Abstract
Activation of endonucleases that cleave chromosomal DNA preferentially at internucleosomal sections is a hallmark of apoptosis. DNA fragmentation revealed by the presence of a multitude of DNA strand breaks, therefore, is considered to be the gold standard for identification apoptotic cells. Several variants of the methodology that is based on fluorochrome-labeling of 3′-OH termini of DNA strand breaks in situ with the use of exogenous terminal deoxynucleotidyl transferase (TdT), commonly defined as the TUNEL assay, have been developed by us. This Chapter describes the variant based on strand breaks labeling with Br-dUTP that is subsequently detected immunocytochemically with Br-dU Ab. Compared with other TUNEL variants the Br-dU-labeling assay offers the greatest sensitivity in detecting DNA breaks. Described also are modifications of the protocol that allow one to use other than Br-dUTP fluorochrome-tagged deoxynucleotides to label DNA breaks. Concurrent staining of DNA with propidium or 4′,6-diamidino-2-phenylindole (DAPI) and multiparameter analysis of cells by flow- or laser scanning- cytometry enables one to correlate induction of apoptosis with the cell cycle phase.
1. Introduction
Extensive fragmentation of nuclear DNA that generates a large number of DNA double – strand breaks is one of the most characteristic events of apoptosis [1,2]. An assay that relies on detection of DNA strand breaks (DSBs) in situ by labeling them with fluorochromes has been developed to identify and quantify apoptotic cells by fluorescence microscopy or cytometry [3-5]. The assay is commonly called TUNEL, the acronym of Terminal deoxynucleotidyl transferase-mediated d-UTP Nick End Labeling. In fact, this acronym is a misnomer because not only d-UTP but variety of other deoxynucleotides, indirectly or directly fluorochrome-tagged, are being used in different variants of the TUNEL assay. Furthermore while DNA nicks are generally recognized as DNA single-strand breaks, in actuality DSBs are being labeled in this assay. Because cellular DNA content is often measured in conjunction with labeling DSBs the bivariate analysis of such data provides information about specificity of apoptosis vis-à-vis DNA ploidy or the cell cycle phase [5,6].
The method presented here can be applied to flow cytometry, and its modification, also included, to cells attached on microscope slides that may be growing on slides or deposited by cytocentrifugation. The attached cells can be analyzed by the laser scanning cytometer (LSC), the instrument that combines advantages of both flow and image cytometry and allows one to measure rapidly (up to 100 cells per second), with high sensitivity and accuracy, multi-wavelength fluorescence of individual cells [7,8]. Cells' staining on slides prevents their loss that otherwise occurs during repeated centrifugations in sample preparation for flow cytometry, and therefore analysis by LSC is advantageous in case of samples with scarcity of cells. Still another virtue of LSC stems from the possibility that after the initial measurement of large cell population and electronic selection (gating) of cells of interest they can be localized for visual inspection or morphometric analysis and their image is recorded. Visual examination is of particular importance because the characteristic changes in cell morphology such as cell shrinkage, nuclear fragmentation and chromatin condensation [9] are considered the gold standard for positive identification of apoptotic cells. Applications of LSC for analysis of apoptosis have been reviewed before [10].
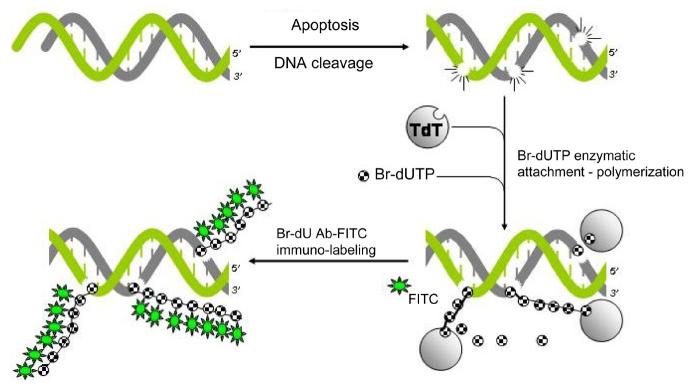
Fixation and permeabilization of the cells are the initial critical steps to successfully label DNA strand breaks. Cells are briefly fixed with a crosslinking fixative such as formaldehyde, and then permeabilized by suspending them in ethanol and/or by using detergents in the subsequent rinses. Formaldehyde prevents extraction of the fragmented DNA by crosslinking low MW DNA fragments to other cell constituents. Omission of this step leads to loss of small DNA fragments during repeated rinses and centrifugations required by this procedure. The 3′OH-termini of the DSBs serve as primers and become labeled in this procedure with BrdU when incubated with Br-dUTP in a reaction catalyzed by exogenous terminal deoxynucleotidyl transferase (TdT) [11,12]. The incorporated Br-dU is immunocytochemically detected by Br-dU antibody conjugated with FITC (Figure 1). The latter is a reagent widely used in studies of cell proliferation to detect Br-dU incorporated during DNA replication [13]. It should be stressed that immunocytochemical detection of Br-dU in the TUNEL assay does not require DNA denaturation, which otherwise is essential for detection of Br-dU incorporation in analysis of cell cycle [13]. The alternate to Br-dU procedures, utilizing digoxygenin, biotin, digoxygenin, or directly fluorochrome-tagged deoxynucleotides [4,14], also are described in the Chapter. It should be noted however that compared to the alternative labeling assays, the overall cost of reagents is significantly lower when Br-dUTP is used as a marker of DSBs. Also, the assay utilizing Br-dUTP is distinctly more sensitive in terms of DSBs detection than other variants of the TUNEL assay [11].
Fig 1.
Schematic illustration of DNA strand breaks labeling with Br-dUTP utilizing exogenous terminal deoxynucleotidyl transferase (TdT) as described in this chapter.
2. Materials
2.1 Glassware and reagents
Phosphate-buffered saline (PBS), pH 7.4
1% Formaldehyde (methanol-free, “ultrapure”, Polysciences, Warrington, PA), in PBS, pH 7.4
70% Ethanol
TdT (Boehringer Mannheim, Indianapolis, IND). TdT 5X reaction buffer: 1M potassium (or sodium) cacodylate, 125 mM HCl, pH 6.6 (this 5X reaction buffer may be purchased from Boehringer Mannheim), 1.25 mg/ml bovine serum albumin (BSA, Sigma, St. Louis, MO).
5-Bromo-2′-deoxyuridine-5′-triphosphate (Br-dUTP) stock solution (50 μl): 2 mM Br-dUTP (Sigma) in 50m mM Tris-HCl, pH 7.5.
10 mM CoCl2 (Boehringer Mannheim)
Rinsing buffer: 0.1% Triton X-100 (Sigma) and 5 mg/ml BSA dissolved in PBS.
FITC- (or Alexa Fluor 488)- conjugated anti Br-dU monoclonal antibody (mAb): Dissolve 0.3 μg of the fluorochrome-conjugated anti Br-dU Ab (Becton Dickinson, BD, Franklin Lakes, NJ) in 100 μl of PBS containing 0.3% Triton X-100 and 1 % (w/v) BSA.
Propidium iodide (PI) staining buffer: 5 μg/ml PI (Invitrogen – Molecular Probes, Eugene, OR), 100 μg/ml of RNase A (DNase-free) (Sigma) in PBS.
Microscope slides (to be used in conjunction with LSC).
Coplin jars (to be used in conjunction with LSC).
Parafilm “M” (American National Can, Greenwich, CT) or ∼2 × 3 cm nylon foil strips (to be used in conjunction with LSC).
Glycerol (to be used in conjunction with LSC).
2.2. Commercial Kits
Several kits for labeling DNA strand breaks are commercially available. The APO-BRDU kit (Phoenix Flow Systems, San Diego, CA, also offered by other vendors) uses a BrdUTP methodology similar to that described in this chapter. As mentioned, this variant of TUNEL methodology offers the most sensitive means of DSBs break detection [11]. The APO-DIRECT kit (also from Phoenix) enables for a single-step labeling of DNA strand breaks with the fluorochrome-tagged deoxynucleotide. It is simpler and more rapid but less sensitive compared to the APO-BRDU [11]. The positive and negative control cells are supplied with each of these Phoenix kits. This is very important component of the kits because it allows the user to assess whether the negative results (no TUNEL labeling in the sample) is due to the genuine lack of apoptosis or to the problems with the kit e.g. such as inactivation of TdT due to improper kit storage or transport. Widely used also is the two-step kit utilizing digoxygenin-dUTP (ApopTag) provided by Roche (Indianapolis, IN).
2.3. Instrumentation
Flow cytometers of different types, offered by several manufacturers, can be used to measure cell fluorescence following staining according to the procedures described below. The manufacturers of the most common flow cytometers are Beckman/Coulter Corporation (Miami, FL), already mentioned Becton Dickinson (BD), Cytomation (Fort Collins, CO) and PARTEC (Zurich, Switzerland). The multiparameter Laser Scanning Cytometer (LSC), including recent iCys model of LSC, is available from CompuCyte, Inc., (Cambridge, MA). Cytospin centrifuge, which is used in conjunction with LSC, is provided by Shandon (Pittsburgh, PA).
The software to deconvolute the DNA content frequency histograms, to analyze the cell cycle distributions, is available from Phoenix Flow Systems (San Diego, CA) and Verity Software House (Topham, MA).
3. Methods
3.1. DNA Strand Break Labeling with BrdUTP for Analysis by Flow Cytometry
Suspend 1-2 × 106 cells in 0.5 ml PBS. Transfer this suspension with a Pasteur pipette into a 5 ml polypropylene tube (see Note 2) containing 4.5 ml of ice cold 1% formaldehyde in PBS (see Note 3). Keep the tube for 15 min on ice.
Centrifuge at 300g for 5 min, resuspend cell pellet in 5 ml of PBS, centrifuge again and resuspend cell pellet in 0.5 ml of PBS. With a Pasteur pipette transfer the suspension to a tube containing 4.5 ml of ice-cold 70% ethanol. The cells can be stored in ethanol for several weeks at −20° C
Centrifuge at 200g for 3 min, remove ethanol, resuspend cells in 5 ml of PBS and centrifuge again at 300g for 5 min.
- Resuspend the pellet in 50 μl of a solution containing:
- 10 μl TdT 5X reaction buffer.
- 2.0 μl of Br-dUTP stock solution.
- 0.5 μl (12.5 units) TdT.
- 5 μl CoCl2 solution.
- 33.5 μl distilled H2O.
Incubate the cells in this solution for 40 min at 37° C (see Notes 4 and 5).
Add 1.5 ml of the rinsing buffer, and centrifuge at 300g for 5 min).
Resuspend cell pellet in 100 μl of FITC- (or Alexa Fluor 488)- conjugated anti-Br-dU mAb solution.
Incubate at room temperature for 1h.
Add 1 ml of PI staining solution.
Incubate for 30 min at room temperature, or 20 min at 37°C, in the dark.
- Analyze cells by flow cytometry.
- excite fluorescence with blue light (488 nm laser line or BG12 excitation filter)
- measure green fluorescence of FITC-( or Alexa Fluor 488)- anti Br-dU Ab at 530 +/− 20 nm.
- measure red fluorescence of PI at >600 nm.
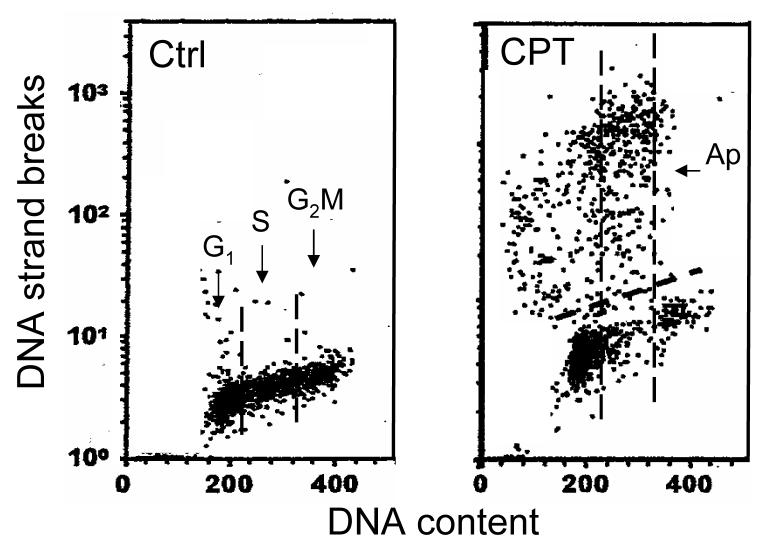
Figure 2 shows apoptotic cells detected by the method described in the protocol. Although the cell fluorescence in this Figure was measured by LSC, nearly identical scatterplots are obtained after measurements by flow cytometry.
Fig 2. Scatterplots illustrating the detection of DNA strand breaks in apoptotic cells by TUNEL assay.
HL-60 cells were untreated (Ctrl) or treated with 200 nM DNA topoisomerase 1 inhibitor camptothecin (CPT) for 3 h , fixed and processed according to the protocol described in this chapter. Cellular DNA was stained with PI while DNA strand breaks were labeled with BrdUTP followed by the FITC-conjugated BrdU Ab. Based on differenced in DNA content one can identify cells in G1 vs S vs G2M phases of the cell cycle as shown in the left panel (separated by the dashed vertical boundaries). Apoptotic cells (Ap) are characterized by very high frequency of DNA strand breaks (note exponential scale of Y coordinate). It is quite evident that CPT induced apoptosis preferentially of S-phase cells.
3.2. DNA Strand Break Labeling with Other Markers for Analysis by Flow Cytometry
As outlined in the Subheading 1 DSBs induced during apoptosis can be labeled with deoxynucleotides tagged with other fluorochromes than listed above. For example, the Invitrogen-Molecular Probes catalog presents several types of dUTP conjugates, including BODIPY dyes (e.g. BODIPY-FL-X-dUTP), fluorescein, Cascade Blue, Texas Red, and dinitrophenol. Several cyanine dyes conjugates (e.g., CY-3-dCTP) are available from Biological Detection Systems (Pittsburgh, PA). Indirect labeling, via biotinylated or digoxygenin conjugated deoxynucleotides offers a multiplicity of commercially available fluorochromes (fluorochrome-conjugated avidin or streptavidin, as well as digoxygenin antibodies) with different excitation and emission characteristics. DNA strand breaks, thus, can be labeled with a dye of any desired fluorescence color and excitation wavelength.
The procedure described in Subheading 3.1 can be adopted to utilize any of these fluorochromes. In the case of the direct labeling [14] the fluorochrome-conjugated deoxynucleotide is included in the reaction solution (0.25 to 0.5 nmoles per 50 μl) instead of BrdUTP, as described in step 4 of Subheading 3.1. Following the incubation step (step 5), omit steps 6 to 8, and stain cells directly with PI (step 9). In the case of the indirect labeling digoxygenin- or biotin-conjugated deoxynucleotides are included into the reaction buffer (0.25 to 0.5 nmoles per 50 μl) instead of BrdUTP at step 4. The cells are then incubated either with the fluorochrome conjugated anti-digoxigenin MAb (0.2 to 0.5 μg per 100 μl of PBS containing 0.1% Triton X-100 and 1% BSA), or with fluorochrome conjugated avidin or streptavidin (0.2 to 0.5 μg per 100 μl, as above) at step 7 and then processed through steps 8-10 as described in the protocol. Analysis by flow cytometry is carried out with excitation and emission wavelengths appropriate to the used fluorochrome.
3.3. DNA Strand Break Labeling for Analysis by LSC or by Fluorescence Microscopy
1. Transfer 300 μl of cell suspension containing ∼20,000 cells (suspended in tissue culture medium, with serum) into a cytospin chamber. Cytocentrifuge at 1,000 rpm for 6 min to deposit the cells on a microscope slide.
2. Without allowing the cytospins to completely dry, prefix the cells by transferring the slides for 15 min to a Coplin jar containing 1% formaldehyde in PBS, cooled to ice temperature.
3. Rinse the slides in PBS and transfer to 70 % ethanol; fix in ethanol for at least 1 h; the cells can be stored in ethanol for weeks at −20°C.
4. Follow steps 4-8 of Subheading 3.1 as described for flow cytometry, with the following modification: Carefully layer small volumes (approximately 100 μl) of the respective buffers, rinses or staining solutions on the cytospin area of the horizontally placed slides. At appropriate times remove these solutions with Pasteur pipette (or vacuum suction pipette). To prevent drying, place a 2×4 cm strip of Parafilm or nylon film on the slide over the cytospin, atop the drop of the solutions used for cell incubations (see Note 8).
9. Replace the PI staining solution with a drop of a mixture of glycerol and PI staining solution (9:1) and mount under the coverslips. To preserve the specimen for longer period of time or transport, seal the coverslip with nail polish or melted paraffin.
- 10. Measure cell fluorescence on LSC (or analyze the cells by fluorescence microscopy).
- excite fluorescence with 488 nm laser line
- measure green fluorescence of FITC- (or Alexa Fluor 488)- anti BrdUrd MAb at 530 +/− 20 nm.
- measure red fluorescence of PI at >600 nm.
The typical results are shown in Figure 1.
3.4. Controls
The procedure of DNA strand break labeling is relatively complex and involves many reagents. Negative results, therefore, may not necessarily mean the absence of DSBs (apoptosis) but may be due to methodological problems, such as loss of TdT activity, degradation of BrdUTP, etc. It is necessary, therefore, to include positive and negative controls. An excellent control provides a sample of HL-60 or other leukemic line cells treated during their exponential growth for 3 to 5 h with 0.2 μM of the DNA topoisomerase I inhibitor camptothecin (CPT). Because CPT under these conditions induces apoptosis selectively during S phase [6], cells in G1and G2/M may serve as TUNEL-negative control populations, while the S phase cells in the same sample, represent the positive control. As mentioned, some kits provide already CPT-treated cells as positive and negative control. A negative control that should also be included is a sample processed identically as described in Subheading 3.1 except that TdT was excluded from step 4.
4. Notes
This method is useful for clinical material, such as obtained from in leukemias, lymphomas and solid tumors [15,16] or tissue sections [16]. The assay can also be combined with surface immunophenotyping as follows: the cells are first immunophenotyped, then fixed with 1% formaldehyde (which stabilizes the antibody bound on the cell surface) and subsequently subjected to the DNA strand break detection assay using different color fluorochromes (see Subheading 3.1) than those used for immunophenotyping.
If the sample contains paucity of cells repeated centrifugations may cause their loss. To minimize cell loss, polypropylene, or siliconized glass tubes are recommended. Transferring cells from one tube to another leads to electrostatic attachment of a large fraction of cells to the surface of each new tube. To minimize the attachment all steps of the procedure (including fixation) should be done in the same tube. Addition of 1 or 2% BSA into rinsing solutions also decreases cell loss. When the sample contains very few cells, the carrier cells, which later can be recognized based on differences in DNA content (e.g., chick erythrocytes) may be included. Analysis by LSC, however, is not plagued by cell loss.
Cell pre-fixation with a crosslinking agent such as formaldehyde is required to prevent extraction of highly fragmented, (mono- and oligo- nucleosomal) DNA, from apoptotic cells [17]. The crosslinking ensures that the DNA content of apoptotic cells (and thus the frequency of DNA strand breaks) is not markedly diminished despite repeated cell washings during the procedure,
Alternatively, incubate at room temperature overnight.
Control cells may be incubated in the same solution, but without TdT.
In most cases is easy to identify apoptotic cells, due to intense labeling with FITC or Alexa Fluor 488 of their DSBs. The high fluorescence intensity often requires use of the exponential scale (logarithmic amplifiers of the flow cytometer or LSC) for data acquisition and display (Fig. 1). Because cellular DNA content of both, apoptotic and nonapoptotic cell populations, is measured the cell cycle distribution and/or DNA ploidy of each of these both populations can be estimated.
While the presence of extensive DNA breakage, detected following DSBs labeling, by the strong fluorescence, is a very characteristic feature of apoptosis, a weak fluorescence may not necessarily indicate for the lack of apoptosis. In some instances DNA fragmentation may stop at 300 - 50 kb size DNA fragments and does not progress into internucleosomal sections [18]. Such apoptotic cells will be characterized by low level of FITC or Alexa Fluor 488 fluorescence. It is advised then to identify apoptotic cells by other marker than the presence of DSBs. Immunocytochemical detection of activated (cleaved) caspase-3 [19] or cleavage of poly(ADP-ribose)polymerase [20] offers an alternative marker of apoptosis is such instances.
It is essential that the incubations are carried out in moist atmosphere to prevent drying at each step of the procedure. This is particularly critical in the case of samples to be measured by LSC. Keep the slides in closed boxes containing moist tissue paper. Even minor drying produces severe artifacts.
This protocol describes labeling DNA with PI. However, other fluorochromes binding to DNA can be used instead of PI. The most commonly used is 4′,6-diamidino-2-phenylindole (DAPI). The protocol is easily modified to accommodate DAPI instead of PI [21,22]. Towards this end substitute the PI staining buffer described in point 9 in Subheading 2.1 by a solution that contains 1μg/ml of DAPI dissolved in PBS and use it in the respective steps of the procedure described above, instead of PI staining solution. Because DAPI fluorescence is excited at UV wavelength, use UV-emitting laser for excitation and measure the DNA-associated fluorescence in blue wavelength (460 - 490 nm)
Acknowledgment
Supported by NCI grant RO1 28704.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nagata S. Exp. Cell Res. 2000;256:12–18. doi: 10.1006/excr.2000.4834. [DOI] [PubMed] [Google Scholar]
- 2.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. Nature. 1998;391:943–50. doi: 10.1038/34112. S. [DOI] [PubMed] [Google Scholar]
- 3.Kajstura M, Halicka HD, Pryjma J, Darzynkiewicz Z. Cytometry A. 2007;71A:125–131. doi: 10.1002/cyto.a.20357. [DOI] [PubMed] [Google Scholar]
- 4.Gorczyca W, Bruno S, Darzynkiewicz RJ, Gong J, Darzynkiewicz Z. Int. J. Oncol. 1992;1:639–648. doi: 10.3892/ijo.1.6.639. [DOI] [PubMed] [Google Scholar]
- 5.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry. 1997;27:1–20. F. [PubMed] [Google Scholar]
- 6.Gorczyca W, Gong JP, Ardelt B, Traganos F, Darzynkiewicz Z. Cancer Res. 1993;53:3186–3192. [PubMed] [Google Scholar]
- 7.Kamentsky LA. Methods Cell Biol. 2001;63:51–87. doi: 10.1016/s0091-679x(01)63007-3. [DOI] [PubMed] [Google Scholar]
- 8.Darzynkiewicz Z, Bedner E, Li X, Gorczyca W, Melamed MR. Exp. Cell Res. 1999:1–12. doi: 10.1006/excr.1999.4477. [DOI] [PubMed] [Google Scholar]
- 9.Kerr JFR, Wyllie AH, Curie AR. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedner E, Li X, Gorczyca W, Melamed MR, Darzynkiewicz Z. Cytometry. 1999;35:181–195. doi: 10.1002/(sici)1097-0320(19990301)35:3<181::aid-cyto1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Darzynkiewicz Z. Cell Proliferation. 1995;28:571–579. doi: 10.1111/j.1365-2184.1995.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Melamed MR, Darzynkiewicz Z. Exp. Cell Res. 1996;222:28–37. doi: 10.1006/excr.1996.0004. [DOI] [PubMed] [Google Scholar]
- 13.Dolbeare F, Selden JR. Methods Cell Biol. 1994;41:297–316. [PubMed] [Google Scholar]
- 14.Li X, Traganos X,F, Melamed MR, Darzynkiewicz Z. Cytometry. 1995;20:172–180. doi: 10.1002/cyto.990200210. [DOI] [PubMed] [Google Scholar]
- 15.Halicka HD, Seiter K, Feldman EJ, Fraganos T, Mittelman A, Ahmed T, Darzynkiewicz Z. Apoptosis. 1997;2:25–39. doi: 10.1023/a:1026431524236. [DOI] [PubMed] [Google Scholar]
- 16.Gorczyca W, Tuziak T, Kram A, Melamed MR, Darzynkiewicz Z. Cytometry. 1994;15:169–175. doi: 10.1002/cyto.990150211. [DOI] [PubMed] [Google Scholar]
- 17.Gong JP, Traganos F, Darzynkiewicz Z. Anal Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. Z. [DOI] [PubMed] [Google Scholar]
- 18.Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozarowski P, Huang X, Halicka DH, Lee B, Johnson G, Darzynkiewicz Z. Cytometry A. 55A(223):50–60. doi: 10.1002/cyto.a.10074. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Darzynkiewicz Z. Exp. Cell Res. 2000;255:125–132. doi: 10.1006/excr.1999.4796. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Cell Proliferation. 2005;38:223–243. doi: 10.1111/j.1365-2184.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurose A, Tanaka T, Huang X, Halicka HD, Traganos F, Dai W, Darzynkiewicz Z. Cytometry A. 2005;68 A:1–9. doi: 10.1002/cyto.a.20186. [DOI] [PubMed] [Google Scholar]