Abstract
Double-stranded RNA deaminase I (ADAR1) contains the Z-DNA binding domain Zα. Here we report the solution structure of free Zα and map the interaction surface with Z-DNA, confirming roles previously assigned to residues by mutagenesis. Comparison with the crystal structure of the (Zα)2/Z-DNA complex shows that most Z-DNA contacting residues in free Zα are prepositioned to bind Z-DNA, thus minimizing the entropic cost of binding. Comparison with homologous (α+β)helix–turn–helix/B-DNA complexes suggests that binding of Zα to B-DNA is disfavored by steric hindrance, but does not eliminate the possibility that related domains may bind to both B- and Z-DNA.
RNA editing in mammals alters codons in mRNA through site-specific deamination of adenosines and cytosines, leading to proteins with modified function. Adenosine to inosine (A → I) editing modulates the calcium permeability of neural glutamate receptors (1) and reduces the G-protein coupling efficacy of serotonin 2C receptors (2). Double-stranded RNA deaminases I and II (ADAR1/2) catalyze these A → I conversions, but unknown auxiliary factors are thought to be involved in the control of editing efficiency in vivo (3). ADAR1, but not ADAR2, has two left-handed Z-DNA binding domains, Zα and Zβ, at its N terminus. These domains may contribute to the control of ADAR1-mediated editing in vivo (4). Z-DNA formation in vivo has been shown to be transcription dependent in prokaryotes and eukaryotes (5). Z-DNA can be generated transiently 5′ to a moving RNA polymerase in alternating purine/pyrimidine sequences (5), thereby providing a transient binding site for Zα and Zβ. Thus, Z-DNA binding may ensure that the catalytic activity of ADAR1 is targeted to sites where nascent pre-mRNA substrates emerge (5).
Here we have determined the solution structure of free Zα and mapped the interaction surface between Zα and a 6-bp d(CG) substrate DNA by two-dimensional (2D) 15N- heteronuclear single quantum correlation (HSQC) NMR spectroscopy. Zα binds this substrate with high affinity (Kd = 30 nM) and a stoichiometry of 2:1 (protein/DNA) (6–10). The map of the interaction surface in solution agrees well with the crystal structure of Zα complexed with Z-DNA (7). Further the structure of Zα free in solution demonstrates that there are only minor conformational changes upon binding Z-DNA. Not only is the overall structure the same, but unexpectedly, most Z-DNA contacting residues are prepositioned in free Zα to fit Z-DNA. This study also examines why Zα preferentially binds Z-DNA rather than B-DNA despite its high structural homology to (α+β) helix–turn–helix (α+βHTH) B-DNA binding proteins.
Materials and Methods
Protein Preparation.
The Zα domain, comprising residues 119–200 of human ADAR1 (GenBank accession no. U10439), has been described (8). Zα was expressed as a fusion protein with a N-terminal (His)6-tag from a pET-21a vector (Novagen) in Escherichia coli strain HM174(DE3). For isotope labeling, bacteria were grown in M9 medium containing 1 g/liter 15NH4Cl and 1.5 g/liter 13C-glucose. Harvested bacteria were resuspended in binding buffer (20 mM Tris⋅HCl, pH 8/150 mM NaCl/0.125 mM PMSF/10 mM β-mercapto-ethanol) and lysed by French pressing. The E. coli lysate was passed over a (His)6-tag affinity column (TALON Metal Affinity Resin, CLONTECH), and the Zα fusion protein was eluted by using a 0–300 mM imidazole gradient. After dialysis against binding buffer, the (His)6 tag was removed by thrombin digestion at room temperature overnight. For the second purification step, the Zα protein was dialyzed into 50 mM Hepes, pH 7.4, 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.125 mM PMSF and loaded on a cation exchange chromatography column (Mono S 5/5, Amersham Pharmacia). The protein was eluted with a 50–1,000 mM NaCl gradient, yielding homogeneous protein of wild-type molecular weight, as indicated by SDS-gel analysis and matrix-assisted laser desorption ionization-time of flight MS. The Zα-C125S mutant was constructed by PCR-based site-directed mutagenesis (QuikChange site-directed mutagenesis kit, Stratagene) in the pET-21a vector harboring the Zα insert. The mutant protein was expressed and purified as described above.
NMR Spectroscopy.
NMR experiments were carried out on a 2 mM sample in 10 mM Na-phosphate buffer, pH 5, 137 mM NaCl, and 0.1 mM NaN3 (10% D2O) at 25°C on 500-, 600-, and 750-MHz NMR spectrometers. 1H, 15N, and 13C resonance assignments were obtained from the following three-dimensional (3D) heteronuclear correlation experiments (11): CBCA(CO)NH, CBCANH, HBHA(CO)NH, H(CCO)NH, HCCH-correlated spectroscopy, HCCH-TOCSY, and HNHA. 3JHNHA coupling constants were measured from a 3D HNHA spectrum (12). Interproton distance restraints were derived from 3D 15N-HSQC-nuclear Overhauser effect spectroscopy (NOESY) (70-, 150-, and 250-ms mixing times), 3D 13C-HSQC-NOESY (40-, 70-, and 100-ms mixing times for aliphatic region; 35- and 70-ms mixing times for aromatic region), and 2D NOESY in D2O and H2O (20-, 40-, 80-, and 160-ms mixing times). Spectra were processed with nmrpipe (13) or xwinnmr (Bruker) and analyzed with felix ’97 (Micron Separations). Spectra were indirectly referenced through the magnetogyric ratios by external calibration on 2,2-dimethyl-2-silapentane-5-sulfonate, sodium salt (14).
Interaction Mapping.
For interaction mapping, 2D 15N-HSQC spectra were recorded on free Zα and (Zα)2/Z-DNA complex in the same buffer as described above at 25°C. The sample of the complex was prepared by titrating Zα into d(CG)3T4(CG)3 DNA at diluted concentrations, and concentrated by using an Amicon stir cell. Free d(CG)3T4(CG)3 forms a hairpin with a 6-bp d(CG)3 stem in the B-DNA conformation in solution (6). The saturation level of two Zα to one hairpin was monitored by one-dimensional 1H-NMR on well-resolved resonances. Spectra were processed and analyzed as described above.
Structure Calculation.
NOE restraints derived from all four types of NOESY experiments were analyzed in the conventional manner by using a lower boundary of 1.8 Å and an upper boundary of 6 Å and 7 Å for 3D and 2D NOESYs, respectively. Structures were calculated by simulated annealing from random coordinates (15) at 2,000 K by using x-plor 3.1 (16) and floating stereospecific assignment. Intraresidual NOEs were omitted. Force constants for NOE and phi angle restraints were 50 kcal mol−1⋅Å−2 and 200 kcal mol−1⋅rad−2, respectively. Force constants for bond lengths, bond angles, and improper angles were 1,000 kcal mol−1⋅Å−2, 500 kcal mol−1⋅rad−2, and 500 kcal mol−1⋅rad−2, respectively.
Results and Discussion
Structure Determination.
The solution structure of the Z-DNA binding domain Zα (residues 119–200) of human ADAR1 was determined by using multidimensional NMR spectroscopy. Because the wild-type construct showed aggregation at concentrations required for NMR spectroscopy, cysteine 125 was mutated to serine. Binding of the C125S-mutant to Z-DNA is indistinguishable from wild type in surface plasmon resonance, CD spectroscopy, and analytical ultracentrifugation experiments (6). In 2D 15N-HSQC spectra, no difference between wild type and the C125S mutant was discernible, suggesting that the C125S substitution has no effect on the protein conformation.
Using triple-resonance NMR experiments, all residues except for the N-terminal glycine were assigned. The chemical shifts of the N-terminal residues 117–135 are not well dispersed. Residues 126–133 show α-helical structure (designated prehelix), whereas the preceding N-terminal residues are unstructured. The 3D structure revealed that the N-terminal residues lie outside of the core fold comprising residues Y136 –A198. The structure of the Zα core is very well defined with an average of 12.7 long-range NOE restraints per residue resulting in a backbone rms deviation of 0.26 Å for the 15 lowest energy structures (Table 1).
Table 1.
Experimental restraints and structural statistics
| Distance restraints from NOE* | |
| Interresidue sequential (|i–j| = 1) | 695 |
| Interresidue medium range (1 < |i–j| < 5) | 669 |
| Interresidue long range (|i–j| > 4) | 800 |
| All | 2,164 |
| Dihedral angle restraints from coupling constants xplor potential energies (kcal mol−1) | 47 |
| Etotal | 143.6 ± 10.1 |
| Ebond | 5.5 ± 0.6 |
| Eangle | 102.5 ± 7.2 |
| Eimproper angles | 11.5 ± 1.0 |
| Erepel | 16.2 ± 1.7 |
| ENOE2 | 7.9 ± 0.8 |
| Ecdih2 | 0.02 ± 0.015 |
| procheck Ramachandran analysis†‡ | |
| Residues in most favored regions | 92.6% |
| Residues in additional allowed regions | 7.4% |
| Residues in generously or disallowed regions | 0% |
| Lennard-Jones potential energies3 (kcal mol−1)§ | |
| 〈SA〉15 structures¶ | −69.8 ± 13.2 |
| Coordinate precision (Å)∥ | |
| Backbone (N, Cα, C, O) | 0.26 |
| All nonhydrogen atoms | 0.65 |
Not including 24 H-bonds in α-helices and 4 H-bonds in the β2β3-sheet set to 1.8 Å ± 0.5.
† No structure shows NOE distance violations greater than 0.2 Å or phi angle violations greater than 2°.
‡ The core domain (residues 136–198) was used.
§ The Lennard-Jones van der Waals energy was calculated with charmm param19/20.
¶Solution-state structures derived by simulated annealing.
∥ The core domain excluding the flexible loop (residues 151–153) was used.
Structure Description.
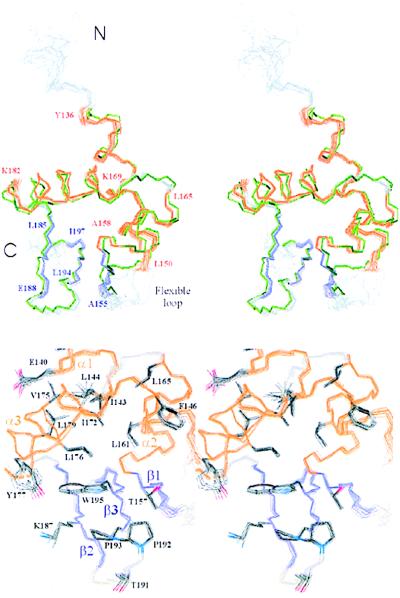
The solution structure of Zα consists of three α-helices (designated α1, α2, and α3) and three β-strands (designated β1, β2, and β3) having an α1β1α2α3β2β3 topology (Fig. 1). The three helices are roughly perpendicular to each other enclosing a hydrophobic core that is packed against the C-terminal antiparallel β2β3-sheet (Fig. 2 Upper). β1 is oriented almost perpendicular to the C-terminal β-sheet, contacting β3 through two backbone hydrogen bonds between T156 and W195. This arrangement of three α-helices and β-strands is classified as the α+βHTH fold, which has been found in numerous eukaryotic and prokaryotic B-DNA binding protein domains (Table 2).
Figure 1.
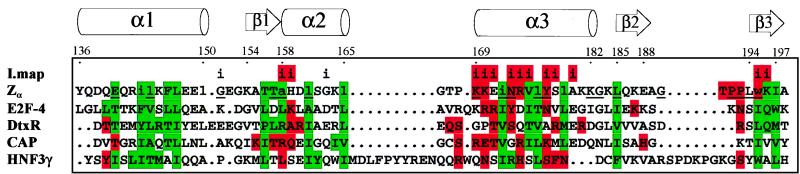
Sequence alignment of structural homologues. The human Zα domain (residues 136–198) is shown with the secondary structure derived from this NMR study. Residues absolutely conserved in human, mouse, rat, bovine, and Xenopus Zα are underlined. Residues of Zα identified as essential for protein stability by scanning mutagenesis are in lowercase. Residues identified by interaction mapping are superscripted with an i, which is shown on red background for those residues forming a roughly contiguous binding surface. Structural homologs were aligned with Zα based on the dali (21) structural alignment. Buried residues (green) were detected by using procheck (26), and DNA contacting residues (red) were taken from the cocrystal structures. CAP, catabolite gene activator protein; HNF3γ, hepatocyte nuclear factor 3γ.
Figure 2.
Solution structure of Zα. (Upper) Stereo view of the backbone atoms of the 15 lowest energy simulated annealing structures (residues 126–200) showing a rigid Zα backbone with one flexible loop between α1 and β1. The terminal residues of α-helices (orange) and β-strands (violet) are labeled as well as the N terminus and C terminus. The superposition with the crystal structure of bound Zα (green) (7) shows that the structures of free and bound Zα are almost identical except for one flexible loop between α1 and β1. (Lower) The close-up stereoview of the hydrophobic core shows the pivotal position of W195 in packing the C-terminal β-sheet against the α-helical core. The side chains of the hydrophobic core residues are well defined in the ensemble of 15 NMR structures.
Table 2.
Structural homologs of Zα
| PDB ID | Z-score* | rms deviation [Å] | LALI† | Protein | α1 length |
|---|---|---|---|---|---|
| — | 12.9 | 0.9 | 63 | Crystal structure of Zα bound to Z-DNA | — |
| 1smt-A | 9.7 | 1.6 | 58 | Transcriptional repressor SmtB | Very short |
| 1hst-A | 8.7 | 1.9 | 61 | Histone H5 | Very short |
| 1lea | 8.4 | 2.0 | 62 | LexA repressor | Short |
| 1bia | 7.5 | 2.2 | 57 | BirA biotin operon repressor | Short |
| 1cf7-A | 7.2 | 2.0 | 60 | Transcription factor E2F4 | Short |
| 2cgp-C | 7.1 | 1.9 | 58 | Catabolite gene activator protein | Very short |
| 1bja-A | 6.9 | 1.4 | 56 | Transcription regulator MotA | Short |
| 1opc | 6.7 | 2.5 | 60 | Omp repressor | Short |
| 2fok-A | 6.3 | 1.8 | 57 | FokI restriction endonuclease‡ | No DNA |
| 1xgs-A | 5.9 | 1.7 | 51 | Methionine aminopeptidase‡ | No DNA |
| 1ecl | 5.7 | 2.3 | 59 | Topoisomerase I‡ | No DNA |
| 1bm9-A | 5.7 | 2.2 | 58 | Replication terminator protein | Short |
| 2tdx | 5.6 | 1.8 | 59 | DtxR | Very short |
| — | 5.3 | 2.3 | 57 | Hepatocyte nuclear factor 3γ | Very short |
The Z-score, calculated by using the program dali (21), describes the similarity between structures. Protein domains with Z-scores <2.0 are structurally dissimilar.
† Length of equivalenced residues. The Zα core domain (63 residues) served as input.
‡ Function other than DNA binding.
Zα has a flexible loop between α1 and β1 and an unexpectedly rigid loop between β2 and β3 in solution (Fig. 2 Upper). The rigidity of the latter can be partially accounted for by the restricted flexibility of the backbone at P192-P193, where P192 forms an unusual cis peptide bond. In addition, long-range NOE restraints between P192 and T157 of β1 define the conformation of this hairpin precisely (Fig. 2 Lower). In contrast, we found no long-range NOEs for the α1β1-loop residues G151-G153. The mutations P192A and P193A strongly reduce Z-DNA affinity (8), suggesting that the rigid proline loop is important for binding Z-DNA.
Comparison with Mutants of the Hydrophobic Core.
Evidence for an important structural role of residues in the hydrophobic core of Zα is obtained from previous mutagenesis studies where changing these residues to alanine enhances the rate of proteolytic degradation within E. coli (8). Such increased degradation suggests either a loss of protein stability or an impairment of correct protein folding. In the solution structure of Zα, α1 is packed against α2 through extensive van der Waals interactions between the buried residues I143 and F146 of α1 and L161 and L165 of α2 (Fig. 2 Lower). All four residues are conserved as hydrophobic residues in human, mouse, rat, bovine, and frog Zα and Zβ domains (4). The mutations I143A, L161G, and L165P resulted in severe proteolytic degradation, suggesting that the interactions observed in the NMR structure are important for the packing between α1 and α2 (8).
Helix α3 forms van der Waals contacts to both hydrophobic and polar side chains on α1. The hydrophobic residues I143 and L144 on α1 interdigitate with I172, L176, and L179 on α3 (Fig. 2 Lower). Mutations in each of these highly conserved hydrophobic residues strongly reduced protein stability (8). Although the aliphatic moieties of the polar residues E140 and Q139 on α1 interleave with V175 and L179 on α3, the mutations E140A and V175G had no effect on protein stability. Thus, the centrally located van der Waals interactions between α3 and the hydrophobic residues on α1 play a more important role for the stability of Zα than those between α3 and the polar residues.
α+βHTH proteins differ from related HTH proteins because they possess an additional C-terminal β-sheet, which is packed against the α-helical core. In Zα the aromatic ring system of W195 is sandwiched between L176 of α3 and K187 of β2, thereby linking the C-terminal β-sheet to the α-helical core (Fig. 2 Lower). The W195A mutation is deleterious to the stability of Zα, and even the conservative W195Y mutation increased proteolytic degradation (8). Mutant W195A showed the lowest affinity for Z-DNA of all mutants screened. The pivotal role of W195 is underscored by its absolute conservation in Zα and Zβ domains. The solution structure of Zα thus provides an explanation for the effects of different mutations on protein stability and for the conservation pattern seen in the sequence alignment of Zα and Zβ. This structure/function analysis of hydrophobic core interactions also may provide valuable insight into packing forces in homologous α+βHTH proteins. These buried residues are markedly conserved throughout this entire class of protein (Fig. 1).
Interaction Map of the Zα/Z-DNA Interface in Solution.
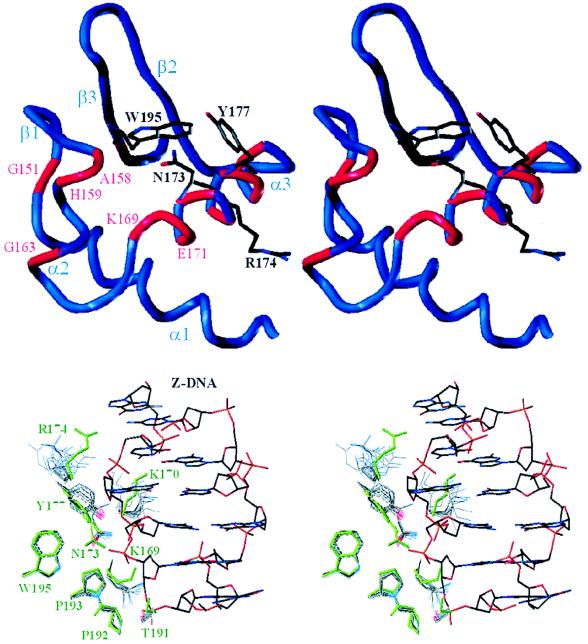
To map the interaction surface between Zα and Z-DNA in solution, we have recorded 2D 15N-HSQC NMR spectra of free and bound Zα. Using this technique, chemical shift changes in backbone and side-chain amides are detected when DNA is bound in the vicinity (17). The superposition of the 15N-HSQC spectra of free Zα and (Zα)2/Z-DNA complex shows that several amide resonances move significantly, a few vanish, and many show insignificant effects as a result of Z-DNA binding (see Fig. 5, which is published as supplementary material on the PNAS web site, www.pnas.org). We considered chemical shift changes with (Δ1H + Δ15N/10) > 0.1 ppm or vanishing cross peaks indicative of closeness to the bound Z-DNA (labeled in capital letters in Fig. 5). Most significant chemical shift changes map to α3 (red in Fig. 3 Upper), which has the characteristics of a recognition helix as shown by mutagenesis (8). Further chemical shift changes are observed at the N terminus of α2 and around W195. The shift changes of G151 and G163 may reflect a slight long-range conformational change resulting from Z-DNA binding. Such effects have been observed for glycines occupying hinge positions in the protein backbone (17). The quality of the chemical shift data is underlined by the good superposition of the N-terminal residues 118–123 (labeled in italics in Fig. 5), which are outside the core domain and do not contact the Z-DNA.
Figure 3.
Interaction map of the Zα/Z-DNA complex. (Upper) The stereo view of residues with NH chemical shift changes in the backbone (red tube) and in the side chain (highlighted) shows that they form a contiguous interaction surface on α3 and part of the β2β3-sheet of Zα, except for two glycines and K196. (Lower) The superposition of the nine Z-DNA contacting residues (green) of Zα complexed with Z-DNA in the cocrystal with those of the 15 lowest energy NMR structures of free Zα shows that seven of these residues are already prepositioned in free Zα to fit Z-DNA. Only two, K170 and R174, are flexible in solution. Y177 shows rotations around χ2 but not χ1. The structures were superimposed as shown in Fig. 2 Upper.
Investigation of the “folded” peaks of imino groups of arginine side chains shows that only R174 shifts upon Z-DNA binding, whereas the other two arginine imino groups present in Zα remain unaffected (lower right box in Fig. 5). In addition, the well-resolved aromatic side chains of the two tyrosines in Zα could be mapped by one-dimensional 1H NMR in D2O (data not shown). Both the Hδ and Hɛ resonances of Y177 vanish when Zα is titrated with substrate DNA, whereas those of Y136 remain unaffected. Taken together, chemical shift changes in four side chains and 13 backbone amides were found by interaction mapping, suggesting that Zα binds Z-DNA mainly through α3 and through additional interactions in the vicinity of W195 and the N terminus of α2.
Comparison with the Crystal Structure of Zα Complexed with Z-DNA.
The NMR structure was determined independently from the recently determined crystal structure of the (Zα)2/Z-DNA complex (7). The superposition of the ensemble of 15 solution structures of free Zα with the crystal structure shows that the structures of free and bound Zα are almost identical (Fig. 2 Upper). The backbone rms deviation is 0.75 Å (residues Y136-E148 and A155-A198), and the nonhydrogen atom rms deviation of buried residues (highlighted in Fig. 1) is 1 Å, underscoring the high similarity of the solution and crystal structures of Zα. Even the flexibility of the α1β1-loop (residues G151-G153) is reflected by the high B factors in the crystal. Small local differences in the backbone of Zα (rms deviation 1–2 Å) are observed between G163 and L165, suggesting that the chemical shift change of G163 reflects a minor rearrangement in the C terminus of α2 when Zα binds Z-DNA. However, overall Zα does not undergo any significant conformational changes upon binding Z-DNA.
The interaction surface between Zα and Z-DNA of the crystal structure also agrees well with that mapped in solution (Fig. 3). N173, R174, Y177, and W195, which show significant side-chain chemical shift alterations, also mediate Z-DNA contacts in the crystal structure. The vicinity of the Z-DNA also is sensed by several backbone amides in α3 and those of A158 and H159 in α2. The NH of A158 coordinates a well-defined water with the NHɛ of W195, and the amide of H159 mediates a phosphate backbone contact relayed by two waters in the crystal structure. The chemical shift changes in these two residues suggest that these waters may be rearranged upon Z-DNA binding. The roles of P192 and P193, which contact Z-DNA in the crystal structure, could not be determined because prolines are insensitive to the 15N-HSQC mapping technique. Overall, the interaction map revealed changes in the medium-range environment of Zα as a result of contact with Z-DNA. These changes in the backbone amides of α3 and α2 complement the short-range information gained from the Zα/Z-DNA contacts in the crystal structure.
Of the nine Z-DNA residues contacting DNA observed in the crystal structure, the side chains of K169, N173, and Y177 are partially ordered and those of T191, P192, P193, and W195 are well ordered in the solution structure of free Zα (Fig. 3 Lower). Only two Z-DNA contacts, K170 and R174, are flexible. NOEs between the methyl group of T191 and P192 (Fig. 2 Lower) lock the T191 side chain in the same orientation as in the crystal structure, even though T191 is totally exposed in free Zα. In the majority of our solution-state structures, NOEs to the δ protons of N173 restrict the orientation of the amide group to that used to mediate a Z-DNA contact in the crystal, suggesting that some of the well-defined waters coordinated by N173 may already be in place in free Zα. Also several NOEs show that the aromatic ring of Y177 is packed against W195 and N173 in free Zα. In the bound state Y177 maintains these hydrophobic contacts, but is restricted in its rotational freedom as a result of van der Waals contact with a guanosine base of Z-DNA. This establishes a chain of contacts between W105 to Y177 to the guanosine base in which the aromatic rings of each residue lie almost perpendicular to the others. This probably relates to the quenching of W195 fluorescence observed when Za binds Z-DNA (10).
Interestingly, the flexibility pattern of the Z-DNA contacting side chains is mirrored by the B factors of bound Zα in the cocrystal (7). The side chains of K169, N173, Y177, P192, P193, and W195 have low B factors, whereas those of K170 and R174 have high ones. Correspondingly, the Z-DNA phosphates contacted by K170 and R174 show high B factors, whereas those contacted by the prepositioned residues show low B factors. These data indicate that a Z-DNA contact flexible in the unbound state maintains some flexibility in the bound state. By alanine scanning mutagenesis it was demonstrated that the flexible Z-DNA contacts had nearly no effect on Z-DNA affinity, whereas the partially ordered Z-DNA contacts K169, N173, and Y177 diminished the binding constant 37-, 168-, and 26-fold, respectively without affecting the overall stability of the protein (8). These data suggest that the contribution of a side chain to binding can be correlated with its relative rigidity in the free and bound state. In the case of K170 and R174, the binding enthalpy gained from forming a hydrogen bond may be roughly balanced by the loss in binding entropy required to partially order a flexible side chain. In contrast, the unexpectedly rigid residues T191, P192, and P193, forming van der Waals contacts with the Z-DNA, may make a significant contribution to the free energy of binding, consistent with the high conservation and uniqueness of these prolines to the Zα/Zβ family. In conclusion, of a total of nine Z-DNA contacts, seven already are prepositioned in free Zα, indicating that Zα is preshaped to fit Z-DNA.
Prepositioned residues within the DNA binding site are not the rule in all HTH domains. A molecular dynamics simulation of the Antennapedia HTH-domain/DNA complex suggests that key DNA contacting side chains, such as Q50, are flexible in the bound state (18). Also, in the crystal structure of the related even-skipped homeodomain/DNA complex, the side chain of the key DNA contacting residue Q50 adopts three different conformations, resulting in flexible DNA recognition (19). The Q50A mutation reduced the DNA affinity only 2.4-fold (20) consistent with our finding that flexible DNA contacts in Zα make minor contributions to the free energy of binding. It is possible that Zα compensates for its significantly smaller interaction surface as compared with other B-DNA binding proteins (7) by using rigid prepositioned Z-DNA contacts.
Homologous α+βHTH Proteins.
A structural similarity search of the protein structure database using the program dali (21) uncovered numerous α+βHTH DNA binding proteins with highly significant Z-scores (Table 2). Of the 14 similar α+βHTH domains, 11 bind to B-DNA, indicating that structural and functional homology correlate well for this fold. Zα and its B-DNA binding homologues agree well in the arrangement of DNA contacting residues in α3, but Zα lacks DNA contacts at the N terminus of α1 (Fig. 1). Furthermore, Zα mediates three DNA contacts through its loop between β2 and β3, whereas the B-DNA binders form only one DNA contact. Another difference is that Zα forms only water mediated backbone/Z-DNA interactions through the N terminus of α2, where B-DNA binding α+βHTH domains have direct side chain/DNA interactions. Overall, Zα and its B-DNA binding homologues agree well in the arrangement of DNA contacts in the recognition helix α3 and agree partially in those of the C-terminal β-sheet, but differ in those of α1 and α2.
Steric Hindrance Disfavors B-DNA Binding by Zα.
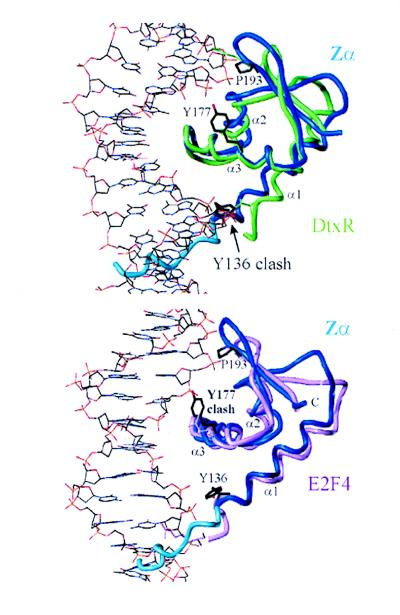
To identify structural discriminants that distinguish between B- and Z-DNA recognition by α+βHTH domains, the 11 B-DNA binding homologues of Table 2 were superimposed with Zα based on the residue matches in α3, β2, and β3 suggested by dali. Five of them show a helix 1 shorter by one turn or more than Zα (designated very short in the final column of Table 2), and six show a helix 1 shorter by less than one turn in the superposition (designated short). Diphtheria toxin repressor (DtxR) is a suitable example for the very short α1 class because it uses nine residues to form contacts to the B-DNA backbone and only one residue for base-specific contacts (22), very similar to Zα. The superposition of the lowest energy NMR structure of Zα with the crystal structure of the DtxR/DNA complex shows that the residues of DtxR preceding α1 are bent out of the way, whereas the N-terminal residues of Zα, including Y136, clash with B-DNA in the minor groove (Fig. 4 Upper). Y136 shows long-range NOE and is defined in all of the three complexes in the asymmetric unit of the crystal structure (7). Therefore Y136 can bend out of the way only at the expense of free energy of binding to accommodate B-DNA binding. In contrast, Y136 and the prehelix have ample space in the distinct binding geometry of the (Zα)2/Z-DNA complex. The superposition of Zα with the crystal structures of the hepatocyte nuclear factor 3γ/DNA (23) and the catabolite gene activator protein/DNA complex (24), additional members of the very short α1 category, also show steric hindrance between Y136 of Zα and the minor groove of B-DNA. Consequently, these comparisons suggest that steric hindrance through the extended helix 1 of Zα may disfavor B-DNA binding by Zα in the binding mode of the very short α1 class of α+βHTHs.
Figure 4.
Steric hindrance disfavors B-DNA binding by Zα. (Upper) The superposition of the lowest energy structure of Zα (blue) with the crystal structure of DtxR (in green) complexed with B-DNA shows that the N terminus of Zα (residues Y136) and possibly also the prehelix of Zα (light blue) cause steric hindrance with B-DNA in the minor groove. For reference, the B-DNA contacting residues of DtxR close to Y136 and P193 of Zα are shown in green. (Lower) The superposition of Zα with the E2F4/DNA complex (pink) shows that Y177 of Zα may clash with the B-DNA backbone in the major groove. The B-DNA contacting residues of E2F4 corresponding to Y136, Y177, and P193 of Zα are represented in pink. P193 is within van der Waals distance to the B-DNA in both superpositions.
The superposition of Zα with the crystal structure of the E2F4/B-DNA complex (25) (Fig. 4 Lower) shows that E2F4 belongs to the short α1 class of α+βHTHs. Here the N terminus of α1 of Zα does not collide with the minor groove. Steric hindrance with the prehelix of Zα may be circumvented by rearranging the loosely folded prehelix. However, the aromatic ring of Y177 of Zα clashes with a phosphate in the major groove. Y177 is the only residue in the crystal structure of the (Zα)2/Z-DNA complex mediating a van der Waals contact with a base in the syn conformation specific for Z-DNA. Moreover, in CD spectroscopy experiments, the Y177A mutant showed a significantly reduced ability to bind specifically to Z-DNA and stabilize this left-handed DNA conformation (8). Taken together, these data suggest that B-DNA binding by Zα also may be disfavored in some cases because of steric hindrance through the phenolic ring of Y177. Indeed, it may be possible to replace Y177 with a more flexible residue to produce a protein that can bind both B- and Z-DNA. The lack of conservation of Y177 in other Zα family members is thus of great interest. Domains capable of recognizing both B- and Z-DNA may bind initially in a B-DNA sequence-specific fashion, e.g., to initiate transcription. The ability to bind Z-DNA allows the interaction with DNA to persist even when negative supercoils arising from the action of enzymes such as RNA polymerase disrupt the B-DNA specific interaction. In this manner targeting can be maintained.
In summary, the specificity of Zα for left-handed Z-DNA probably results from two different structural mechanisms. First, B-DNA binding by Zα is disfavored by steric hindrance. Second, Z-DNA binding by Zα is favored because seven of the nine Z-DNA contacting residues are prepositioned to bind the distinct backbone of Z-DNA. These structural modifications of the α+βHTH fold may enable Zα to recognize Z-DNA in the presence of excess B-DNA in the nucleus of a living cell.
Supplementary Material
Acknowledgments
We thank Thomas Schwartz for providing the crystal structure of the (Zα)2/Z-DNA complex, Steve Unger at Micron Separations for help with felix ’97, Frank Delaglio and Dan Garrett at the National Institutes of Health for making nmrpipe available, and Mark Kelly and Linda Ball for critical reading of the manuscript. This work was supported by a fellowship of the Boehringer Ingelheim Fonds to M.S., National Institutes of Health Grant RR-00995 to The Center of Magnetic Resonance at the Massachusetts Institute of Technology, and grants from the National Institutes of Health, National Science Foundation, and the National Foundation for Cancer Research to A.R.
Abbreviations
- ADAR1/2
double-stranded RNA deaminases I and II, respectively
- 2D
two-dimensional
- HSQC
heteronuclear single quantum correlation
- HTH
helix–turn–helix
- 3D
three-dimensional
- NOESY
nuclear Overhauser effect spectroscopy
- DtxR
diphtheria toxin repressor
Footnotes
Data deposition: The atomic coordinates of the 15 lowest energy structures of a total of 200 have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1qgp).
References
- 1.Sommer B, Kohler M, Sprengel R, Seeburg P H. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 2.Burns C M, Chu H, Rueter S M, Hutchinson L K, Canton H, Sanders-Bush E, Emeson R. Nature (London) 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 3.Polson A G, Bass B L. EMBO J. 1994;13:5701–5711. doi: 10.1002/j.1460-2075.1994.tb06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbert A, Alfken J, Kim Y G, Mian S, Nishikura K, Rich A. Proc Natl Acad Sci USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbert A, Rich A. J Biol Chem. 1996;271:11595–11598. doi: 10.1074/jbc.271.20.11595. [DOI] [PubMed] [Google Scholar]
- 6.Schade M, Behlke J, Lowenhaupt K, Herbert A, Rich A, Oschkinat H. FEBS Lett. 1999;458:27–36. doi: 10.1016/s0014-5793(99)01119-9. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz T, Rould M A, Lowenhaupt K, Herbert A, Rich A. Science. 1999;284:1841–1845. doi: 10.1126/science.284.5421.1841. [DOI] [PubMed] [Google Scholar]
- 8.Schade M, Turner C J, Lowenhaupt K, Rich A, Herbert A. EMBO J. 1999;18:470–479. doi: 10.1093/emboj/18.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbert A, Schade M, Lowenhaupt K, Alfken J, Schwartz T, Shlyakhtenko L S, Lyubchenko Z L, Rich A. Nucleic Acids Res. 1998;26:3486–3493. doi: 10.1093/nar/26.15.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger I, Winston W, Manoharan R, Schwartz T, Alfken J, Kim Y G, Lowenhaupt K, Herbert A, Rich A. Biochemistry. 1998;37:13313–13321. doi: 10.1021/bi9813126. [DOI] [PubMed] [Google Scholar]
- 11.Cavanagh J, Fairbrother W J, Palmer A G, Skelton N J. Protein NMR Spectroscopy. San Diego: Academic; 1996. [Google Scholar]
- 12.Vuister W G, Bax A. J Am Chem Soc. 1993;115:7772–7777. [Google Scholar]
- 13.Delaglio F, Grzesiek S, Vuister G, Zhu G, Pfeifer J, Bax A. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 14.Markley J L, Bax A, Arata Y, Hilbers C W, Kapein R, Sykes B D, Wright P E, Wuthrich K. J Mol Biol. 1998;280:933–952. doi: 10.1006/jmbi.1998.1852. [DOI] [PubMed] [Google Scholar]
- 15.Kuszewski J, Nilges M, Brunger A T. J Biomol NMR. 1992;2:33–56. doi: 10.1007/BF02192799. [DOI] [PubMed] [Google Scholar]
- 16.Brünger A T. x-plor, Version 3.1: A System for X-ray Crystallography and NMR. New Haven: Yale Univ. Press; 1993. [Google Scholar]
- 17.Foster M P, Wuttke D S, Clemens K R, Jahnke W, Radhakrishnan I, Tennant L, Reymond M, Chung J, Wright P E. J Biomol NMR. 1998;12:51–71. doi: 10.1023/a:1008290631575. [DOI] [PubMed] [Google Scholar]
- 18.Billeter M, Guntert P, Luginbuhl P, Wuthrich K. Cell. 1996;85:1057–1065. doi: 10.1016/s0092-8674(00)81306-9. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch J A, Aggarwal A K. EMBO J. 1995;14:6280–6291. doi: 10.1002/j.1460-2075.1995.tb00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades S E, Sauer R T. Biochemistry. 1994;33:9187–9194. doi: 10.1021/bi00197a022. [DOI] [PubMed] [Google Scholar]
- 21.Holm L, Sander C. Science. 1996;273:595–602. doi: 10.1126/science.273.5275.595. [DOI] [PubMed] [Google Scholar]
- 22.White A, Ding X, vanderSpek J C, Murphy J R, Ringe D. Nature (London) 1998;394:502–506. doi: 10.1038/28893. [DOI] [PubMed] [Google Scholar]
- 23.Clark K L, Halay E D, Lai E, Burley S K. Nature (London) 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 24.Schulz S C, Shields G C, Steitz T A. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 25.Zheng N, Fraenkel E, Pabo C O, Pavletich N P. Genes Dev. 1999;13:666–674. doi: 10.1101/gad.13.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.