Abstract
The American lobster Homarus americanus is a decapod crustacean with both high economic and scientific importance. To facilitate physiological investigations of peptide transmitter/hormone function in this species, we have used matrix-assisted laser desorption/ionization Fourier transform mass spectrometry (MALDI-FTMS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS) and nanoscale liquid chromatography coupled to electrospray ionization quadrupole time-of-flight tandem mass spectrometry (nanoLC-ESI-Q-TOF-MS/MS) to elucidate the peptidome present in its nervous system and neuroendocrine organs. In total, 84 peptides were identified, including 27 previously known H. americanus peptides (e.g. VYRKPPFNGSIFamide [Val1-SIFamide]), 23 peptides characterized previously from other decapods, but new to the American lobster (e.g. pQTFQYSRGWTNamide [Arg7-corazonin]), and 34 new peptides de novo sequenced/detected for the first time in this study. Of particular note are a novel B-type allatostatin (TNWNKFQGSWamide) and several novel FMRFamide-related peptides, including an unsulfated analog of sulfakinin (GGGEYDDYGHLRFamide), two myosuppressins (QDLDHVFLRFamide and pQDLDHVFLRFamide), and a collection of short neuropeptide F isoforms (e.g. DTSTPALRLRFamide, and FEPSLRLRFamide). Our data also include the first detection of multiple tachykinin-related peptides in a non-brachyuran decapod, as well as the identification of potential individual-specific variants of orcokinin and orcomyotropin-related peptide. Taken collectively, our results not only expand greatly the number of known H. americanus neuropeptides, but also provide a framework for future studies on the physiological roles played by these molecules in this commercially and scientifically important species.
Keywords: Homarus americanus, matrix-assisted laser desorption/ionization-Fourier transform mass spectrometry (MALDI-FTMS), electrospray ionization quadrupole time-of-flight tandem mass spectrometry (ESI-Q-TOF MS/MS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), neuropeptide, neurohormone, peptide sequencing, supraoesophageal ganglion, suboesophageal ganglion, thoracic ganglia, abdominal ganglia, eyestalk ganglia (ESG), stomatogastric ganglion (STG), sinus gland (SG), pericardial organ (PO), ventral nerve cord (VNC)
1. Introduction
The American lobster Homarus americanus provides a useful model organism in many areas of physiology, including the study of neuroendocrine control/modulation of behavior. For example, this species has long served as a model for studies of hormonal control of aggression (Kravitz, 1988; Huber et al., 1997; Kravitz 2000). Likewise the neural circuits contained within the H. americanus cardiac ganglion and stomatogastric nervous system (STNS) have served as important models for the study of modulatory control of rhythmic behavior, specifically cardiac output and the swallowing, chewing and filtering of food items, respectively (Cooke, 2002; Clarac and Pearlstein, 2007; Marder and Bucher, 2007).
One surprising limitation, given the commercial and biological importance of H. americanus, is the lack of a complete catalog of the signaling molecules that lobsters use to affect their behavior, particularly their complement of neuropeptides. In fact, only about two-dozen neuropeptides have been fully characterized from this species (e.g. Schwarz et al., 1984; Trimmer et al., 1987; Chang et al., 1990; Tensen et al., 1991a; Tensen et al., 1991b; Tensen et al., 1991c; Li et al., 2002; Skiebe et al., 2003; Stemmler et al., 2005; Fu et al., 2005a; Christie et al., 2006; Stemmler et al., 2006; Dickinson et al., 2007a; Dickinson et al., 2007b; Dickinson et al., 2007c; Stemmler et al., 2007a). Since complete knowledge of the neuromodulators/hormones present in an organism is necessary for an accurate assessment of its behavioral control mechanisms, we have undertaken a mass spectral characterization of the peptide transmitters/hormones present in the H. americanus nervous system using a combination of matrix-assisted laser desorption/ionization Fourier transform mass spectrometry (MALDI-FTMS), matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and nanoscale liquid chromatography coupled to electrospray ionization quadrupole time-of-flight tandem mass spectrometry (nanoLC-ESI-Q-TOF-MS/MS). This combined approach identified 84 neuropeptides, including 27 known H. americanus peptides, 23 peptides that were known previously from other arthropods, but new to the American lobster, and 34 peptides sequenced/detected here for the first time. Some of these data have appeared previously in abstract form (Li et al., 2007).
2. Materials and methods
2.1. Animals
American lobsters, H. americanus, were purchased from Downeast Lobster Pound (Trenton, ME) or Commercial Lobster and Seafood Company (Boston, MA) and were maintained in flow-through natural seawater aquaria at ambient seawater temperature (approximately 8–12 °C) or recirculating artificial seawater aquaria at 10–14°C. In total, approximately 150 individuals, including both males and females, were used in this study.
2.2. Tissue collection
All animals were anesthetized by packing them in ice for 30–60 minutes, after which the dorsal carapace was removed from each individual and its CNS (eyestalk ganglia [ESG], supraoesophageal ganglia [brain] and ventral nerve cord [VNC; consisting of the suboesophageal, thoracic and abdominal ganglia]), neuroendocrine tissues (sinus gland [SG] and pericardial organ [PO]), and/or stomatogastric ganglion (STG) dissected free from surrounding muscle and connective tissues in chilled (approximately 10 °C) physiological saline (composition in mM: 479.12 NaCl, 12.74 KCl, 13.67 CaCl2, 20.00 MgSO4, 3.91 Na2SO4, 5.00 HEPES [pH 7.4]). Following dissection, tissue samples were either immediately assayed or placed in acidified methanol (90% methanol [Fisher Scientific, Pittsburgh, PA]: 9% glacial acetic acid [Fisher]: 1% deionized water) and stored at −80°C until utilized for peptide extraction or direct tissue mass spectral analysis.
2.3. Tissue extraction and HPLC fractionation
For some experiments, tissues were pooled, homogenized, and extracted with acidified methanol (see 2.2). Extracts were dried in a Savant SC 110 SpeedVac concentrator (Thermo Electron Corporation, West Palm Beach, FL) and re-suspended in approximately 100 µl of 0.1% formic acid. The re-suspended extracts were then vortexed and briefly centrifuged. The resulting supernates were subsequently fractionated via high performance liquid chromatography (HPLC).
HPLC separations were performed using a Rainin Dynamax HPLC system (Rainin Instrument Inc., Woburn, MA) equipped with a Dynamax UV-D II absorbance detector. The mobile phases were: deionized water containing 0.1% formic acid (Solution A), and acetonitrile (HPLC grade, Fisher Scientific) containing 0.1% formic acid (Solution B). For each separation, 20 µl of extract was injected onto a Macrosphere C18 column (2.1 mm i.d. × 250 mm length, 5 µm particle size; Alltech Assoc. Inc., Deerfield, IL). The separation consisted of a 120 minute gradient of 5%–95% Solution B with fractions automatically collected every two minutes using a Rainin Dynamax FC-4 fraction collector.
2.4. Formaldehyde derivatization
For some experiments, peptides in HPLC fractions were derivatized with formaldehyde prior to mass spectral analysis. Specifically, 0.3 µl of a given fraction was spotted on the MALDI plate, followed by the addition and mixing of 0.3 µl of 26 mM sodium cyanoborohydride (Sigma-Aldrich, St. Louis, MO), and subsequent addition of 0.3 µl of formaldehyde (20% in H2O vol/vol, Sigma-Aldrich). The droplet was left at room temperature for 5 minutes, after which 0.3 µl of 50 mM ammonium bicarbonate solution was added to the reaction mixture. Finally, 0.3 µl of a saturated 2,5-dihydroxybenzoic acid (DHB; ICN Biomedical Corp., Costa Mesa, CA) matrix (150 mg/ml in a 50:50 v/v mixture of deionized water and purge and trap grade methanol [Fisher Scientific]) was added to the droplet and crystallized at room temperature.
2.5. MALDI-FTMS and sustained off resonance irradiation-collision induced dissociation
MALDI-FTMS experiments were performed on an IonSpec ProMALDI Fourier transform mass spectrometer (Lake Forest, CA) equipped with a 7.0 Tesla actively-shielded superconducting magnet. This FTMS instrument contains a high pressure MALDI source where ions from multiple laser shots can be accumulated in the external hexapole storage trap before the ions are transferred to the ICR cell via a quadrupole ion guide. A 337 nm nitrogen laser (Laser Science, Inc., Franklin, MA) was used for ionization/desorption. The ions were excited prior to detection with an rf sweep beginning at 7050 ms with a width of 4 ms and amplitude of 150 V base to peak. The filament and quadrupole trapping plates were initialized to 15 V, and both were ramped to 1V from 6500 to 7000 ms to reduce baseline distortion of peaks. Detection was performed in broadband mode from m/z 108.00 to 4500.00.
Peptide fragmentation was accomplished by sustained off resonance irradiation-collision induced dissociation (SORI-CID). An arbitrary waveform from 2000 ms to 2131 ms with a ±10 Da isolation window was introduced to isolate the ion of interest. Ions were excited with SORI Burst excitation (2.648V, 2500–3000 ms). A pulse of nitrogen gas was introduced through a pulse valve from 2500 to 2750 ms to introduce collision activation.
For direct tissue analysis, tissue fragments were desalted by briefly rinsing in a solution of DHB prepared in deionized water (10 mg/ml). The tissue was then placed onto the MALDI sample plate along with 0.3 µl of saturated DHB matrix (prepared as described in 2.4) before allowing the DHB spot to crystallize at room temperature.
Off-line analysis of HPLC fractions (see 2.3) was performed by spotting 0.3 µl of saturated DHB on the MALDI sample plate and adding 0.3 µl of the HPLC fraction of interest. The resulting mixture was allowed to crystallize at room temperature. The MALDI-FTMS analysis was then performed as described above.
2.6. MALDI-TOF MS
MALDI-TOF mass spectra were obtained using a Voyager DE STR (Applied Biosystems, Framingham, MA) time-of-flight mass spectrometer equipped with delayed ion extraction. A pulsed nitrogen laser (337 nm) was used as the desorption/ionization source, and positive-ion mass spectra were acquired using both linear and reflectron modes. Each representative mass spectrum shown is the smoothed average of 128–256 laser pulses. Mass calibration was performed externally using a mixture of synthetic peptide standards (angiotensin II and bovine insulin, Sigma-Aldrich, St. Louis, MO). Mass accuracy was better than 0.01%.
2.7. Capillary LC-ESI-Q-TOF MS/MS
Nanoscale LC-ESI-Q-TOF MS/MS was performed using a Waters capillary LC system coupled to a Q-TOF Micro mass spectrometer (Waters Corp., Milford, MA). Chromatographic separations were performed on a C18 reverse phase capillary column (75 µm internal diameter ×150 mm length, 3 µm particle size; Micro-Tech Scientific Inc., Vista, CA). The mobile phases used were: deionized water with 5% acetonitrile and 0.1% formic acid (A); acetonitrile with 5% deionized water and 0.1% formic acid (B); deionized water with 0.1% formic acid (C). An aliquot of 6.0 µl of an HPLC fraction (see 2.3) was injected and loaded onto the trap column (PepMap™ C18; 300 µm column internal diameter × 1 mm, 5 µm particle size; LC Packings, Sunnyvale, CA, USA) using mobile phase C at a flow rate of 30 µl/min for 3 minutes. Following this, the stream select module was switched to position the trap column in line with the analytical capillary column, and a linear gradient of mobile phases A and B was initiated. A splitter was added between the mobile phase mixer and the stream select module to reduce the flow rate from 15 µl/min to 200 nl/min.
The nanoflow ESI source conditions were set as follows: capillary voltage 3200 V, sample cone voltage 35 V, extraction cone voltage 1 V, source temperature 120°C, cone gas (N2) 10 l/hr. Data dependent acquisition was employed for the MS survey scan and the selection of precursor ions and subsequent MS/MS of the selected parent ions. The MS scan range was from m/z 300–2000 and the MS/MS scan was from m/z 50–1800. The MS/MS de novo sequencing was performed with a combination of manual sequencing and automatic sequencing by PepSeq software (Waters Corp.).
2.8. Figure production
MALDI-FTMS figures were produced by converting the spectra obtained using IonSpec version 7.0 software (IonSpec Corp.) to a bitmap image using Boston University Data Analysis (BUDA) software (version 1.4; Boston University, Boston, MA). The BUDA files were then pasted into Fireworks MX 2004 (Macromedia, Inc., San Francisco, CA) and resampled to improve the resolution. All MS/MS figures were produced using a combination of Fireworks MX 2004 and Microsoft Windows paint tool (Microsoft Corporation, Redmond, WA).
3. Results
3.1. Strategy for the mass spectral analysis of peptides in the nervous system of H. americanus
The characterization of a neuropeptidome is inherently difficult due to its extreme chemical complexity and the often-minute amounts of the expressed peptides. Here, we have used a strategy combining MALDI-based high resolution mass profiling (direct tissue and off-line HPLC fraction analysis; Fig. 1) and nanoscale biochemical separation/derivatization coupled to tandem mass spectrometric sequencing (Fig. 2–Fig. 4) to characterize the peptide complement of the H. americanus nervous system. Our analyses include all of the major components of the ventral nerve cord, as well as the stomatogastric ganglion and two prominent neurosecretory organs (the sinus gland and the pericardial organ). For ease of later discussion, we have grouped the identified peptides into families of related isoforms, whenever possible, and these are presented below in alphabetical order based on family name. Novel peptides for which there are no known families are presented at the end of the Results section. The tissues that express each identified peptide are given in Table 1. It is important to note that the methodologies used in this study do not differentiate between isobaric amino acids, e.g. isoleucine and leucine, and thus for some peptide sequences, e.g. most of the novel FMRF-related peptides, the assigned amino acid(s) were predicted and assigned based on homology to related family members. Thus, it is possible that some leucine residues have been mis-assigned, and care should be taken by readers in interpreting these sequences until they are confirmed biochemically and/or genetically.
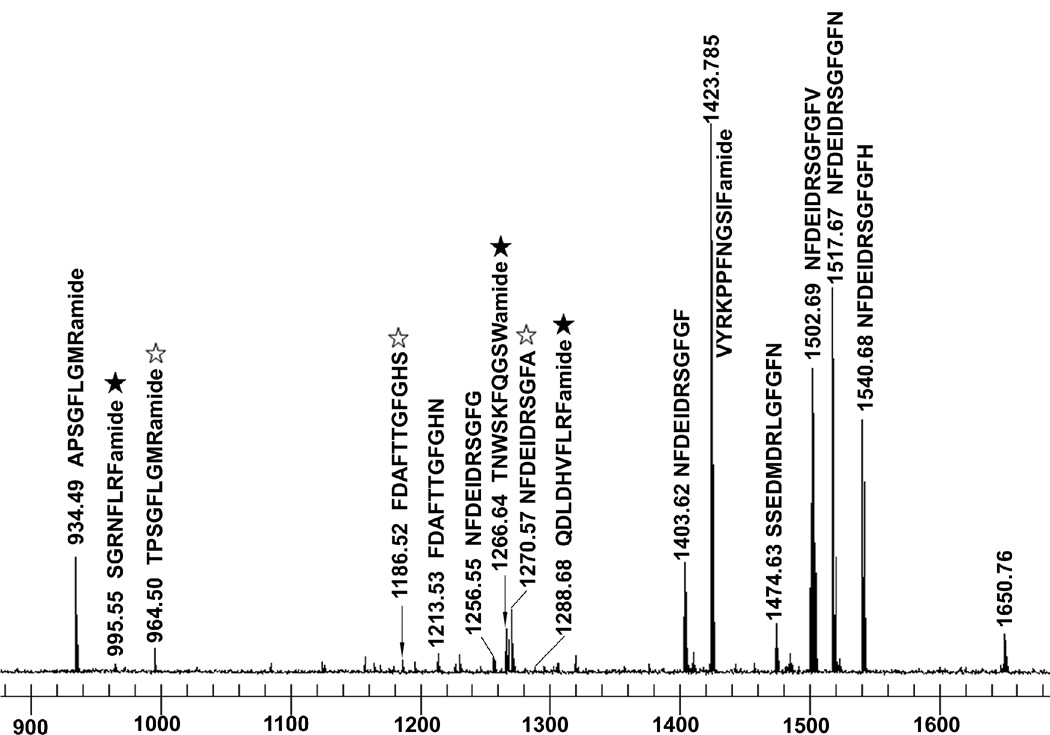
Figure 1.
Direct tissue peptide profiling of H. americanus brain by MALDI FTMS. Signals correspond to the protonated molecular ions, [M+H]+, where M is the molecular weight of each peptide. Peptide identity was assigned based on accurate mass measurement. Novel peptides are indicated by stars with open stars indicating known peptides in other decapods but new to H. americanus and filled stars showing new peptides reported for the first time in this study.
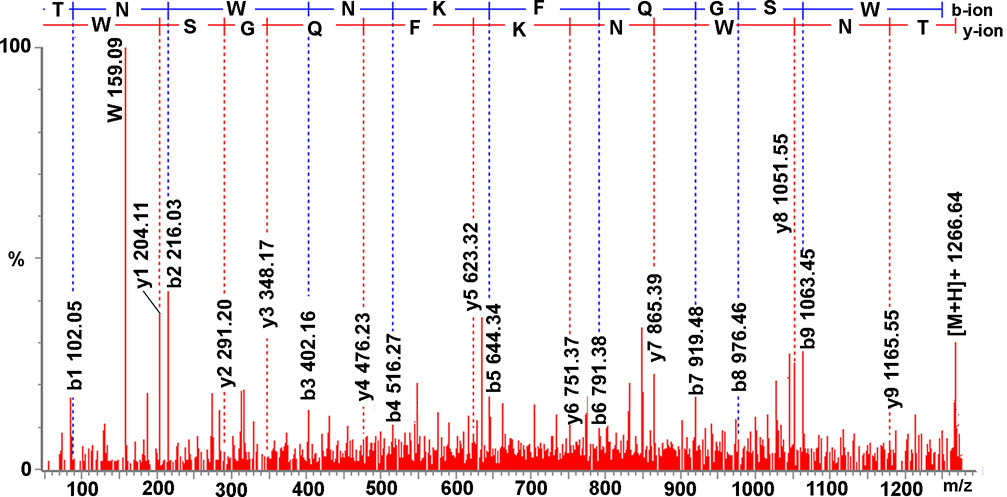
Figure 2.
Collision-induced dissociation spectrum of a de novo sequenced B-type allatostatin, TNWNKFQGSWamide. The sequence-specific b-type and y-type fragment ions and immonium ion characteristic of tryptophan are labeled, with derived amino acid sequence listed above the fragmentation spectrum.
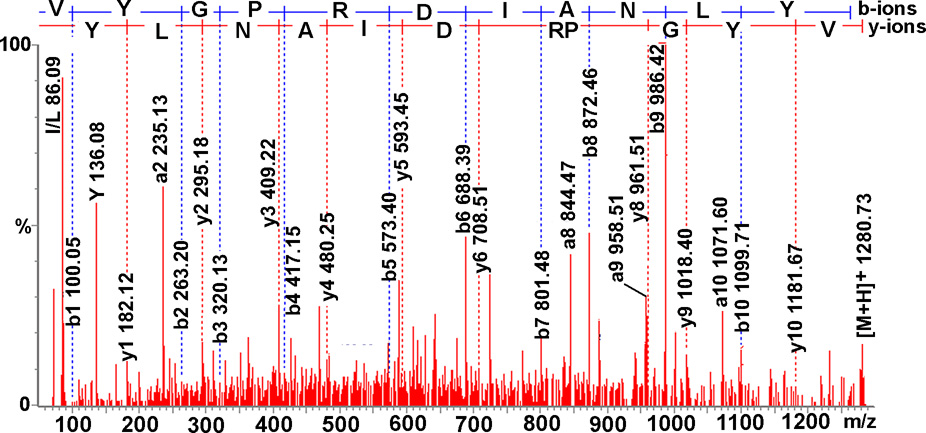
Figure 4.
Collision-induced dissociation spectrum of a de novo sequenced orcokinin-precursor related peptide VYGPRDIANLY. The identities of isoleucine and leucine are determined based on knowledge of orcokinin prepro-hormone sequence (Dickinson et al., 2007a).
Table 1.
Neuropeptides detected in the nervous system and neuroendocrine organs of the American lobster Homarus americanus
| Neuropeptide Family | [M+H]+ | Sequence | FTMS | QTOF | MALDI-TOF | Tissue location |
|---|---|---|---|---|---|---|
| AST-A type | 795.40 | EPYAFGLamide | − | + | − | Br |
| 753.39 | SPYAFGLamide | − | + | − | Br | |
| 810.41 | SGPYAFGLamide | − | + | + | Br/STG | |
| 826.41 | SGPYSFGLamide | − | + | − | Br | |
| 824.43 | ASPYAFGLamide | − | + | + | Br/STG | |
| 794.42 | AGPYAFGLamide | − | + | − | Br/VNC | |
| 822.45 | VGPYAFGLamide | − | + | − | Br | |
| 854.44 | TPSYAFGLamide | − | + | + | Br/STG | |
| 814.30 | SQYTFGLamide | − | + | − | Br | |
| 841.42 | AGGAYSFGLamide | − | + | − | Br | |
| AST-B type | 1266.64 | TNWNKFQGSWamide | + | + | + | Br/PO/STG |
| Corazonin | 1369.65 | pQTFQYSRGWTNamide | + | − | − | Br/ESG |
| CCAP | 956.38 | PFCNAFTGCamide | + | + | + | Br/PO |
| CCAP precursor related peptide | 1074.53 | DIGDLLEGKD* | − | + | − | Br/VNC |
| CHH | 8577.89 | pCHH-A[pQ61−V132amide] | + | − | + | SG/PO/STG |
| 8633.20 | pCHH-B[pQ61−V132amide] | + | − | − | SG | |
| MIH | 8478.76 | pMIH [pQ61−M131] | − | − | + | STG |
| CPRP | 3604.77 | RSVEGASRMEKLLSSSNSPSSTPLGFLSQDHSVN | + | + | + | SG/ESG/PO |
| 3544.81 | RSVEGVSRMEKLLSSISPSSTPLGFLSQDHSVN | + | + | − | SG/ESG | |
| Truncated | 1199.61 | PLGFLSQDHSV | − | + | − | SG |
| CPRPs | 1313.65 | PLGFLSQDHSVN | − | + | − | SG |
| 1149.57 | RSVEGVSRME | − | + | − | SG | |
| 1362.72 | RSVEGASRMEKL | − | + | − | SG | |
| 1503.83 | RSVEGASRMEKLL | − | + | − | SG | |
| 1562.83 | RSVEGASRMEKLLS | − | + | − | SG | |
| 1590.86 | RSVEGVSRMEKLLS | − | + | − | SG | |
| 1649.86 | RSVEGASRMEKLLSS | − | + | − | SG | |
| 1576.85 | RSVEGASRMEKLLT | − | + | − | SG | |
| 1604.88 | RSVEGVSRMEKLLT | − | + | − | SG | |
| FaRPs | 695.40 | NFLRFamide | − | + | − | Br/VNC |
| 965.54 | GGRNFLRFamide | − | + | + | Br/VNC/PO/STG | |
| 995.55 | SGRNFLRFamide | + | + | + | Br/VNC/PO/STG | |
| 1022.56 | GNRNFLRFamide | − | + | + | Br/PO/STG | |
| 1023.55 | GDRNFLRFamide | − | + | + | Br/VNC/PO/STG | |
| 1147.65 | APQRNFLRFamide | − | − | + | STG | |
| 1208.63 | DQNRNFLRFamide | + | + | + | Br/VNC/PO/STG | |
| 1271.68 | pQDLDHVFLRFamide | + | + | + | Br/VNC/ESG/PO/STG | |
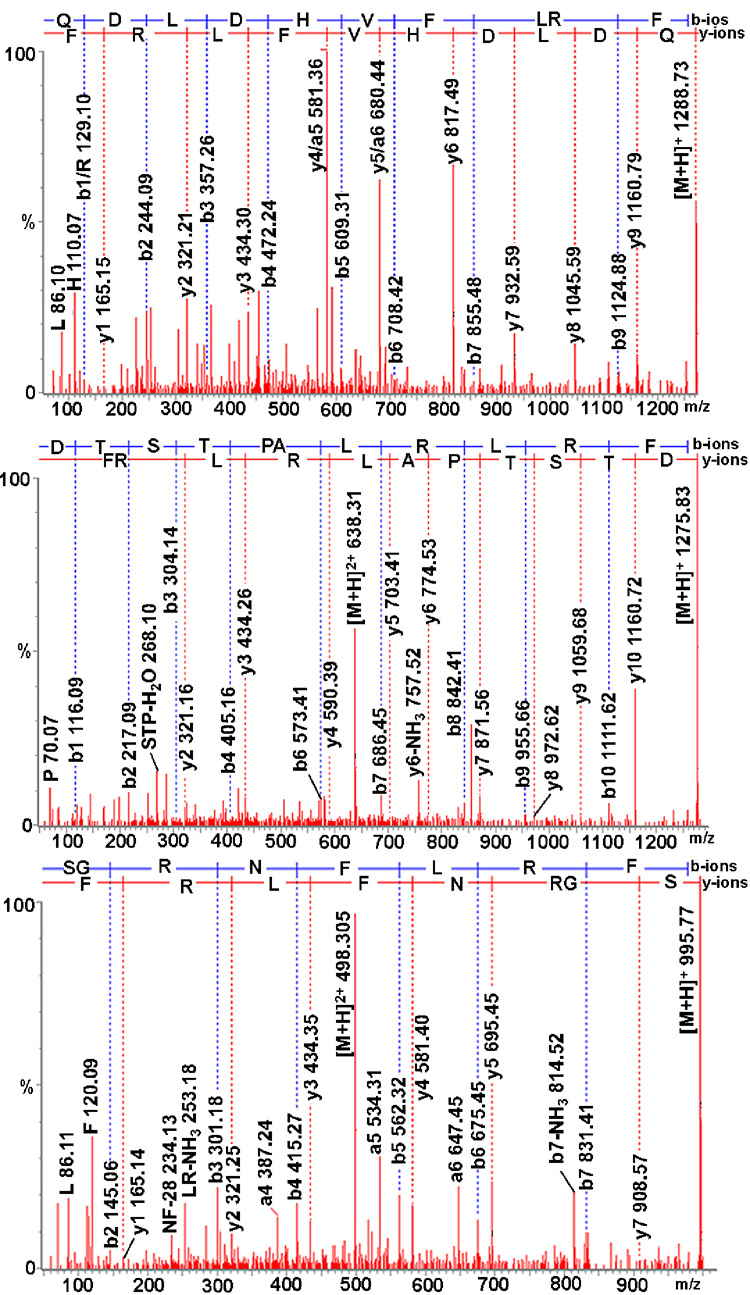
| 1288.68 | QDLDHVFLRFamide | + | + | + | Br/VNC/ESG/STG | |
| 887.56 | PSLRLRFamide | − | + | − | Br/VNC | |
| 1041.63 | GPPSLRLRFamide | + | + | + | Br/VNC/STG | |
| 1105.63 | SMPSLRLRFamide | + | + | + | Br/VNC/SG/PO/STG | |
| 1121.62 | SM(O)PSLRLRFamide | + | + | − | Br/VNC | |
| 1163.67 | FEPSLRLRFamide | − | + | − | VNC | |
| 1275.72 | DTSTPALRLRFamide | + | + | + | Br/VNC/STG | |
| 1069.55 | SDRNYLRFamide | − | + | − | Br | |
| 1104.61 | GAHKNYLRFamide | + | − | + | Br/PO/STG | |
| 1289.64 | GYSDRNYLRFamide | + | + | + | Br/VNC/STG | |
| 1484.66 | GGGEYDDYGHLRFamide‡ | + | − | − | VNC | |
| Orcokinin | 1198.55 | NFDEIDRSGFamide | − | + | + | Br/VNC/SG/STG |
| 1199.53 | NFDEIDRSGF | − | + | − | SG | |
| 1270.57 | NFDEIDRSGFA | + | + | − | Br/VNC/SG/ESG | |
| 1256.55 | NFDEIDRSGFG | + | + | + | Br/VNC/SG/ESG/STG | |
| 1403.62 | NFDEIDRSGFGF | + | + | + | Br/VNC/SG/ESG/STG | |
| 1502.69 | NFDEIDRSGFGFV | + | − | + | Br/VNC/SG/ESG/STG | |
| 1517.67 | NFDEIDRSGFGFN | + | − | + | Br/VNC/SG/ESG/STG | |
| 1540.68 | NFDEIDRSGFGFH | + | + | + | Br/VNC/SG/ESG/STG | |
| 1554.70 | NFDEIDRSSFGFN | + | + | + | Br/VNC/STG | |
| Orcokinin | 1474.63 | SSEDMDRLGFGFN | + | + | + | Br/VNC/SG/ESG/STG |
| related | 1490.62 | SSEDM(O)DRLGFGFN | − | + | − | Br/VNC |
| peptides | 1213.50 | SSEDMDRLGFG | − | + | + | Br/VNC/SG/STG |
| 1227.52 | SSEDMDRLGFA | + | + | − | Br/VNC/SG | |
| 904.42 | FDAFTTGFamide | − | + | − | Br | |
| 1213.53 | FDAFTTGFGHN | + | + | + | Br/VNC/SG/STG | |
| 1186.52 | FDAFTTGFGHS | + | − | − | Br/ESG | |
| 1280.66 | VYGPRDIANLY# | + | + | + | Br/VNC/SG/STG | |
| β-PDH | 1927.03 | NSELINSILGLPKVMNDAamide | − | + | − | Br/SG |
| Proctolin | 649.37 | RYLPT | − | − | + | PO |
| Pyrokinin | 618.37 | FSPRLamide | − | + | − | Br/VNC |
| RPCH | 930.47 | pQLNFSPGWamide | − | + | − | SG |
| SIFamide | 1423.78 | VYRKPPFNGSIFamide | − | + | + | Br/VNC/ESG/STG |
| 1161.65 | RKPPFNGSIFamide | − | + | − | Br | |
| Tachykinin | 950.49 | APSGFLGM(O)Ramide | − | + | − | Br/VNC |
| 980.50 | TPSGFLGM(O)Ramide | − | + | − | Br/VNC | |
| 934.49 | APSGFLGMRamide | − | + | + | Br/VNC/SG/STG | |
| 964.50 | TPSGFLGMRamide | − | + | − | Br/VNC | |
| 766.40 | SGFLGMRamide | − | + | − | Br/VNC | |
| 863.46 | PSGFLGMRamide | − | + | − | Br/VNC | |
| 992.50 | APSGFLGMRG | − | + | − | Br/VNC | |
| Other peptide | 1099.64 | DLPKVDTALK | − | + | − | Br/VNC |
| 858.54 | KPKTEKK | − | + | − | Br | |
| 1254.81 | AVLLPKKTEKK | − | + | − | Br | |
| 1363.67 | EVEEPEAPAPPAK | − | + | − | Br | |
| 1372.79 | LRVAPEEHPVLL | − | + | − | Br | |
| 1103.56 | GPSGGFNGALAR | − | + | − | Br | |
Br, brain; VNC, ventral nerve cord; ESG, eyestalk ganglia; PO, pericardial organ; SG, sinus gland; STG, stomatogastric ganglion. AST, allatostatin; CCAP, crustacean cardioactive peptide; CHH, crustacean hyperglycemic hormone; CPRP, crustacean hyperglycemic hormone precursor related peptide; FaRP, FMRFamide-related peptide; MIH, molt inhibiting hormone; PDH, pigment dispersing hormone; RPCH, red pigment concentrating hormone
Previously known H. americanus peptides are shown in black; peptides previously described from other decapods, but new to H. americanus are shown in blue; novel peptides are shown in red.
Predicted to exist in Homarus gammarus (Chung et al., 2006), but de novo sequenced here for the first time
predicted to exist in H. americanus (Dickinson et al., 2007c), but detected in neural tissue for the first time here
predicted and de novo sequenced from H. americanus simultaneously and independently in Dickinson et al. (2007a)
3.2. A-type allatostatins
Ten peptides possessing -YXFGLamide C-termini (where X is a variable amino acid) were sequenced via ESI-Q-TOF MS/MS from the brain of H. americanus (Table 1). This C-terminus classifies these peptides as members of the A-type allatostatin (A-type AST) family (Stay and Tobe, 2007). Among these peptides, EPYAFGLamide, SPYAFGLamide, SGPYAFGLamide, ASPYAFGLamide, AGPYAFGLamide, TPSYAFGLamide, SQYTFGLamide, and AGGAYSFGLamide are identical in structure to previously identified A-type ASTs from the crabs Carcinus maenas and Cancer productus or the shrimp Penaeus monodon (Duve et al., 1997; Duve et al., 2002; Fu et al., 2005b), but are new to the American lobster. The remaining two isoforms, SGPYSFGLamide and VGPYAFGLamide, are described here for the first time.
Outside of the brain, AGPYAFGLamide was also found in the VNC and SGPYAFGLamide, ASPYAFGLamide and TPSYAFGLamide were detected in the STG (Table 1).
3.3. B-type allatostatins
A novel peptide possessing the C-terminal motif -W(X)6 Wamide (X indicating variable amino acids), which is the hallmark of the B-type allatostatin (B-type AST) family (Stay and Tobe, 2007), was sequenced via ESI-Q-TOF MS/MS from the brain of H. americanus (Fig. 2 and Table 1). The newly characterized B-type AST is TNWNKFQGSWamide, whose C-terminus -WNKFQGSWamide is identical to that of two previously identified Cancer productus B-type allatostatins, i.e. GNWNKFQGSWamide and NWNKFQGSWamide (Fu et al., 2005b). This peptide was also detected via direct tissue/off line HPLC fraction MALDI-FTMS analysis of brain tissue/extract (Fig. 1), and was detected in both the PO and STG using direct tissue MALDI-TOF MS (Table 1).
It should be noted that the initial MS/MS de novo sequencing of TNWNKFQGSWamide revealed ambiguity at the N-terminus, which could have been either TN- or SK-. This sequence discrepancy was resolved using a combination of chemical derivatization and MALDI-FTMS internal calibration. Specifically, upon formaldehyde labeling, a 56 Da mass shift was observed, which indicated that there are two primary amine groups in the peptide, thus suggesting a TN-rather than SK- N-terminus. An N-terminus of TN- rather than SK- was further substantiated by the internal calibration of MALDI-FTMS spectra, which showed mass measurement accuracies (MMAs) of 4.3 versus 24.4 ppm, respectively, for the two possible termini. Thus, TNWNKFQGSWamide, rather than SKWNKFQGSWamide, was derived as the correct sequence.
3.4. Corazonins
The peptide pQTFQYSRGWTNamide, commonly referred to as Arg7-corazonin (Veenstra, 1989), was identified via direct tissue MALDI-FTMS analysis of both brain and eyestalk ganglia tissue fragments (Table 1). Internal calibration of the spectra containing this peptide showed MMA of approximately 1.1 ppm, which strongly supported this attribution. While Arg7-corazonin has been identified previously from the PO of the crab Cancer borealis (Li et al., 2003), here we report the first detection of this peptide in H. americanus.
3.5. Crustacean cardioactive peptide
The peptide PFCNAFTGCamide, commonly referred to as crustacean cardioactive peptide or CCAP (Stangier et al., 1987), was sequenced from the brain of H. americanus via ESI-Q-TOF MS/MS (Table 1). This peptide was also detected in the brain via direct tissue/off-line HPLC fraction MALDI-FTMS analysis and in the PO via direct tissue MALDI-TOF MS (Table 1). While authentic CCAP has been detected/predicted in the nervous systems of a number of decapod species (Stangier et al., 1987; Chung et al., 2006), and has been shown to be capable of modulating neural output in the STNS of H. americanus (Richards and Marder, 2000), this study reports the first direct demonstration that this peptide is present in authentic form in the American lobster.
3.6. CCAP precursor-related peptides
In addition to CCAP itself, another peptide, DIGDLLEGKD, was de novo sequenced from the H. americanus brain and VNC via ESI-Q-TOF MS/MS (Table 1). This peptide is identical to one predicted from the European lobster Homarus gammarus prepro-hormone encoding CCAP (Chung et al., 2006), but is shown to exist for the first time here.
3.7. Crustacean hyperglycemic hormone (CHH) superfamily members
In decapods, the crustacean hyperglycemic hormone (CHH) superfamily consists of a large group of structurally-related 70+ amino acid peptides that possess six conserved cysteine residues which form three characteristic disulfide bridges (Fanjul-Moles, 2006). In addition to the CHHs proper, this superfamily also encompasses moult-inhibiting hormones (MIHs), gonad-inhibiting hormones (GIHs), vitellogenesis-inhibiting hormones (VIHs) and mandibular organ-inhibiting hormones (MOIHs). In H. americanus, multiple CHH superfamily members have been identified/predicted previously, including several isoforms of CHH (Tensen et al., 1991a; Tensen et al., 1991b; de Kleijn et al., 1995), an isoform of MIH (Chang et al., 1990) and an isoform of GIH (de Kleijn et al., 1994). Here, via direct tissue MALDI-FTMS peptides with mass similar to that of H. americanus CHH A and CHH B (Tensen et al., 1991a) were detected in the SG with average mass errors of 0.01% and 0.02% (Table 1). CHH A isoform was also detected in the PO and STG using MALDI-TOF. Likewise, a peptide with a mass corresponding to that of H. americanus moult-inhibiting hormone MIH (Chang et al., 1990) was detected in the STG via direct tissue MALDI-TOF with an average mass error of 0.01% (Table 1).
3.8. CHH precursor-related peptides (CPRPs)
In addition to encoding an isoform of CHH, all CHH precursor proteins also contain an isoform of a second peptide, crustacean hyperglycemic hormone precursor-related peptide or CPRP (Fanjul-Moles, 2006). To date, numerous CPRPs have been identified from decapod species, including several isoforms from H. americanus (Tensen et al., 1991a; Tensen et al., 1991c; Fu et al., 2005a). Here, two of the previously identified H. americanus CPRPs, RSVEGASRMEKLLSSSNSPSSTPLGFLSQDHSVN and RSVEGVSRMEKLLSSISPSSTPLGFLSQDHSVN (Tensen et al., 1991a; Tensen et al., 1991c; Fu et al., 2005a), were sequenced via ESI-Q-TOF MS/MS and direct tissue MALDI-FTMS analysis from both the SG and eyestalk ganglia (Table 1). The former peptide was also detected in the PO via MALDI-TOF MS (Table 1). In addition to these full-length CPRPs, ten truncations were also sequenced from the SG via ESI-Q-TOF MS/MS (Table 1): PLGFLSQDHSV, PLGFLSQDHSVN, RSVEGVSRME, RSVEGASRMEKL, RSVEGASRMEKLL, RSVEGASRMEKLLS, RSVEGVSRMEKLLS, RSVEGASRMEKLLSS, RSVEGASRMEKLLT and RSVEGVSRMEKLLT, the former eight peptides being previously identified from the H. americanus SG (Fu et al., 2005a), with the latter two being identified here for the first time.
3.9. FMRFamide-related peptides
The FMRFamide family is a large and diverse grouping of peptides found in both invertebrates and vertebrates (Zajac and Mollereau, 2006). In arthropods many subfamilies have been identified, including the sulfakinins, the myosuppressins and the neuropeptide Fs (Brown et al., 1999; Nichols, 2003; Garczynski et al., 2006). In our study, 19 FMRFamide-related peptides were sequenced/detected from H. americanus neural tissues using a combination of ESI-Q-TOF MS/MS sequencing, direct tissue/off line HPLC fraction MALDI-FTMS analysis and/or direct tissue MALDI-TOF MS (Table 1). One peptide, GGGEYDDYGHLRFamide, possessed the C-terminal motif -YGHM/LRFamide, which clearly places it within the sulfakinin subfamily. This peptide was previously predicted from an H. americanus prepro-sulfakinin cDNA (Dickinson et al., 2007c), but is shown to exist for the first time here (Table 1). Two de novo sequenced peptides, QDLDHVFLRFamide (Fig. 1 and Fig. 3) and pQDLDHVFLRFamide, possess the C-terminal motif -HVFLRFamide, which places them into the myosuppressin subfamily (Table 1). The presence of the N-terminal pyroglutamine residue in the latter isoform was confirmed by formaldehyde labeling, where no mass shift was observed for the peptide after derivatization. Six peptides, SMPSLRLRFamide, SM(O)PSLRLRFamide (where M(O) represents an oxidized methionine residue), GPPSLRLRFamide, PSLRLRFamide, FEPSLRLRFamide and DTSTPALRLRFamide (Fig. 3), exhibit -RXRFamide C-termini (where X represents a variable residue), which places them into the short neuropeptide F (NPF) subfamily. This subfamily has been proposed to be the invertebrate homolog of the vertebrate neuropeptide Ys (McVeigh et al., 2005). All of the H. americanus NPFs are novel to the species, with all but SMPSLRLRFamide, which was identified previously in both the shrimp Penaeus monodon and the crab Cancer borealis (Sithigorngul et al., 2002; Huybrechts et al., 2003), being sequenced here for the first time. Of the remaining 10 peptides, seven possess C-terminal sequence -NFLRFamide, with the remaining three exhibiting -YLRFamide C-termini. Four of the -NFLRFamide isoforms, GGRNFLRFamide, GNRNFLRFamide, GDRNFLRFamide and APQRNFLRFamide, were identified previously from the PO of the crab Cancer productus (Fu et al., 2005b), but are described for the first time here from H. americanus. Three other NFLRFamide-containing peptides, SGRNFLRFamide (Fig. 1 and Fig. 3), DQNRNFLRFamide and NFLRFamide were de novo sequenced for the first time from any crustacean species. Among the identified -YLRFamide, SDRNYLRFamide and GYSDRNYLRFamide were novel peptides sequenced in this study, while GAHKNYLRFamide has been previously described from several species of Cancer crabs (Cruz-Bermudez et al., 2006), but is new to H. americanus.
Figure 3.
Collision-induced dissociation spectra of three de novo sequenced FMRFamide-related peptides QDLDHVFLRFamide, DTSTPALRLRFamide, and SGRNFLRFamide. All precursor ions are doubly charged. The sequence-specific b-type and y-type fragment ions and immonium ions are labeled.
3.10. Orcokinins
Four full-length orcokinins, NFDEIDRSSFGFN, NFDEIDRSGFGFV (Fig. 1), NFDEIDRSGFGFN (Fig. 1) and NFDEIDRSGFGFH (Fig. 1), and four putative orcokinin truncations, NFDEIDRSGF, NFDEIDRSGFG (Fig. 1), NFDEIDRSGFGF (Fig. 1), and NFDEIDRSGFA (Fig. 1), were characterized from H. americanus neural tissues via ESI-Q-TOF MS/MS, direct tissue/off line HPLC fraction MALDI-FTMS analysis and/or direct tissue MALDI-TOF MS (Table 1). Each of these peptides has been described previously from crustacean neural tissues (Yasuda-Kamatani et al., 2000; Fu et al., 2005), with all but NFDEIDRSSFGFN and NFDEIDRSGFA identified previously from H. americanus (Li et al., 2002; Skiebe et al., 2003; Stemmler et al., 2005; Dickinson et al., 2007a). In addition, a novel amidated truncation, NFDEIDRSGFamide, was de novo sequenced via ESI-Q-TOF MS/MS from the brain, VNC and SG (Table 1). This peptide was also detected via MALDI-TOF MS in the STG (Table 1).
3.11. Orcokinin-precursor-related peptides
Simultaneous with our study, several cDNAs encoding H. americanus prepro-orcokinins were identified and characterized (Dickinson et al., 2007a). In addition to the full-length orcokinin isoforms NFDEIDRSGFGFV, NFDEIDRSGFGFN and NFDEIDRTGFGFH, several other peptides were predicted from the encoded prepro-hormones, including the orcokinin-like peptide SSEDMDRLGFGFN, and the orcomyotropin-related peptide FDAFTTGFGHN, as well as the five additional peptides which bear no sequence homology to any known peptide family: APARSSPQQDAAGYTDGAPV (encoded within prepro-orcokinin I), GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV (encoded within prepro-orcokinin II), VYGPRDIANLY, GDYDVYPE, SAE (Dickinson et al., 2007a). Direct tissue MALDI-FTMS analysis showed that all of these peptides, save APARSSPQQDAAGYTDGAPV, GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV and SAE, are detectable in H. americanus neural tissues (Dickinson et al., 2007a).
In our study, ESI-Q-TOF MS/MS sequencing, direct tissue/off line HPLC fraction MALDI-FTMS analysis and/or MALDI-TOF MS identified SSEDMDRLGFGFN in the brain (Fig. 1), VNC, eyestalk ganglia, sinus gland and STG (Table 1). Moreover, a novel methionine oxidized form of this peptide, SSEDM(O)DRLGFGFN was de novo sequenced via ESI-Q-TOF MS/MS from both the brain and VNC (Table 1). In addition, two related, putative truncations, SSEDMDRLGFG and SSEDMDRLGFA, were also identified by ESI-Q-TOF MS/MS sequencing, direct tissue/off line HPLC fraction MALDI-FTMS analysis and/or MALDI-TOF MS from the brain, VNC and SG; the former peptide was described previously from H. americanus (Fu et al., 2005a), however the latter peptide was de novo sequenced here as a new peptide. SSEDMDRLGFG was also detected in the STG via direct tissue MALDI-TOF MS (Table 1).
The peptide FDAFTTGFamide, described previously from crayfish Orconectes limosus (Dircksen et al., 2000) and named orcomyotropin, was also sequenced from the brain of H. americanus via ESI-Q-TOF MS/MS (Table 1), which was the first identification of this peptide from the American lobster. In addition, FDAFTTGFGHN, was identified in the brain, VNC, SG and STG via ESI-Q-TOF MS/MS sequencing, direct tissue/off line HPLC fraction MALDI-FTMS analysis (Fig. 1) and/or MALDI-TOF MS (Table 1). Interestingly, a second isoform of orcomyotropin-related peptide, FDAFTTGFGHS, was also detected in our study, specifically in the brain and eyestalk ganglia via direct tissue/off line HPLC fraction MALDI-FTMS analysis (Fig. 1 and Table 1). This peptide is identical in sequence to an isoform described previously from numerous brachyuran species, one thalassinidean, and the crayfish Cherax quadricarinatus and Pacifastacus leniusculus (Skiebe et al., 2003), but is described here from H. americanus for the first time.
The orcokinin precursor-related peptide VYGPRDIANLY was identified in the brain, VNC, SG and STG via ESI-Q-TOF MS/MS sequencing (Fig. 4), direct tissue/off line HPLC fraction MALDI-FTMS analysis and/or MALDI-TOF MS (Table 1). This peptide is described for the first time simultaneously in our study and by Dickinson et al. (2007a). It should be noted that the identities of isoleucine and leucine, as reported here, were determined based on knowledge of orcokinin prepro-hormone sequence described in Dickinson et al. (2007a).
3.12. Pigment dispersing hormones
The peptide NSELINSILGLPKVMNDAamide, commonly known as β-pigment dispersing hormone (β-PDH; Rao et al., 1985), was sequenced via ESI QTOF MS/MS from both the SG and brain (Table 1). NSELINSILGLPKVMNDAamide is a previously known H. americanus peptide (Fu et al., 2005a).
3.13. Proctolin
The pentapeptide RYLPT, commonly referred to as proctolin (Brown, 1975; Starratt and Brown, 1975), was dectected in the PO of H. americanus via direct tissue MALDI-TOF MS (Table 1). The presence of RYLPT in the American lobster nervous system has been described previously (Schwarz et al., 1984).
3.14. Pyrokinins
Recently, peptides possessing the C-terminal motif -FXPRLamide (where X is a variable amino acid) were identified from the penaeid shimp Penaeus vannamei and the crab Cancer borealis (Torfs et al., 2001; Saideman et al., 2007). This sequence places them in the pyrokinin/(PBAN) family of peptides (Torfs et al., 2001). Here, we have de novo sequenced the pyrokinin FSPRLamide using ESI-Q-TOF MS/MS from both the brain and VNC of H. americanus (Table 1).
3.15. Red pigment concentrating hormone
The peptide pQLNFSPGWamide, commonly known as red pigment concentrating hormone or RPCH (Fernlund, 1974), was sequenced via ESI-Q-TOF MS/MS from the SG of H. americanus (Table 1). The presence of RPCH in the H. americanus nervous system has been described previously (Jaffe et al., 1984; Fu et al., 2005a; Stemmler et al., 2006).
3.16. SIFamides
The peptide VYRKPPFNGSIFamide (Val1-SIFamide; Christie et al., 2006) was identified via ESI-Q-TOF MS/MS and/or direct tissue/offline HPLC fraction MALDI-FTMS analysis (Fig. 1) from the brain, VNC and eyestalk ganglia of H. americanus (Table 1). This peptide was also detected via MALDI-TOF MS in the STG of this species (Table 1). This isoform of SIFamide is a known H. americanus variant (Christie et al., 2006). In addition to this full-length isoform, the putative truncation RKPPFNGSIFamide was de novo sequenced via ESI-Q-TOF MS/MS from the brain (Table 1).
3.17. Tachykinin-related peptides
Two full-length tachykinin-related peptide (TRP) isoforms, APSGFLGMRamide and TPSGFLGMRamide, their methionine oxidized forms APSGFLGM(O)Ramide and TPSGFLGM(O)Ramide, and two putative truncated forms, PSGFLGMRamide and SGFLGMRamide, were sequenced via ESI-Q-TOF MS/MS from the brain and VNC of H. americanus (Table 1). Likewise, the putative precursor of APSGFLGMRamide, APSGFLGMRG, was sequenced from both tissues via ESI-Q-TOF MS/MS (Table 1). APSGFLGMRamide was also sequenced from the SG via ESI-Q-TOF MS/MS and was detected via direct tissue MALDI-TOF MS in the STG (Table 1). APSGFLGMRamide, TPSGFLGMRamide, APSGFLGMRG, and SGFLGMRamide are previously described crustacean peptides (Christie et al., 1997; Stemmler et al., 2007b), though all except APSGFLGMRamide (Stemmler et al., 2007a) are described here from the lobster for the first time. PSGFLGMRamide and the methionine oxidized isoforms APSGFLGM(O)Ramide and TPSGFLGM(O)Ramide were de novo sequenced.
3.18. Other peptides
In addition to the above mentioned peptides, six peptides that do not fit into any known peptide family were de novo sequenced via ESI-Q-TOF MS/MS from the brain of H. americanus: DLPKVDTALK, KPKTEKK, AVLLPKKTEKK, EVEEPEAPAPPAK, LRVAPEEHPVLL and GPSGGFNGALAR (Table 1). DLPKVDTALK was also sequenced from the VNC via this technique (Table 1).
4. Discussion
4.1. Peptide discovery in H. americanus using high-resolution mass profiling and tandem mass spectrometric sequencing
The American lobster H. americanus is arguably one of the most important decapod species, given its combined economic and scientific significance. As investigations of peptidergic control of behavior are among the major uses of this species scientifically, and are of critical importance for understanding the physiological control of this species in terms of its fishery, it is significant to note that little work has focused on identifying the lobster neuropeptidome prior to this and several contemporaneous molecular studies (Dickinson et al., 2007a; Dickinson et al., 2007b; Dickinson 2007c). Here, we have used a combination of mass spectral techniques to identify the peptide complement present in the nervous system and neuroendocrine organs of H. americanus, complementing and augmenting the ongoing molecular analyses.
In our study, neuropeptides were identified using a strategy combining MALDI-based high resolution mass profiling (direct tissue and off-line HPLC analysis) and nanoscale biochemical separation/derivatization coupled to tandem mass spectrometric sequencing. Specifically, the highly sensitive and accurate mass measurements provided by MALDI-FTMS and MALDI-TOF-MS (both performed with internal calibration) were used to identify known peptides based on predicted m/z, while the sequencing power of nanoLC-ESI-Q-TOF MS/MS was exploited to confirm the identity of the known peptides and to de novo sequence novel ones. This combined approach resulted in the identification of 84 peptides from 17 peptide families in the lobster nervous system, including 27 previously known H. americanus peptides (e.g. β-PDH [Fu et al., 2005a], proctolin [Schwarz et al., 1984] and Val1-SIFamide [Christie et al., 2006]), 23 peptides identified in other species, but new to the American lobster (e.g. Arg7-corazonin [Li et al., 2003] and crustacean cardioactive peptide [Stangier et al., 1987; Chung et al., 2006]), and 34 new peptides that were de novo sequenced/detected for the first time (e.g. the pyrokinin isoform FSPRLamide). The truncated forms and the methionine oxidized forms were also included. The origin of these peptides is unknown. It could be either enzymatic processing product or due to sample preparation artifact. Collectively, these data have nearly tripled the number of fully characterized H. americanus neuropeptides, and thus provide a stronger framework for future investigations of the physiological roles these molecules play in this species. Moreover, in combination with several recently constructed H. americanus cDNA libraries with ESTs (i.e. Stepanyan et al., 2006; Towle and Smith, 2006), these data will provide a strong foundation for future peptide discovery, as well as studies directed at the expression and regulation of peptide modulators/hormones in the American lobster.
4.2. Most, but not all, known H. americanus peptides were detected using combined mass spectral analyses
As stated earlier, approximately two dozen neuropeptides had been characterized from H. americanus prior to our study, and the majority of those were re-identified here. Interestingly, however, several well-known lobster peptides were not detected in our study, including the FMRFamide-related peptides TNRNFLRFamide and SDRNFLRFamide (Trimmer et al., 1987). The lack of detection of these two peptides is of particular note as members of the FMRFamide family were by far the largest single group of peptides identified in our study (19 in total), and the tissues assayed included those used for the original biochemical isolation and subsequent sequence analysis of both peptides.
The reason for the lack of detection of TNRNFLRFamide and SDRNFLRFamide in our study remains unknown. It is possible that the different approaches to peptide discovery used here versus those employed by Trimmer et al (1987) may be at play; however both peptides possess structures that should make them readily ionizable and detectable via the MS methods we used, and both freshly dissected tissues and tissue extracts were assayed. Moreover, FMRFamide-related peptides with very similar structures to both TNRNFLRFamide and SDRNFLRFamide were identified, e.g. GNRNFLRFamide, GDRNFLRFamide and SDRNYLRFamide. It is possible that the three N-terminal residues in each peptide were in some way labile under the conditions used here and that the two peptides were truncated to NFLRFamide, which we did sequence via ESI-Q-TOF MS/MS. Again, this seems unlikely given the sequencing of SDRNYLRFamide, which possesses the same three N-terminal residues seen in SDRNFLRFamide. It is also possible that TNRNFLRFamide and SDRNFLRFamide are regional-specific variants that were not present in the population of animals used in our study; however, this possibility seems remote as some of the animals used in our study were from the same general geographic area as those used by Trimmer et al (1987). Clearly additional experiments will be needed to determine why these two well-known H. americanus peptides were absent in our study; however, their lack of detection does impart a cautionary note that, while extensive, the catalog of H. americanus peptides described here undoubtedly represents only subset of the total peptidome of H. americanus as a species, missing peptides that possess structures that are not readily ionizable, one in very minute abundance, as well as those that may be population- and/or individual-specific variants.
4.3. Correspondence of mass spectrally-identified peptides with previous immunohistochemical data
While sequence data on H. americanus neuropeptides was limited prior to our study, many immunohistochemical studies had been conducted on neuronal tissues from this species, and thus a wealth of data exists on the putative localization of many of the peptides identified here. For the most part, there is good correspondence between the tissues from which we identified peptides and the extant anatomical data concerning their putative tissue distributions (e.g. Siwicki and Bishop, 1986; Kobierski et al., 1987; Goldberg et al., 1988; Mortin and Marder, 1991; Bungart et al., 1994; Skiebe, 1999; Li et al., 2002; Christie et al., 2005), however, discrepancies were also noted (e.g. Marder et al., 1986; Bungart et al., 1994; Skiebe, 1999; Li et al., 2002; Pulver and Marder, 2002). For example, in a recent study, A-type AST-, CCAP-, FMRFamide-related peptide-, orcokinin-, proctolin- and TRP-like immunoreactivities were seen in the H. americanus PO (Pulver and Marder, 2002). While we detected CCAP, proctolin and multiple isoforms of FMRFamide-related peptide in this tissue, no A-type ASTs, orcokinins or TRPs were identified. It is possible that our failure to identify members of these peptide families via mass spectrometry resulted from their low abundance in the PO and a lack of sufficient sensitivity to detect them via our MS instrumentation. It is also possible that the endogenous isoforms of these families possess sequences that do not ionize efficiently and hence were not detected on our instruments. Alternatively, it is possible that no authentic members of the A-type AST, orcokinin and TRP families exist in the H. americanus PO, and that the antibodies used for their detection in the PO were simply cross-reacting with structurally unrelated peptides, though this scenario seems unlikely for at least the A-type ASTs as the PO has been shown to be a rich source of them in other decapods (e.g. Fu et al., 2005b). Clearly additional studies will be required to resolve these and other discrepancies that exist between our mass spectral and previous anatomical studies.
4.4. The presence of B-type allatostatins appears broadly conserved in decapod species
The B-type allatostatins are a family of peptides possessing the C-terminal motif - WX6Wamide (where X6 is six variable amino acids; Stay and Tobe, 2007). While these peptides bear no sequence identity to the A-type allatostatins (characterized by -Y/FXFGLamide C-termini [where X is also a variable amino acid]), they do inhibit the biosynthesis of juvenile hormone in crickets, where they were first identified, and hence have been named accordingly due to this function (Stay and Tobe, 2007). Since their original description, B-type allatostatins have been identified in a number of other insect groups, though they do not necessarily appear to inhibit juvenile hormone synthesis in many of these species (Stay and Tobe, 2007).
Recently, we demonstrated the existence of peptides exhibiting the -WX6Wamide C-terminal motif in decapod crustaceans (Fu et al., 2005b). Specifically, we characterized several B-type allatostatins from the pericardial organs of the crabs Cancer productus and Cancer borealis (Fu et al., 2005b; Fu et al., 2007). In the latter species, the peptide VPNDWAHFRGSWamide was identified and shown to exhibit inhibitory action on the pyloric motor pattern, which drives the rhythmic filtering of food between the foregut and midgut (Fu et al., 2007). Interestingly this inhibitory action was described as essentially identical to that of A-type allatostatin, though, again, the two families share no sequence homology (Fu et al., 2007). The presence of bioactive B-type allatostatins in Brachyurans raised the question as to the prevalence of these peptides in members of other decapod infraorders. In our study, we identified the peptide TNWNKFQGSWamide from H. americanus, showing that B-type allatostatin isoforms are present in at least some Astacidean decapods. Moreover, we also identified an EST encoding a putative B-type AST precursor from the shrimp Marsupenaeus japonicus during a recent in silico search for unannotated neuropeptide encoding transcripts (Christie et al., 2008). Thus, with the identification of B-type ASTs in members of three decapod infraorders, which include both a basal and two derived taxa, it would appear that this family of peptides is broadly conserved within the decapods.
4.5. The presence of multiple tachykinin-related peptides is not limited to Brachyuran species
The invertebrate homologs of the vertebrate tachykinins are characterized by the C-terminal motif –FX1GX2Ramide, where the Xs are variable amino acids (Nässel, 1999). In insects, large families of species-specific TRP isoforms are common (Nässel, 1999), while in decapod crustaceans only two full-length isoforms are known: the ubiquitously conserved APSGFLGMRamide and TPSGFLGMRamide, thus far identified only from Cancer crabs, and not detected by molecular cloning or mass spectrometry in either the crayfish Procambarus clarkii or the spiny lobster Panulirus interruptus (Christie et al., 1997; Yasuda-Kamatani and Yasuda, 2004; Stemmler et al., 2007a; Stemmler et al., 2007b). While the phylogeny of the decapods is controversial, it is generally agreed that the Brachyurans are more derived than either the Palinurans (spiny lobsters) or the Astacideans (clawed lobsters and freshwater crayfish). Thus, it was proposed that APSGFLGMRamide represented the common ancestral decapod TRP with TPSGFLGMRamide being an evolutionary advancement in the derived Brachyurans (Stemmler et al., 2007b).
Here, we have identified both APSGFLGMRamide and TPSGFLGMRamide in the nervous system of H. americanus. This finding is in sharp contrast to the hypothesis proposed above, as this species is a member of the same infraorder as P. clarkii (Astacidea), where no TPSGFLGMRamide is present (Yasuda-Kamatani and Yasuda, 2004). Moreover, it is generally agreed that the freshwater crayfish are derived from marine ancestors (Crandall et al., 2000), and hence the derived Astacidean lacks the peptide whereas the more basal species is the one possessing it. Clearly additional mass spectral and molecular studies will be needed to determine the evolutionary origin and complexity of the tachykinin-related peptides in decapods.
4.6. Identification of orcokinin and orcomyotropin-related peptide isoforms not encoded on the known H. americanus prepro-hormone
As stated in Sections 3.10 and 3.11, cDNAs encoding H. americanus prepro-orcokinin were recently identified and characterized (Dickinson et al., 2007a). Present within the predicted prepro-hormones are the orcokinin isoforms NFDEIDRSGFGFH, NFDEIDRSGFGFN and NFDEIDRSGFGFV, the orcokinin-like peptide SSEDMDRLGFGFN, and the orcomyotropin-related peptide FDAFTTGFGHN, as well as the five additional peptides which bare no sequence homology to any known peptide family: APARSSPQQDAAGYTDGAPV or GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV, VYGPRDIANLY, GDYDVYPE, SAE (Dickinson et al., 2007a). Direct tissue MALDI-FTMS analysis of H. americanus neural tissues confirmed the production of all peptides from the prepro-hormones except APARSSPQQDAAGYTDGAPV and GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV (Dickinson et al., 2007a).
In our study, the same complement of peptides identified by Dickinson et al. (2007a), save GDYDVYPE and SAE, was identified from the lobster CNS. In addition, we also identified the full-length orcokinin NFDEIDRSSFGFN, the orcokinin truncation NFDEIDRSGFA, the C-terminally truncated orcokinin-like peptide SSEDMDRLGFA and the orcomyotropin-related peptide FDAFTTGFGHS within the H. americanus nervous system. NFDEIDRSSFGFN, NFDEIDRSGFA and FDAFTTGFGHS have been identified previously from other decapod species (Bungart et al., 1995; Yasuda-Kamatani and Yasuda, 2000), whereas SSEDMDRLGFA is novel. The identification of these four peptides in H. americanus is curious as none are encoded in any of the fully characterized prepro-orcokinins (Dickinson et al., 2007a), and none have been identified in previous mass spectral studies focusing on this species (Li et al., 2002; Skiebe et al., 2003; Fu et al., 2005a; Stemmler et al., 2005; Dickinson et al., 2007a). One possible origin for the discrepancy between previous molecular and mass spectral analysis, and the data we present here, is that additional alleles of the orcokinin prepro-hormone exist. If one or more alleles encoding NFDEIDRSSFGFN, NFDEIDRSGFA, FDAFTTGFGHS and/or SSEDMDRLGFA are present at low frequency in the population, it is possible that individuals possessing them might have been missed during the previous studies, which used far fewer animals than were included in the starting material used for our study. Direct tissue MALDI-FTMS of individual sinus glands of the crab C. productus has shown that this situation exists for isoforms of CPRP in this species, with individual crabs exhibiting one of three distinct patterns of CPRP isoforms, one pattern being present over 60% of the population while another was found in only 10% of the individuals assayed (Stemmler et al., 2007c). It is also possible that variant isoforms are derived from individual-specific mutations in the gene(s) encoding prepro-orcokinin and that single variant animals were included in our pooled tissue. Mass spectrometric detection of an individual-specific peptide has recently been described for a variant of Gly1-SIFamide, i.e. Gly1-PIFamide, in the hermit crab Pagurus pollicarus (Cashman et al., 2007).
Clearly additional studies will be needed to determine the origin of the orcokinin and orcokinin-precursor related peptides detected here, but not reported in the earlier studies (Li et al., 2002; Skiebe et al., 2003; Fu et al., 2005a; Stemmler et al., 2005; Dickinson et al., 2007a). Regardless, our detection of them again raises a cautionary note with regard to interpreting the presence of large families of peptides in a species as being ubiquitously present in all individuals of that species rather than a result of the inclusion of animals potentially possessing distinct peptidomes in the starting material used for a peptide survey (also see 4.2).
Acknowledgements
We thank the University of Wisconsin School of Pharmacy Analytical Instrumentation Center for access to the MALDI-FTMS instrument. We wish to thank Dr. Michael Nusbaum (University of Pennsylvania School of Medicine) for his generous donation of the Rainin Dynamax HPLC system to the Li laboratory. Dr. Peter O’Connor from Boston University is thanked for the use of BUDA software to make FTMS figures. Christopher Cazzolla from the Goy laboratory is acknowledged for technical assistance with lobster tissue collection. L.L. acknowledges financial support provided by the University of Wisconsin School of Pharmacy, Wisconsin Alumni Research Foundation, National Science Foundation CAREER Award CHE-0449991, National Institutes of Health through grant 1R01DK071801 and a research fellowship from the Alfred P. Sloan Foundation. M.F.G. acknowledges support by a National Science Foundation grant (IBN 0236320), and A.E.C. thanks grants from the National Center for Research Resources’ Maine INBRE Program (NIH P20 RR-016463 to Mount Desert Island Biological Laboratory [MDIBL]) and the National Science Foundation’s Research Experience for Undergraduates Program (NSF DBI-0453391; to the MDIBL REU site), as well as the MDIBL High School Fellowship Research Program and an MDIBL New Investigator Award (from the Salisbury Cove Research Fund provided through the Thomas H. Maren Foundation).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown BE. Proctolin: a peptide transmitter candidate in insects. Life Sci. 1975;17:1241–1252. doi: 10.1016/0024-3205(75)90133-2. [DOI] [PubMed] [Google Scholar]
- Brown MR, Crim JW, Arata RC, Cai HN, Chun C, Shen P. Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides. 1999;20:1035–1042. doi: 10.1016/s0196-9781(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Bungart D, Dircksen H, Keller R. Quantitative determination and distribution of the myotropic neuropeptide orcokinin in the nervous system of astacidean crustaceans. Peptides. 1994;15:393–400. doi: 10.1016/0196-9781(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Bungart D, Hilbich C, Dircksen H, Keller R. Occurrence of analogues of the myotropic neuropeptide orcokinin in the shore crab, Carcinus maenas: evidence for a novel neuropeptide family. Peptides. 1995;16:67–72. doi: 10.1016/0196-9781(94)00145-v. [DOI] [PubMed] [Google Scholar]
- Cashman CR, Hsu YA, Messinger DI, Christie AE, Dickinson PS, de la Iglesia HO, Stemmler EA. Program No. 140.4. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007; 2007. Identification of individual-specific variations in peptide complement of crustacean neural tissues using direct tissue MALDI-FTMS. Online. [Google Scholar]
- Chang ES, Prestwich GD, Bruce MJ. Amino acid sequence of a peptide with both molt-inhibiting and hyperglycemic activities in the lobster, Homarus americanus. Biochem. Biophys. Res. Commun. 1990;171:818–826. doi: 10.1016/0006-291x(90)91219-i. [DOI] [PubMed] [Google Scholar]
- Christie AE, Cashman CR, Brennan HR, Ma M, Sousa GL, Li L, Stemmler EA, Dickinson PS. Identification of putative crustacean neuropeptides using in silico analyses of publicly accessible expressed sequence tags. Gen. Comp. Endocrinol. Submitted. 2008 doi: 10.1016/j.ygcen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Christie AE, Lundquist CT, Nassel DR, Nusbaum MP. Two novel tachykinin-related peptides from the nervous system of the crab Cancer borealis. J. Exp. Biol. 1997;200:2279–2294. doi: 10.1242/jeb.200.17.2279. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stemmler EA, Peguero B, Messinger DI, Provencher HL, Scheerlinck P, Hsu YW, Guiney ME, de la Iglesia HO, Dickinson PS. Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val1-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus. J. Comp. Neurol. 2006;496:406–421. doi: 10.1002/cne.20932. [DOI] [PubMed] [Google Scholar]
- Chung JS, Wilcockson DC, Zmora N, Zohar Y, Dircksen H, Webster SG. Identification and developmental expression of mRNAs encoding crustacean cardioactive peptide (CCAP) in decapod crustaceans. J. Exp. Biol. 2006;209:3862–3872. doi: 10.1242/jeb.02425. [DOI] [PubMed] [Google Scholar]
- Clarac F, Pearlstein E. Invertebrate preparations and their contribution to neurobiology in the second half of the 20th century. Brain Res. Rev. 2007;54:113–161. doi: 10.1016/j.brainresrev.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Cooke IM. Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol. Bull. 2002;202:108–136. doi: 10.2307/1543649. [DOI] [PubMed] [Google Scholar]
- Crandall KA, Harris DJ, Fetzner JW. The monophyletic origin of freshwater crayfish estimated from nuclear and mitochondrial DNA sequences. Proc. R. Soc. Lond. B. 2000;267:1679–1686. doi: 10.1098/rspb.2000.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Bermudez ND, Fu Q, Kutz-Naber KK, Christie AE, Li L, Marder E. Mass spectrometric characterization and physiological actions of GAHKNYLRFamide, a novel FMRFamide-like peptide from crabs of the genus Cancer. J. Neurochem. 2006;97:784–799. doi: 10.1111/j.1471-4159.2006.03747.x. [DOI] [PubMed] [Google Scholar]
- de Kleijn DP, de Leeuw EP, van den Berg MC, Martens GJ, Van Herp F. Cloning and expression of two mRNAs encoding structurally different crustacean hyperglycemic hormone precursors in the lobster Homarus americanus. Biochim. Biophys. Acta. 1995;1260:62–66. doi: 10.1016/0167-4781(94)00173-z. [DOI] [PubMed] [Google Scholar]
- de Kleijn DP, Sleutels FJ, Martens GJ, Van Herp F. Cloning and expression of mRNA encoding prepro-gonad-inhibiting hormone (GIH) in the lobster Homarus americanus. FEBS Lett. 1994;353:255–258. doi: 10.1016/0014-5793(94)01055-2. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Cashman CR, Rus S, Brennan HR, Stemmler EA, McClintock TS, Christie AE. Molecular and mass spectral analyses of orcokinins and orcokinin precursor-related peptides in the American Lobster Homarus americanus and red swamp crayfish Procambarus clarkii. Gen. Comp. Endocrinol. Submitted. 2007a doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS, Stemmler EA, Cashman CR, Brennan HR, Dennison B, Huber KE, Peguero B, Rabacal W, Goiney CC, Smith CM, Towle DW, Christie AE. SIFamide peptides in clawed lobsters and freshwater crayfish (Crustacea, Decapoda, Astacidea): a combined molecular, mass spectrometric and electrophysiological investigation. Gen. Comp. Endocrinol. Submitted. 2007b doi: 10.1016/j.ygcen.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Stevens JS, Rus S, Brennan HR, Goiney CC, Smith CM, Li L, Towle DW, Christie AE. Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus. J. Exp. Biol. 2007c;210:2278–2289. doi: 10.1242/jeb.004770. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Burdzik S, Sauter A, Keller R. Two orcokinins and the novel octapeptide orcomyotropin in the hindgut of the crayfish Orconectes limosus: identified myostimulatory neuropeptides originating together in neurones of the terminal abdominal ganglion. J. Exp. Biol. 2000;203:2807–2818. doi: 10.1242/jeb.203.18.2807. [DOI] [PubMed] [Google Scholar]
- Duve H, Johnsen AH, Maestro JL, Scott AG, Jaros PP, Thorpe A. Isolation and identification of multiple neuropeptides of the allatostatin superfamily in the shore crab Carcinus maenas. Eur. J. Biochem. 1997;250:727–734. doi: 10.1111/j.1432-1033.1997.00727.x. [DOI] [PubMed] [Google Scholar]
- Duve H, Johnsen AH, Scott AG, Thorpe A. Allatostatins of the tiger prawn, Penaeus monodon (Crustacea: Penaeidea) Peptides. 2002;23:1039–1051. doi: 10.1016/s0196-9781(02)00035-9. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles ML. Biochemical and functional aspects of crustacean hyperglycemic hormone in decapod crustaceans: review and update. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;142:390–400. doi: 10.1016/j.cbpc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Fernlund P. Structure of the red-pigment-concentrating hormone of the shrimp, Pandalus borealis. Biochim. Biophys. Acta. 1974;371:304–311. doi: 10.1016/0005-2795(74)90027-0. [DOI] [PubMed] [Google Scholar]
- Fu Q, Goy MF, Li L. Identification of neuropeptides from the decapod crustacean sinus glands using nanoscale liquid chromatography tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2005a;337:765–778. doi: 10.1016/j.bbrc.2005.09.111. [DOI] [PubMed] [Google Scholar]
- Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005b;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- Fu Q, Tang LS, Marder E, Li L. Mass spectrometric characterization and physiological actions of VPNDWAHFRGSWamide, a novel B type allatostatin in the crab, Cancer borealis. J. Neurochem. 2007;101:1099–1107. doi: 10.1111/j.1471-4159.2007.04482.x. [DOI] [PubMed] [Google Scholar]
- Garczynski SF, Brown MR, Crim JW. Structural studies of Drosophila short neuropeptide F: Occurrence and receptor binding activity. Peptides. 2006;27:575–582. doi: 10.1016/j.peptides.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Nusbaum MP, Marder E. Substance P-like immunoreactivity in the stomatogastric nervous systems of the crab Cancer borealis and the lobsters Panulirus interruptus and Homarus americanus. Cell Tissue Res. 1988;252:515–522. doi: 10.1007/BF00216638. [DOI] [PubMed] [Google Scholar]
- Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav. Evol. 1997;50 Suppl 1:60–68. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- Huybrechts J, Nusbaum MP, Bosch LV, Baggerman G, De Loof A, Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab, Cancer borealis. Biochem. Biophys. Res. Commun. 2003;308:535–544. doi: 10.1016/s0006-291x(03)01426-8. [DOI] [PubMed] [Google Scholar]
- Jaffe H, Loeb M, Hayes DK, Talbot N, Garvick S. Isolation of crustacean erythrophore concentrating hormone from nerve tissue of Homarus americanus. Comp. Biochem. Physiol. C. 1984;78:397–401. doi: 10.1016/0742-8413(84)90105-1. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Kobierski LA, Beltz BS, Trimmer BA, Kravitz EA. FMRFamide-like peptides of Homarus americanus: distribution, immunocytochemical mapping, and ultrastructural localization in terminal varicosities. J. Comp. Neurol. 1987;266:1–15. doi: 10.1002/cne.902660102. [DOI] [PubMed] [Google Scholar]
- Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J. Neurochem. 2003;87:642–645. doi: 10.1046/j.1471-4159.2003.02031.x. [DOI] [PubMed] [Google Scholar]
- Li L, Ma M, Chen R, Christie AE. Program No. 315.17. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007; 2007. Peptidomic analyses of the central nervous systems of the American lobster Homarus americanus and the European green crab Carcinus maenas. Online. [Google Scholar]
- Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E. Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J. Comp. Neurol. 2002;444:227–244. doi: 10.1002/cne.10139. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu. Rev. Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Hooper SL, Siwicki KK. Modulatory action and distribution of the neuropeptide proctolin in the crustacean stomatogastric nervous system. J. Comp. Neurol. 1986;243:454–467. doi: 10.1002/cne.902430403. [DOI] [PubMed] [Google Scholar]
- McVeigh P, Kimber MJ, Novozhilova E, Day TA. Neuropeptide signalling systems in flatworms. Parasitology. 2005;131:S41–S55. doi: 10.1017/S0031182005008851. [DOI] [PubMed] [Google Scholar]
- Mortin LI, Marder E. Differential distribution of beta-pigment-dispersing hormone (beta PDH)-like immunoreactivity in the stomatogastric nervous system of five species of decapod crustaceans. Cell Tissue Res. 1991;265:19–33. doi: 10.1007/BF00318135. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Tachykinin-related peptides in invertebrates: a review. Peptides. 1999;20:141–158. doi: 10.1016/s0196-9781(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Nichols R. Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides. Annu. Rev. Entomol. 2003;48:485–503. doi: 10.1146/annurev.ento.48.091801.112525. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Marder E. Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus. J. Comp. Neurol. 2002;451:79–90. doi: 10.1002/cne.10331. [DOI] [PubMed] [Google Scholar]
- Rao KR, Riehm JP, Zahnow CA, Kleinholz LH, Tarr GE, Johnson L, Norton S, Landau M, Semmes OJ, Sattelberg RM, Jorenby WH, Hintz MF. Characterization of a pigment-dispersing hormone in eyestalks of the fiddler crab Uca pugilator. Proc. Natl. Acad. Sci. USA. 1985;82:5319–5322. doi: 10.1073/pnas.82.16.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KS, Marder E. The actions of crustacean cardioactive peptide on adult and developing stomatogastric ganglion motor patterns. J. Neurobiol. 2000;44:31–44. [PubMed] [Google Scholar]
- Saideman SR, Ma M, Kutz-Naber KK, Cook A, Torfs P, Schoofs L, Li L, Nusbaum MP. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- Schwarz TL, Lee GM, Siwicki KK, Standaert DG, Kravitz EA. Proctolin in the lobster: the distribution, release, and chemical characterization of a likely neurohormone. J. Neurosci. 1984;4:1300–1311. doi: 10.1523/JNEUROSCI.04-05-01300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithigorngul P, Pupuem J, Krungkasem C, Longyant S, Chaivisuthangkura P, Sithigorngul W, Petsom A. Seven novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant tiger prawn Penaeus monodon. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002;131:325–337. doi: 10.1016/s1096-4959(01)00499-7. [DOI] [PubMed] [Google Scholar]
- Skiebe P. Allatostatin-like immunoreactivity in the stomatogastric nervous system and the pericardial organs of the crab Cancer pagurus, the lobster Homarus americanus, and the crayfish Cherax destructor and Procambarus clarkii. J. Comp. Neurol. 1999;403:85–105. [PubMed] [Google Scholar]
- Skiebe P, Dreger M, Borner J, Meseke M, Weckwerth W. Immunocytochemical and molecular data guide peptide identification by mass spectrometry: orcokinin and orcomyotropin-related peptides in the stomatogastric nervous system of several crustacean species. Cell. Mol. Biol. (Noisy-le-grand) 2003;49:851–871. [PubMed] [Google Scholar]
- Stangier J, Hilbich C, Beyreuther K, Keller R. Unusual cardioactive peptide (CCAP) from pericardial organs of the shore crab Carcinus maenas. Proc. Natl. Acad. Sci. USA. 1987;84:575–579. doi: 10.1073/pnas.84.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starratt AN, Brown BE. Structure of the pentapeptide proctolin, a proposed neurotransmitter in insects. Life Sci. 1975;17:1253–1256. doi: 10.1016/0024-3205(75)90134-4. [DOI] [PubMed] [Google Scholar]
- Stay B, Tobe SS. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 2007;52:277–299. doi: 10.1146/annurev.ento.51.110104.151050. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Cashman CR, Messinger DI, Dickinson PS, Christie AE. High mass resolution direct-tissue MALDI-FTMS reveals broad conservation of three neuropeptides (APSGFLGMRamide, GYRKPPFNGSIFamide and pQDLDHVFLRFamide) across members of seven decapod crustaean infraorders. Peptides. 2007a doi: 10.1016/j.peptides.2007.08.019. In press. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Gardner NP, Guiney ME, Bruns EA, Dickinson PS. The detection of red pigment-concentrating hormone (RPCH) in crustacean eyestalk tissues using matrix-assisted laser desorption/ionization-Fourier transform mass spectrometry: [M + Na]+ ion formation in dried droplet tissue preparations. J. Mass Spectrom. 2006;41:295–311. doi: 10.1002/jms.989. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Hsu YW, Cashman CR, Messinger DI, de la Iglesia HO, Dickinson PS, Christie AE. Direct tissue MALDI-FTMS profiling of individual Cancer productus sinus glands reveals that one of three distinct combinations of crustacean hyperglycemic hormone precursor-related peptide (CPRP) isoforms are present in individual crabs. Gen Comp Endocrinol. 2007c doi: 10.1016/j.ygcen.2007.06.025. In press. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J. Neurochem. 2007b;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Provencher HL, Guiney ME, Gardner NP, Dickinson PS. Matrix-assisted laser desorption/ionization fourier transform mass spectrometry for the identification of orcokinin neuropeptides in crustaceans using metastable decay and sustained off-resonance irradiation. Anal. Chem. 2005;77:3594–3606. doi: 10.1021/ac0502347. [DOI] [PubMed] [Google Scholar]
- Stepanyan R, Day K, Urban J, Hardin DL, Shetty RS, Derby CD, Ache BW, McClintock TS. Gene expression and specificity in the mature zone of the lobster olfactory organ. Physiol. Genomics. 2006;25:224–233. doi: 10.1152/physiolgenomics.00276.2005. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Bishop CA. Mapping of proctolin-like immunoreactivity in the nervous systems of lobster and crayfish. J. Comp. Neurol. 1986;243:435–453. doi: 10.1002/cne.902430402. [DOI] [PubMed] [Google Scholar]
- Tensen CP, de Kleijn DP, Van Herp F. Cloning and sequence analysis of cDNA encoding two crustacean hyperglycemic hormones from the lobster Homarus americanus. Eur. J. Biochem. 1991a;200:103–106. doi: 10.1111/j.1432-1033.1991.tb21054.x. [DOI] [PubMed] [Google Scholar]
- Tensen CP, Janssen KP, Soyez D, Van Herp F. Comparative characterization of hyperglycemic neuropeptides from the lobster Homarus americanus. Peptides. 1991b;12:241–249. doi: 10.1016/0196-9781(91)90006-b. [DOI] [PubMed] [Google Scholar]
- Tensen CP, Verhoeven AH, Gaus G, Janssen KP, Keller R, Van Herp F. Isolation and amino acid sequence of crustacean hyperglycemic hormone precursor-related peptides. Peptides. 1991c;12:673–681. doi: 10.1016/0196-9781(91)90119-a. [DOI] [PubMed] [Google Scholar]
- Torfs P, Nieto J, Cerstiaens A, Boon D, Baggerman G, Poulos C, Waelkens E, Derua R, Calderon J, De Loof A, Schoofs L. Pyrokinin neuropeptides in a crustacean. Isolation and identification in the white shrimp Penaeus vannamei. Eur. J. Biochem. 2001;268:149–154. doi: 10.1046/j.1432-1327.2001.01858.x. [DOI] [PubMed] [Google Scholar]
- Towle DW, Smith CM. Gene discovery in Carcinus maenas and Homarus americanus via expressed sequence tags. Int. Comp. Biol. 2006;46:912–918. doi: 10.1093/icb/icl002. [DOI] [PubMed] [Google Scholar]
- Trimmer BA, Kobierski LA, Kravitz EA. Purification and characterization of FMRFamidelike immunoreactive substances from the lobster nervous system: isolation and sequence analysis of two closely related peptides. J. Comp. Neurol. 1987;266:16–26. doi: 10.1002/cne.902660103. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 1989;250:231–234. doi: 10.1016/0014-5793(89)80727-6. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. Identification of orcokinin gene-related peptides in the brain of the crayfish Procambarus clarkii by the combination of MALDI-TOF and on-line capillary HPLC/Q-Tof mass spectrometries and molecular cloning. Gen. Comp. Endocrinol. 2000;118:161–167. doi: 10.1006/gcen.1999.7453. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. APSGFLGMRamide is a unique tachykinin-related peptide in crustaceans. Eur. J. Biochem. 2004;271:1546–1556. doi: 10.1111/j.1432-1033.2004.04065.x. [DOI] [PubMed] [Google Scholar]
- Zajac JM, Mollereau C. RFamide peptides. Introduction. Peptides. 2006;27:941–942. doi: 10.1016/j.peptides.2005.12.005. [DOI] [PubMed] [Google Scholar]