 (0.00 to 0.73) | 0.03 (−0.03 to 0.09)* |
| No. of snails infected | 73 | 67 | |
| No. (%) of sites with infected snails | 30 (33.0) | 25 (44.6) | 0.12 (−0.05 to 0.28)* |
| No. of infected snails m−2 (range) | 1.87 ( 0.00 to 25.62) | 2.79 ( 0.00 to 25.62) | −0.06 (−0.24 to 0.11)** |
| Median proportion of snails infected per site (range) | |||
| Total snails | 0.00 ( 0.00 to 0.19) | 0.00 ( 0.00 to 0.27) | −0.01 (−0.02 to 0.00) |
| Small snails | 0.00 ( 0.00 to 0.05) | 0.00 ( 0.00 to 0.13) | −0.00 (−0.01 to 0.00) |
| Large snails | 0.00 ( 0.00 to 0.27) | 0.02 ( 0.00 to 0.38) | −0.01 (−0.02 to 0.01) |
95% CI calculated using a binomial distribution
difference on a log(10)-scale
Most of the factors studied did not correlate with density of snails; only shading by trees had a slight positive correlation with snail density (Table 2). This effect tended to remain in a multivariate analysis, even though it became not strictly significant. In brief, each increase in the percentage of ring samples taken under a tree cover was associated with an increase of snail density of 100.11 or 1.29 per m2. Since the average density of the colonies was of the order of 200 m2, this effect is negligible.
Table 2.
Univariate and multivariate linear regression coefficient estimates of environmental factors associated with the logarithm in base 10 of O. quadrasi snail density in 50 villages of Samar Province, the Philippines, 2003–2004.
| Factor | Reference | Univariable models | Multi-variable model | ||
|---|---|---|---|---|---|
| Regression coefficient | 95%CI | Regression coefficient | 95%CI | ||
| Irrigation | |||||
| Irrigated | Rain-fed | 0.02 | −0.07 to 0.10 | 0.02 | −0.07 to 0.11 |
| Habitat | |||||
| Canal | Stream, spring | −0.03 | −0.14 to 0.08 | 0.00 | −0.11 to 0.11 |
| Other | Stream, spring | −0.04 | −0.16 to 0.07 | −0.01 | −0.13 to 0.10 |
| Substratum | |||||
| Plants or organic debris | Stones, sand, clay | 0.06 | −0.02 to 0.14 | 0.02 | −0.07 to 0.12 |
| Proportion of ring samples located in an area where shade is provided by | |||||
| Tree cover | 0.14 | 0.02 to 0.25 | 0.11 | −0.01 to 0.24 | |
| Grass | −0.03 | −0.15 to 0.10 | −0.01 | −0.15 to 0.13 | |
| Palm trees | −0.18 | −0.66 to 0.29 | −0.12 | −0.62 to 0.37 | |
| Other vegetation | −0.07 | −0.18 to 0.04 | −0.07 | −0.19 to 0.05 | |
The logistic regression assessing factors associated with the presence of at least one infected snail in the colony showed that the habitat modified the effect of irrigation. Indeed, the prevalence of infected colonies among streams and springs was 5.76 times higher (95% CI: 1.31 to 25.42) in irrigated villages as compared to rain-fed villages. Such effect was not found in other habitats. This should be interpreted with care though since the 95%CI is very wide.
Discussion
This study is the most extensive snail survey recently conducted in one defined region of the Philippines. We inspected 825 potential snail sites located in 50 villages of Samar Province, the Philippines. Nearly one-quarter of all sites inspected harboured O. quadrasi colonies.
Our results suggest that once O. quadrasi populations have established, densities are similar in irrigated and rain-fed villages and across macro-habitats. Soil factors, which were not measured in this study may also be important in determining distribution and density of O. quadrasi (Nihei et al., 1998).
One important finding of our study is that the habitat where colonies are found seem to modify the effect of the village-level irrigation scheme on the prevalence of infection of snails. We found that among streams and springs, snails located in irrigated villages tended to be more infected than snails in rain-fed villages. One reason for this could be that habitats are more permanent in irrigated villages than in rain fed villages and therefore have longer period for infections to accumulate. This finding will need to be confirmed with more data as the confidence interval for this effect was still fairly wide. However, it could have a very important impact on snail control strategies which may need to be modified according to the type of irrigation found in different villages.
Locations with O. quadrasi are often of rather limited extension and in nearly one-quarter of the sites where snails were found, there were too few for a quantitative survey to be conducted. Whether these represent establishing populations requires revisiting the sites, which was done months after the initial visit and will be reported in future analyses. There is no doubt that such small habitat patches are vulnerable to agricultural activities, which can temporarily destroy the site as snail habitat, and that many of these sites probably represent new colonisations. It is nonetheless likely that considerable transport of O. quadrasi occurs around the village and that these minor populations constitute a potential source for reestablishment of populations. Snails may spread in the area by water currents or in mud attached to animals or agricultural tools. O. quadrasi has a rather low reproduction and populations are rather stable in stable habitats (Pesigan et al., 1958), i.e. it is much more of a K-strategist than aquatic snails.
More than one third of the colonies harboured S. japonicum infections which suggests active transmission. These sites may be important for mammalian infection as cercariae can travel downstream to habitats where mammals’ water contacts take place (Pesigan et al. 1958). However, we did not find any significant association between infection in snails or the presence of snail colonies and the intensity of infection in humans (analyses not shown). Further analyses accounting for space at a finer scale than the village-level will need to be conducted to confirm this trend.
A previous study has shown that rats contribute significantly to contamination of the environment with S. japonicum eggs (Fedorko 1999) and prevalence of infection can be up to 81.9% in Rattus rattus mindanensis in Dagami, Leyte (Kamiya et al. 1980) and up to 95% in rats in Samar (Fernandez et al., 2007). This could lead to increased infection levels in snails. However, we did not find any significant association between the prevalence of infection in rats and the presence of snail colonies or the infection of snails in this study (analyses not shown). Also, statistical evidence does not suggest that infection in rats is associated with infection in humans in Samar province (McGarvey et al., 2006). However, a recent transmission dynamics model does suggest that there may be some link between infection in rats and humans (Steven Riley, personal communication). If this association is confirmed, it would resemble the situation in the West Indies where the prevalence of S. mansoni in rats (Rattus rattus) reached 100% during some seasons (Pointier & Théron 1979) and contributed to transmission. In this study, control efforts against human infection in the area resulted in an adaptation of the parasite to the murine host through a change in allelic frequencies and the cercarial emergence pattern (Théron et al. 1992). Other animals, such as dogs or cats, are infected as well and could be potential reservoir hosts, as we found a statistical association between infection in dogs and cats and humans (McGarvey et al., 2006). Interestingly, however, infection in water buffaloes is very rare in Samar with an average adjusted prevalence of 2.1% and present in only 11 of 50 villages (Fernandez et al., 2007). This contrasts findings in China where water buffaloes are considered as a major reservoir host (Jiang et al., 1996; Guo et al., 2001, Spear et al., 2004). Previous studies have suggested that people primarily become infected in larger habitats such as streams, rivers, and canals by cercariae transported into these by drainage from their catchment area that contain established colonies of O. quadrasi (Pesigan et al., 1958). Some transmission could occur during land preparation for agriculture (primarily rice culture). Based on evidence (sentinel mice exposure) presented by Pesigan et al. (1958) we judge that a “catchment area” of a transmission site could be up to a radius of about 2 km, but could possibly be larger.
Due to the nature of their habitat, O. quadrasi snails are particularly sensitive to environmental control measures. Such measures include stream channelization, seepage control, canal lining, canal relocation with deep burial of snails, proper drainage (either surface or subsurface drainage) in irrigation schemes, vegetation removal, earth filling, ponding, and improved agricultural practices. These measures may be expensive to implement but they can control Oncomelania effectively (Yokogawa, 1976; Makiya et al., 1982, 1986; Ohmae et al., 2003). According to Blas (1976) vegetation clearance in combination with drainage could reduce snail density by 96% of the original number.
A number of molluscicides have been used against Oncomelania hupensis ssp., the most common being niclosamide, copper sulphate, Frescon, sodium pentachlorophenate, Yurimin, and B-2. The fact that snails can descend into the top sediment or climb up on vegetation (Pesigan et al., 1958) and hence possibly out of reach of molluscicide may make these less efficient. Although environmental management has proven quite effective in controlling snail populations, there is a general impression that they are expensive to implement and maintain and hence not sustainable. The methods applicable, however, would have the added benefit of increasing agricultural productivity.
Because the snail populations in Samar are of rather limited size, we feel that it would be realistic to attempt to control them by environmental management, primarily drainage (either subsurface or by ditches) and vegetation control. It is important that the methods be applied by the local farmers in order to be sustainable, and therefore the programme of environmental snail control must be designed in close cooperation with the local communities. The main obstacles against their broad application are lack of sustainability and it may be difficult to convince farmers to invest time in these measures if schistosomiasis is not seen as a major problem for them. Fortunately, however, many of these methods coincide with methods of good agricultural practices and maybe if these benefits became obvious to the farmers there might be a greater willingness to implement the methods. In order to ensure optimization of the management strategy we will need to involve agricultural engineers in the deliberations with the communities and in the supervision of the activities.
Table 3.
Prevalence odds ratios of environmental factors associated with the prevalence of infection in snail colonies obtained from a multivariate logistic regression with an interaction term between irrigation and habitat, Samar Province, the Philippines, 2003–2004.
| Factor | Reference | Prevalence proportion ratio | 95%CI |
|---|---|---|---|
| Irrigation | |||
| Irrigated among stream, spring | Rain-fed among stream, spring | 5.76 | 1.31 to 25.42 |
| Irrigated among canal | Rain-fed among canal | 0.79 | 0.28 to 2.22 |
| Irrigated among other habitat | Rain-fed among other habitat | 1.84 | 0.52 to 6.50 |
| Habitat | |||
| Canal among rain-fed villages | Stream/spring among rain-fed villages | 2.26 | 0.70 to 7.38 |
| Other habitat among rain-fed villages | Stream/spring among rain-fed villages | 0.54 | 0.19 to 1.50 |
| Canal among irrigated villages | Stream/spring among irrigated villages | 0.32 | 0.08 to 1.22 |
| Other habitat among irrigated villages | Stream/spring among irrigated villages | 0.39 | 0.09 to 1.67 |
Acknowledgements
Funding was provided by the NSF/NIH joint program in ecology of infectious diseases, NIH grant R01 TW01582. We would also like to acknowledge the hadr work of the staff at the Schistosomiasis Control Univer of Samar (Center for Health and Development Region VII) and the Schistosomiasis Research and Training Division in Palo, Leyte who highly contributed to the field snail survey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blas BL. Agro-engineering and sanitation improvement in the control of schistosomiasis japonica in the Philippines: a review. Southeast Asian J. Trop. Med. Pub. Hlth. 1976;7:341–345. [PubMed] [Google Scholar]
- Carabin H, Balolong E, Joseph L, McGarvey ST, Johansen MV, Fernandez T, Willingham AL, Olveda R Schistosomiasis Transmission and Ecology in the Philippines (STEP) Project. Assessing the validity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs and rats in Western Samar and Sorsogon Provinces, The Philippines. Int. J. Parasitol. 2005;35:1517–1524. doi: 10.1016/j.ijpara.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Fedorko JM. Schistosoma japonicum in the black rat, Rattus rattus mindanensis, from Leyte, Philippines in relation to Oncomelania snail colonies with reference to other endoparasites. Southeast Asian J. Trop. Med. Pub. Hlth. 1999;30:343–349. [PubMed] [Google Scholar]
- Fernadez TJ, Jr, Petilla T, Banez B. An epidemiological study on Schistosoma japonicum in domestic animals in Leyte, Philippines. Southeast Asian J. Trop. Med. Pub. Hlth. 1982;13:575–579. [PubMed] [Google Scholar]
- Fernandez TJ, Jr, Tarafder MR, Balolong E, Jr, Joseph L, Willingham AL, III, Bélisle P, Webster JP, Olveda RM, McGarvey ST, Carabin H. Prevalence of Schistosoma japonicum infection among animals in 50 villages of Samar Province, The Philippines. Vector-Borne Zoonotic Dis. 2007;7:147–155. doi: 10.1089/vbz.2006.0565. [DOI] [PubMed] [Google Scholar]
- Frandsen F, Christensen NØ. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984;41:181–202. [PubMed] [Google Scholar]
- Jiang Q, Zhang S, Yuan H, Liu Z, Zhao G, Brinkmann U. The effect of a combined approach to schistosomiasis control on the transmission of Schistosoma japonicum in Xingzi of Poyang Lake area, China. Southeast Asian J. Trop. Med. Public Health. 1996;27:535–541. [PubMed] [Google Scholar]
- Kamiya H, Tada Y, Matsuda H, Tanaka H, Blas BL, Noseñas JS, Santos AT., Jr Annual fluctuation of Schistosoma japonicum infection in field rats, Rattus rattus mindanensis, in Dagami, Leyte, Philippines. Japan J. Exp. Med. 1980;50:375–382. [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. 2nd Ed. Belmont: Duxbury Press; 1988. Applied Regression analysis and other multivariable methods. [Google Scholar]
- Guo JG, Ross AG, Lin DD, Williams GM, Chen HG, Li Y, Davis GM, Feng Z, McManus DP, Sleigh AC. A baseline study on the importance of bovines for human Schistosoma japonicum infection around Poyang Lake, China. Am. J. Trop. Med. Hyg. 2001;65:272–278. doi: 10.4269/ajtmh.2001.65.272. [DOI] [PubMed] [Google Scholar]
- Lewert RM, Yogore MG, Jr, Blas BL. Schistosomiasis japonica in Barrio San Antonio, Basey, Samar, The Philippines. Am. J. Trop. Med. Hyg. 1979;28:1010–1025. doi: 10.4269/ajtmh.1979.28.1010. [DOI] [PubMed] [Google Scholar]
- Lipayon IL, Blas BL, Balonga IP. Schistosomiasis and irrigation systems in Leyte, Philippines. RTRMF Journal. 2002;2:60–70. [Google Scholar]
- Makiya K, Tanaka H, Banez E, Blas BL, Perez A, Kumada N, Santos AT., Jr Population studies on Oncomelania quadrasi, the snail intermediate host of Schistosoma japonicum, in the Philippines. 4. Evaluation of drainage for snail control. Japan J. Exp. Med. 1982;52:173–181. [PubMed] [Google Scholar]
- Makiya K, Tanaka H, Banez E, Blas BL, Santos AT., Jr Population studies on Oncomelania quadrasi, the snail intermediate host of Schistosoma japonicum, in the Philippines. 5. Quantitative analysis on successful snail control by land reclamation. Japan J. Exp. Med. 1986;56:81–87. [PubMed] [Google Scholar]
- McGarvey ST, Carabin H, Balolong E, Jr, Bélisle P, Fernandez T, Joseph L, Tallo V, Gonzales R, Tarafder M, Alday P, Olveda R. Schistosomiasis Transmission and Ecology in the Philippines. A cross-sectional survey of the association between animal and human intensity of infection with Schistosoma japonicum in Samar Province, the Philippines. Bull. World Health Organ. 2006;84:446–452. doi: 10.2471/blt.05.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei N, Kanazawa T, Blas BL, Saitoh Y, Itagaki H, Pangilinan R, Matsuda H, Yasuraoka K. Soil factors influencing the distribution of Oncomelania quadrasi, the intermediate host of Schistosoma japonicum, on Bohol Island, Philippines. Ann. Trop. Med. Par. 1998;92:699–710. doi: 10.1080/00034989859168. [DOI] [PubMed] [Google Scholar]
- Ohmae H, Iwanaga Y, Nara T, Matsuda H, Yasuraoka K. Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol. Int. 2003;52:409–417. doi: 10.1016/s1383-5769(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Pointier J-P, Théron A. La schistosomose intestinale dans les forêts marécageuses à Pterocarpus de Guadeloupe (Antilles françaises). Ecologie du mollusque vecteur, Biomphalaria glabrata et de son parasite Schistosoma mansoni. Ann Parasitol. 1978;54:43–56. [PubMed] [Google Scholar]
- Spear RC, Seto E, Liang S, Birkner M, Birkner M, Hubbard A, Qiu D, Yang C, Zhong B, Xu F, Gu X, Davis GM. Factors influencing the transmission of Schistosoma japonicum in the mountains of Sichuan Province of China. Am. J. Trop. Med. Hyg. 2004;70:48–56. [PubMed] [Google Scholar]
- StataCorp L.P. Stata/SE 9.2 for Windows. College Station: StataCorp; 2006. [Google Scholar]
- Tarafder MR, Balolong E, Carabin H, Bélisle P, Tallo V, Joseph L, Alday P, Gonzales RO, Riley S, Olveda R, McGarvey ST. A cross-sectional study of the prevalence of intensity of infection with Schistosoma japonicum in 50 irrigated and rain-fed villages in Samar Province, the Philippines. BMC Public Health. 2006 March 9;6:61 doi: 10.1186/1471-2458-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théron A, Pointier JP, Morand S, Imbert-Establet D, Borel G. Long-term dynamics of natural populations of Schistosoma mansoni among Rattus rattus in patchy environment. Parasitol. 1992;104:291–298. doi: 10.1017/s0031182000061734. [DOI] [PubMed] [Google Scholar]
- Viyanant V, Upatham ES, Blas BL, Yuan HC. Analysis of allozymes by electrofocusing in schistosome snail hosts (Oncomelania hupensis) from China and the Philippines. Malacol. Rev. 1987;20:91–96. [Google Scholar]
- Woodruff DS, Staub KC, Upatham ES, Viyanant V, Yuan H-C. Genetic variation in Oncomelania hupensis: Schistosoma japonicum transmitting snails in China and the Philippines are distinct species. Malacologia. 1988;29:347–361. [Google Scholar]
- Yasuraoka K. Schistosomiasis and water resource development. Southeast Asian J. Trop. Med. Public Health. 1979;10:630–633. [PubMed] [Google Scholar]
- Yokogawa M. Programme of schistosomiasis control in Japan. Southeast Asian J. Trop. Med. Public Health. 1976;7:322–329. [PubMed] [Google Scholar]