Abstract
We report a case of a 64 year-old man whose clinical presentation and neuroimaging findings strikingly resembled those found in Takayasu’s Arteritis which is characterized by the triad of absent radial pulses, ischemic retinopathy, and carotid sinus hyperreflexia causing syncopes. Angiographically, the patient exhibited severe atherosclerotic changes of the supraaortic large vessels. Stent-assisted angioplasty resulted in both clinical improvement and increased cerebral blood flow as measured by angiography and ultrasound.
Keywords: Takayasu’s arteritis, atherosclerosis, angiography, stent, angioplasty
Introduction
Takayasu’s Arteritis (TA) is an idiopathic, inflammatory, granulomatous vasculopathy of the aorta and its main branches (Liang and Hoffman 2005). It is reported to be more frequent in women but a decline in the female to male ratio from Eastern Asia towards the West is discussed (Johnston et al 2002). TA commonly presents during the second and third decade of life. The disease is infrequent in the elderly population although the correct diagnosis is often delayed from months to years from onset of first symptoms. We report the case of a male patient of old age with severe atherosclerotic stenosing disease of the supraaortal vessels whose clinical and neuroimaging presentation is strikingly similar to that found in TA.
Case report
This male patient aged 64 years was referred to our service with a history of repeated (pre-) syncopal episodes. An extensive workup for the first syncope two years earlier done by an internal medicine service had concluded high grade coronary artery disease to be the main cause of these symptoms. An occlusion of the left internal carotid artery (ICA) was already recorded at that time. Vascular risk factors included essential hypertension, hyperlipidemia, and excessive nicotine abuse for several years. The patient was treated with 300 mg of aspirin daily. Our examination revealed no neurological symptoms and no signs of ischemic retinopathy by fundoscopy, but absence of both radial and ulnar artery pulses. The results of an extended laboratory workup revealed a vitamin B12 deficiency with macrocytic anemia and thrombocytopenia. Markers for systemic inflammation such as erythrocyte sedimentation rate, C-reactive protein were negative. The most striking finding was a syncope during a doppler examination provoked by a compression manoeuvre of the right carotid artery with the ultrasound probe.
Brain MRI (T2- and diffusion-weighted) showed a pattern of white matter lesions suggestive for microangiopathy but no apparent diffusion abnormalities. The tilt table test revealed an orthostatic hypoadrenergic hypotension during head-up tilt combined with a decreased cerebral blood flow (CBF) in the left posterior cerebral artery (LPCA) of 33% and in the right middle cerebral artery (RMCA) of 23% as measured by transcranial doppler (TCD) when tilted. The reserve capacity was depleted as of 1.1%/mmHg in the LPCA and normal as of 2.88%/mmHg in the RMCA demonstrating a threatening low CBF in the vertebrobasilar system under orthostatic stress. TCD detected no microembolic signals in the LPCA or RMCA.
Transfemoral digital substraction angiography revealed severe supraaortic atherosclerotic changes with occlusion of the proximal left subclavian artery, the left ICA and the right vertebral artery. Additionally, a high grade stenosis of the proximal brachiocephalic trunk (BCT) was present. The left common carotid artery was the only supraaortic vessel that was not affected. A complex pattern of collateralization is illustrated in Figure 1.
Figure 1.
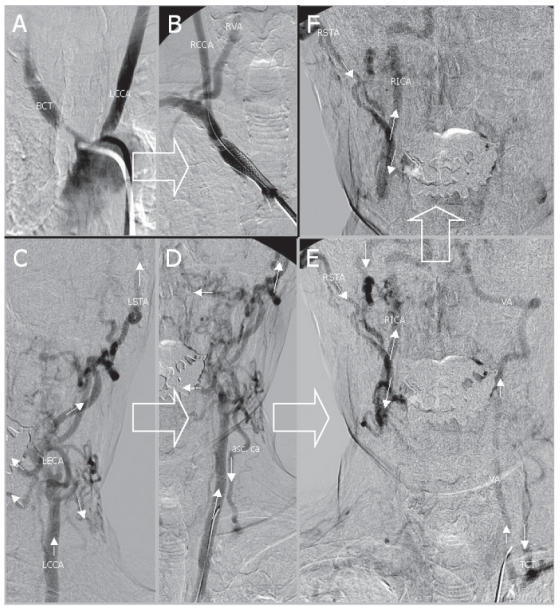
Transfemoral digital subtraction angiography of the stenosed brachiocephalic trunk (BCT) and of the left common carotid artery (LCCA) before (A) and after (B) stent assisted angioplasty. The complex pattern of collateral flow is depicted in C-F: 1) to the left vertebral artery (VA) via a left sided subclavian steal with flow reversal of the ascending cervical artery (asc. ca) and thyrocervical trunk (TCT) and 2) to the contralateral internal carotid artery (RICA) via numerous collaterals from the left external carotid artery (LECA) crossing the midline such as the left and the right superficial temporal artery (LSTA and RSTA). The direction of blood flow is indicated by arrows. Large arrows indicate chronological order.
After the patient signed a written informed consent form, stent assisted angioplasty of the BCT was successfully performed without complications. In the sequel, the right radial and ulnar artery pulses were palpable again. On the tilt table, the decrease of the CBF improved to 23% in the LPCA and to 20% in the RMCA while the depleted reserve capacity of the LPCA remained nearly unchanged. The patient was discharged with a medication of clopidogrel 75 mg lifelong in combination with aspirin 100 mg for four weeks.
Discussion
The pattern of extracranial supraaortal atherosclerotic lesions presented in this case features several similarities with those often found with TA. The subclavian arteries are the arteries most commonly involved in TA, followed by the aorta, and the common carotid arteries (Kerr et al 1994). TA is a rare acute and chronic form of granulomatous panarteritis of the large vessels that frequently affects the aorta and its branches thus causing severe stenosis or even vessel occlusion (Johnston et al 2002). In contrast with other types of vasculitis, TA seems to affect preferentially young women in Asian countries. Lately, however, the incidence of TA in middle aged patients seems to be increasing, and in these patients chronic vasculitis latently progresses with atherosclerosis (Seyahi et al 2006). Almost all patients have ischemic disorders of the involved vessels (de Franciscis et al 2007). As in our patient, intracranial stenoses are uncommon in this disease and hemodynamic abnormalities such as low flow pulsatility of the CBF and collateral circulation due to the severe extracranial involvement have been demonstrated by TCD (Cantu et al 2000). Duplex ultrasonography reveals characteristic long segments of smooth, homogeneous, midechoic, concentric wall thickening (Schmidt et al 2002; Ringleb et al 2005). Vasculitis can be easily differentiated from arteriosclerotic lesions, which are heterogeneous and irregular with calcifications (Schmidt 2007). Delayed gadolinium-enhanced MRI may be useful in monitoring disease activity or inflammation of the arterial wall (Seko 2007).
Clinical presentation of TA is characterized by constitutional symptoms such as myalgias, arthralgias, fever, and weight loss (Liang and Hoffman 2005). Vascular inflammation may lead to pain such as carotidynia. However, most of the neurological symptoms such as headache, seizures, TIA, or orthostatic syncopes and finally ischemic brain infarctions relate to fibrosis or occlusion of vessels (Johnston et al 2002). In Western countries this disease is also known as “pulseless disease,” because the pulse is frequently absent due to the obstruction of subclavian or brachial arteries (Numano et al 2000). Our patient also showed absence of both radial and ulnar artery pulses with improvement after stent-assisted angioplasty of the BCT. Twenty percent of patients with TA will have a self-limited, monophasic inflammatory episode (Kerr et al 1994). In most of the patients, months or years later, a progressive or relapsing/remitting course requires immunosuppressive treatment (Liang and Hoffman 2005).
Interestingly, patients with TA have a higher rate of atherosclerotic carotid plaques compared with healthy controls (Seyahi et al 2006). The association of atherosclerosis and chronic inflammatory diseases such as systemic lupus erythematosus and rheumatoid arthritis is well known (Bruce 2005; Chung et al 2007). Systemic inflammation is increasingly being considered central to atherogenesis and an important risk factor for vascular disease (Numano et al 2000; Abou-Raya and Abou-Raya 2006). However, the present case does not meet the diagnostic guidelines of TA as reported by the American College of Rheumatology (Arend et al 1990), and the difference from TA is obvious. Similar to atherosclerotic disease, several treatment modalities are available for TA comprising surgical and endovascular interventions in addition to immunosuppressive therapy (Liang et al 2004). Management of TA remains a therapeutic challenge, and relapses are frequent (de Franciscis et al 2007). There is increasing evidence that emerging therapeutic options such as antitumor necrosis factor (TNF)-alpha therapy can be at least an alternative treatment for patients refractory to conventional immunosuppressive therapy (Seko 2007). Hence, for the individual patient with TA the treatment of choice is often empirically based. Percutaneous transluminal angioplasty (PTA) in patients with TA is rarely reported and is associated with a higher rate of re-stenoses than in atherosclerotic disease (Joseph et al 1994; Tyagi et al 1998). More stable long-term results after stent deployment (stent PTA) have been reported (Takahashi et al 2002). However, the experiences of other authors have been to the contrary (Liang et al 2004). Conventional stents may be associated with a high rate of failure, as suggested by studies with long-term follow-up (Liang and Hoffman 2005). Bypass surgery is reported to be the procedure with the best long-term patency rates. Fortunately, this treatment option is associated with low morbidity and mortality (Liang and Hoffman 2005). Therefore, the indication for neurovascular or surgical intervention in both the inflammatory and the atherosclerotic disease entities should be based on an exact hemodynamic assessment using ultrasound techniques including reserve capacity, angiography, and MRI. In our case, we decided to treat the stenosis of the BCT by stent PTA mainly because of the threatening decrease in CBF under orthostatic stress and the highly depleted reserve capacity of the posterior circulation in order to prevent brain ischemia.
Acknowledgments
The authors have nothing to disclose.
References
- Abou-Raya A, Abou-Raya S. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev. 2006;5:331–7. doi: 10.1016/j.autrev.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Clin Radiol. 1990;42:177–82. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- Bruce IN. Atherogenesis and autoimmune disease: the model of lupus. Lupus. 2005;14:687–90. doi: 10.1191/0961203305lu2201oa. [DOI] [PubMed] [Google Scholar]
- Chung CP, Avalos I, Raggi P, et al. Atherosclerosis and inflammation: insights from. rheumatoid arthritis. Clin Rheumatol. 2007;2 doi: 10.1007/s10067-007-0548-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cantu C, Pineda C, Barinagarrementeria F, et al. Noninvasive cerebrovascular assessment of Takayasu arteritis. Stroke. 2000;31:2197–202. doi: 10.1161/01.str.31.9.2197. [DOI] [PubMed] [Google Scholar]
- de Franciscis S, Serra R, Luongo A. The Management of Takayasu’s Arteritis: Personal Experience. Ann Vasc Surg. 2007;16 doi: 10.1016/j.avsg.2007.03.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol. 2002;55:481–6. doi: 10.1136/jcp.55.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S, Mandalam KR, Rao VR, et al. Percutaneous transluminal angioplasty of the subclavian artery in nonspecific aortoarteritis: results of long-term follow-up. J Vasc Interv Radiol. 1994;5:573–80. doi: 10.1016/s1051-0443(94)71556-6. [DOI] [PubMed] [Google Scholar]
- Kerr GS, Hallahan CW, Giordano J, et al. Takayasu’s arteritis. Ann Intern Med. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- Liang P, Tan-Ong M, Hoffman GS. Takayasu’s arteritis: vascular interventions and outcomes. J Rheumatol. 2004;31:102–6. [PubMed] [Google Scholar]
- Liang P, Hoffman GS. Advances in the medical and surgical treatment of Takayasu arteritis. Curr Opin Rheumatol. 2005;17:16–24. doi: 10.1097/01.bor.0000146607.65808.37. [DOI] [PubMed] [Google Scholar]
- Numano F, Kishi Y, Tanaka, et al. Inflammation and atherosclerosis. Atherosclerotic lesions in Takayasu arteritis. Ann N Y Acad Sci. 2000;902:65–76. doi: 10.1111/j.1749-6632.2000.tb06301.x. [DOI] [PubMed] [Google Scholar]
- Ringleb PA, Strittmatter EI, Loewer M, et al. Cerebrovascular manifestations of Takayasu arteritis in Europe. Rheumatology. 2005;44:1012–5. doi: 10.1093/rheumatology/keh664. [DOI] [PubMed] [Google Scholar]
- Schmidt WA, Nerenheim A, Seipelt E, et al. Diagnosis of early Takayasu arteritis by colour Doppler ultrasonography. Rheumatology. 2002;41:496–502. doi: 10.1093/rheumatology/41.5.496. [DOI] [PubMed] [Google Scholar]
- Schmidt WA. Technology Insight: the role of color and power Doppler ultrasonography in rheumatology. Nat Clin Pract Rheumatl. 2007;3:35–42. doi: 10.1038/ncprheum0377. [DOI] [PubMed] [Google Scholar]
- Seko Y. Giant cell and Takayasu arteritis. Curr Opin Rheumatol. 2007;19:39–43. doi: 10.1097/BOR.0b013e3280119866. [DOI] [PubMed] [Google Scholar]
- Seyahi E, Ugurlu S, Cumalir R, et al. Atherosclerosis in Takayasu arteritis. Ann Rheum Dis. 2006;65:1202–7. doi: 10.1136/ard.2005.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JC, Sakai N, Manaka H, et al. Multiple supra-aortic stenting for Takayasu arteritis: extensive revascularization and two-year follow-up. AJNR Am J Neuroradiol. 2002;23:790–3. [PMC free article] [PubMed] [Google Scholar]
- Tyagi S, Verma PK, Gambhir DS, et al. Early and long-term results of subclavian angioplasty in aortoarteritis (Takayasu disease): comparison with atherosclerosis. Cardiovasc Intervent Radiol. 1998;21:219–24. doi: 10.1007/s002709900248. [DOI] [PubMed] [Google Scholar]


