Summary
In this study we show that GATA-6 is a novel repressor of TN-C gene expression. We demonstrated that overexpression of GATA-6 in fibroblasts inhibited basal levels, as well as markedly decreased IL-4- and TGF-β-induced TN-C mRNA and protein levels. A GATA-6 response element was mapped to position −467~ −460 of the TN-C promoter. In addition, we showed that GATA-6 binds this site both in vitro and in vivo.
Keywords: Tenascin-C, transcriptional regulation, GATA-6
1. Introduction
TN-C is a modular extracellular matrix glycoprotein composed of a series of epidermal growth factor-like repeats, fibronectin type III-like repeats and a C-terminal fibrinogen-like globular domain [1]. TN-C is highly expressed during development in organogenesis and at sites of epithelial-mesenchymal transition [2]. In the adult, TN-C expression is less abundant, but is induced during wound healing and in pathological conditions such as tumorigenesis, vascular hypertension and myocardial infarction [3–6]. TN-C is an important regulator of cell adhesion, migration and proliferation during tumorgenesis and vascular remodeling. In cell culture, TN-C interferes with integrin-dependent spreading of most cell types by binding to fibronectin and preventing its interaction with syndecan-4 [7]. Syndecan-4, a transmembrane heparan sulfate proteoglycan, works in synergy with integrin α5β1 to activate Rho signaling and subsequently, actin stress fiber assembly and cell spreading on fibronectin [8]. Disruption of syndecan-4 signaling in cancer cells stimulates a migratory behavior and proliferation [9, 10]. TN-C also plays a critical role in the vascular remodeling during pulmonary arterial hypertension (PAH) by promoting proliferation and survival of vascular smooth muscle cell (VSMC) via its ability to cross-modulate the activity of EGF and FGF-2 receptors [11]. Given the importance of TN-C during the development and progression of tumorigenesis and vascular disease, identification of factors that regulate TN-C expression is important in understanding the site-specific and transient nature of its expression during these pathological conditions.
The GATA family of transcription factors is an evolutionary conserved family of DNA-binding proteins that contain two tandem zinc fingers that interact with other transcriptional regulators and bind to the canonical DNA motif (G/A)GATA(A/T) [12]. Six family members have been identified in vertebrates and based on their sequence homology and expression patterns are divided into two subfamilies: GATA-1, -2, and -3, which are involved mainly in the development of hematopoietic cells and GATA-4, -5 and -6, which function in the development of mesoderm- and endoderm-derived organs such as the heart and gastrointestinal tract, respectively [13]. Human GATA-6 is expressed in a wide array of adult tissues including heart, lung, liver, kidney, pancreas, spleen, ovary and small intestine, where it is believed to maintain the differentiated phenotypes of cells within these tissues [12, 14]. Loss of GATA-6 in ovarian carcinomas leads to a loss of epithelial-specific markers like laminin and Disabled-2 (Dab2) [15]. It has been suggested that GATA-6 is a regulator of the cell cycle in vascular myocytes and embryonic fibroblasts [14, 16, 17]. In VSMCs, GATA-6 mRNA is downregulated after mitogenic stimulation and forced expression of GATA-6 inhibits cell proliferation [18]. GATA-6 has also been shown to be downregulated in balloon-injured rat carotid arteries and when restoration of GATA-6 levels was performed with transduction of a GATA-6-encoding adenovirus, vessels exhibited a higher degree of VSMC differentiation and a reduced level of intimal hyperplasia [18]. Furthermore, GATA-6 has been shown to regulate genes involved in cell-cell and cell-matrix interactions associated with synthetic VSMC function, such as the response to vascular injury [19]. Therefore, GATA-6 may be one of the key regulators of the VSMC phenotype during proliferative vascular diseases like PAH and atherosclerosis.
Because TN-C and GATA-6 have been shown to be key players in both cancer and vascular remodeling, and the human TN-C promoter contains seven putative GATA binding sites, we decided to investigate whether GATA-6 can regulate TN-C gene expression. In this study, we demonstrate that GATA-6 is a functional repressor of TN-C transcription and binds to at least one GATA site within the TN-C promoter in vivo.
2. Materials and Methods
2.1 Cells
Human foreskin fibroblast cultures were obtained from foreskins of healthy newborns and propagated as previously described [20]. 293T cells were purchased from ATCC (Manassas, VA, USA) and grown in the same conditions.
2.2 Adenovirus and Immunoblotting
Gata-6 adenovirus (Ad-CMV-GATA-6) was purchased from Vector biolabs. Cell extracts were prepared in radioimmune precipitation buffer and subjected to sodium dodecyl sulfate acrylamide gel electrophoresis (SDS-PAGE). Nitrocellulose membranes were blocked with either 0.1% BSA or 1% nonfat dry milk in Tween/Tris-buffered saline for TN-C and GATA-6, respectively and then probed overnight with anti-TN-C (kindly provided by Dr. Stan Hoffman) or anti-GATA-6 (Santa Cruz) at 4°C. The blots were incubated for one hour with the appropriate horseradish peroxidase coupled-secondary antibodies (1:3000) and developed using the Chemiluminescent detection kit (Pierce). Band intensities were determined by densitometric analysis.
2.3 Plasmids, Transient transfections and chloramphenicol acetyltransferase assays
A series of 5′ deletions of the TN-C promoter linked to the chloramphenicol acetyltransferase reporter gene were generated as previously described (Shirasaki et al., 1999). The site-specific mutation of the −516 TNC-CAT deletion construct was generated using the Site-directed mutagenesis kit (Stratagene). Briefly, the GATA site was mutated from TTATCT to TTACTT using the following 5′ oligo: GCTTTTGAAGGGCTTTACTTCCTCTTTCCAGGAACTGG. pcDNA3.GATA-6 was a gift from Dr. Mike Xu (Fox Chase Cancer Center).
Human foreskin fibroblasts were transfected using Fugene6 (Roche Applied Bioscience) with 2 μg of various TNC-promoter-CAT constructs and 0.5 μg of pCDNA3.GATA-6. PSV-β-galactosidase control vector (Promega) was co-transfected to normalize transfection efficiencies. CAT assays were performed at least three times, as previously described [21]. The T-test was used to determine statistical significance.
2.4 Quantitative Reverse Transcription-PCR
Real time PCR was performed as previously described [20]. TN-C primer sequences used were: forward 5′-TTTCTGACATAACTCCCGAGAGC-3′ and reverse 5′-AGATATGGGCAGTTCGTTCAGC-3′.
2.5 DNA affinity precipitation assay (DAPA)
DAPA was performed as previously described [21] using RIPA extracts from 293T cells transfected with 2 μg of pCDNA3.GATA-6. Four oligonucleotides labeled with biotin on the 5′end of the sense strand were used in this assay. The following biotin-labeled oligonucleotides were used: (1) 3XGATA oligo, 5′-TCGAGAGCAGATAACAAGGAGCAGATAACAAGGAGCAGATAACACTCGAG, a trimer of the GATA consensus binding site; (2) 3XGATA-M oligo, 5′-TCGAGAGCAGTAAACAAGGAGCAGTAAACAAGGAGCAGTAAACACTCGAG, the trimer of the GATA consensus binding site with the core GATA site mutated; (3) TN-C oligo, 5′-AACCCCCCAGGCTTTTGAAGGGCTTTATCTCCTCTTTCCAGGAACT GGGCTCAGTCCC, which contains positions −491 to −434bp of the human TN-C promoter; (4) TN-C-M oligo, 5′-AACCCCCCAGGCTTTTGAAGGGCTTTACTTCC TCTTTCCAGGAACTGGGCTCAGTCCC, which has a mutated GATA motif in the TN-C oligo. Protein-DNA-streptavidin-agarose complexes were washed and subjected to SDS-PAGE. GATA-6 was detected with the anti-GATA-6 antibody (1:500).
2.6 Chromatin Immunoprecipitation
ChIP was performed as previously described [20]. A 170-base pair region (−507 to −338) of the human TN-C promoter, which contains a putative GATA site, was amplified using the following primers: Forward, 5′ GCCCAGAGAAACCTGAAA CC 3′; Reverse, 5′ TGGCTCCCCTCTTGTACTTG 3′. Band intensities were determined by densitometric analysis.
3. Results
3.1 GATA-6 is a negative regulator of TN-C gene expression
To investigate the potential role of GATA-6 in TN-C gene regulation, fibroblasts were transduced with a GATA-6 adenovirus. As shown in Figure 1a, overexpression of GATA-6 decreased the levels of TN-C protein in a dose-dependent manner. Previous reports have shown that interleukin-4 (IL-4) and transforming growth factor-β (TGF-β) are inducers of TN-C gene expression [22, 23]. Therefore, we investigated whether GATA-6 can modulate the induction of TN-C by IL-4 and TGF-β. From the dose-response experiment (Figure 1a), we selected the 10 MOI condition and demonstrated that at this concentration GATA-6 was able to again inhibit basal levels, as well as markedly reduce IL-4 and TGF-β-induced levels of TN-C gene expression (Figure 1b). In addition to the protein levels, the mRNA levels of TN-C were measured by quantitative RT-PCR (Figure 1c). GATA-6 overexpression significantly lowered mRNA basal levels of TN-C (35%), IL-4-induced levels (36%), and TGF-β-induced levels (25%). These data suggest that GATA-6 may be a negative regulator of the TN-C gene.
Figure 1.
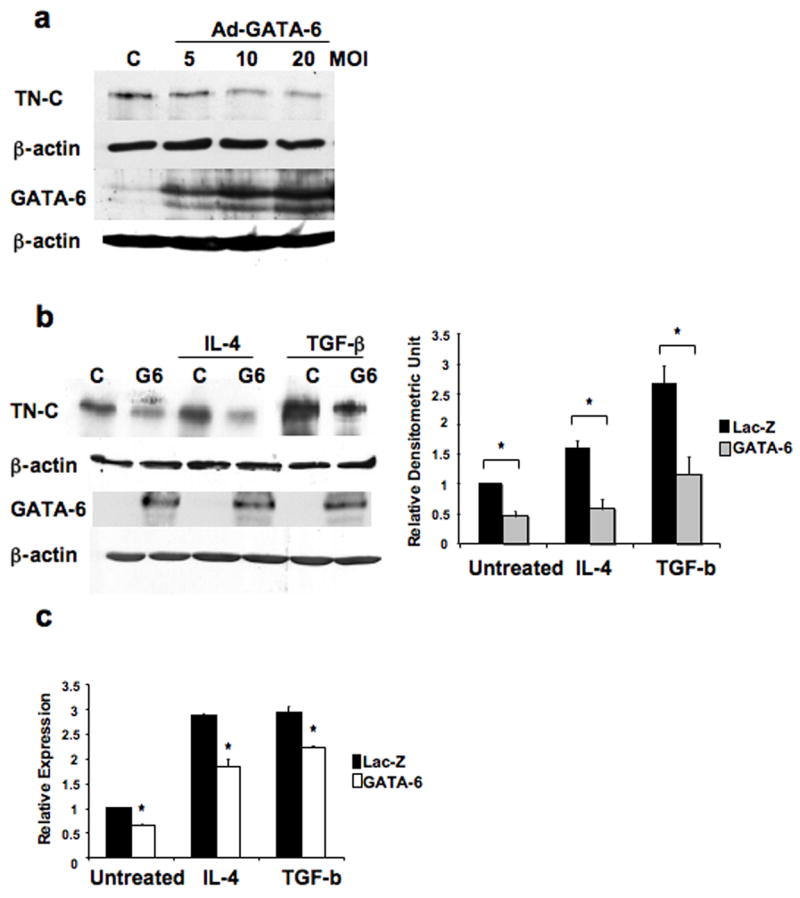
GATA-6 negatively regulates TN-C gene expression. (a) Cell lysates from fibroblasts transduced with 5, 10, and 20 MOI of Ad-GATA-6 or 20 MOI of Ad-LacZ (control) for 48 hours were subjected to immunoblot analysis with antibodies against TN-C and GATA-6. (b) Fibroblasts were transduced with 10 MOI of Ad-GATA-6 or control virus for 24 hours, then treated with IL-4 (10 ng/ml) or TGF-β (2.5 ng/ml) for 24 hours. Samples were assayed by immunoblot analysis with antibodies against TN-C and GATA-6 or (c) quantitative RT-PCR to determine TN-C mRNA levels. One representative Western blot of four independent experiments is shown. Band intensities were quantitated by densitometric analysis and are shown relative to the level of untreated fibroblasts transduced with control virus. * P < 0.01.
3.2 TN-C promoter is negatively regulated by GATA-6
Examination of the TN-C promoter (−2271~ +75bp) revealed that it contains seven potential GATA cis-acting elements (Figure 2a). To narrow our focus of potential binding sites, a series of 5′-deletions of the TN-C promoter linked to the chloramphenicol acetyltransferase reporter gene were cotransfected with a GATA-6 expression plasmid. In the presence of GATA-6, the basal promoter activity of the base pair (bp) −2100~ +75 construct, which contains five putative GATA sites was reduced by 60% (Figure 2b). GATA-6 had even a more significant repressive effect on the −1300~ +75 bp (87%) and −516~ +75 bp (77%) constructs, which contain three and one putative GATA site, respectively. To determine if GATA-6 is mediating its repressive effects via the putative GATA site located at −467 within the −516~ +75 bp contruct, we mutated the GATA core consensus sequence. Mutation of the GATA site completely abrogated the inhibitory effect of GATA-6 on the TN-C −516~ +75 bp promoter. These data further support the evidence that GATA-6 is a negative regulator of TN-C gene expression and reveal that the TN-C promoter contains at least one functional GATA-6 response element.
Figure 2.
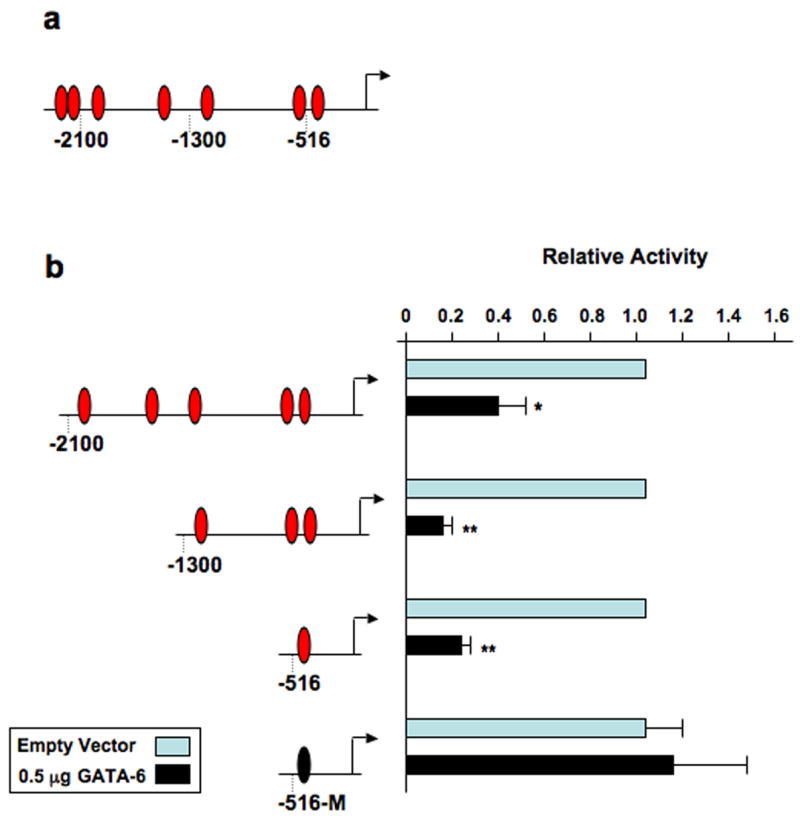
The TN-C promoter contains at least one functional GATA-6 response element. (a) The TN-C promoter contains seven putative GATA-binding sites. (b) Fibroblasts were cotransfected with 2 μg of the indicated TN-C promoter deletion constructs and 0.5 μg of GATA-6 expression vector. CAT assays were performed as described in “Materials and Methods”. Note: The −516-M deletion construct contains a mutation in the putative GATA site (TTATCT to TTACTT). * P < 0.05, ** P < 0.01.
3.3 GATA-6 binds the TN-C promoter in vitro and in vivo
Although it is known that GATA factors bind the consensus GATA motif (A/T)GATA(A/G), a detailed study involving polymerase chain reaction site selection with GATA-6 protein demonstrated an order of binding site preference to be GATA>GATT>GATC and that adenines on both sides of the core confer strong binding (AGAT(A/T)A) [24]. The putative GATA site within the −516~ +75 promoter region therefore, contains a potentially high affinity GATA-6 binding site (AGATAA). To determine if GATA-6 directly binds to the TN-C promoter, we employed the DNA affinity precipitation assay, using a TN-C oligo containing the AGATAA sequences positioned at −467~ −460 on the TN-C promoter (Figure 3a). As a positive control we used the 3XGATA oligo, which is a trimer of the consensus GATA motif and as a negative control we also used the 3XGATA-M oligo in which the GATA motifs of the 3XGATA oligo were mutated as follows: AGATAA was changed to AGTAAA. An additional control we used was the TN-C-M oligo in which the GATA motif of the TN-C oligo was mutated from AGATAA to AAGTAA. The results showed that GATA-6 strongly bound to the 3XGATA oligo and the TN-C oligo, whereas the 3XGATA-M and TN-C-M oligo were not able to bind GATA-6 with the same high affinity (Figure 3b). This in vitro study demonstrates that GATA-6 binds the AGATAA sequences of the TN-C promoter (position −467~ −460).
Figure 3.
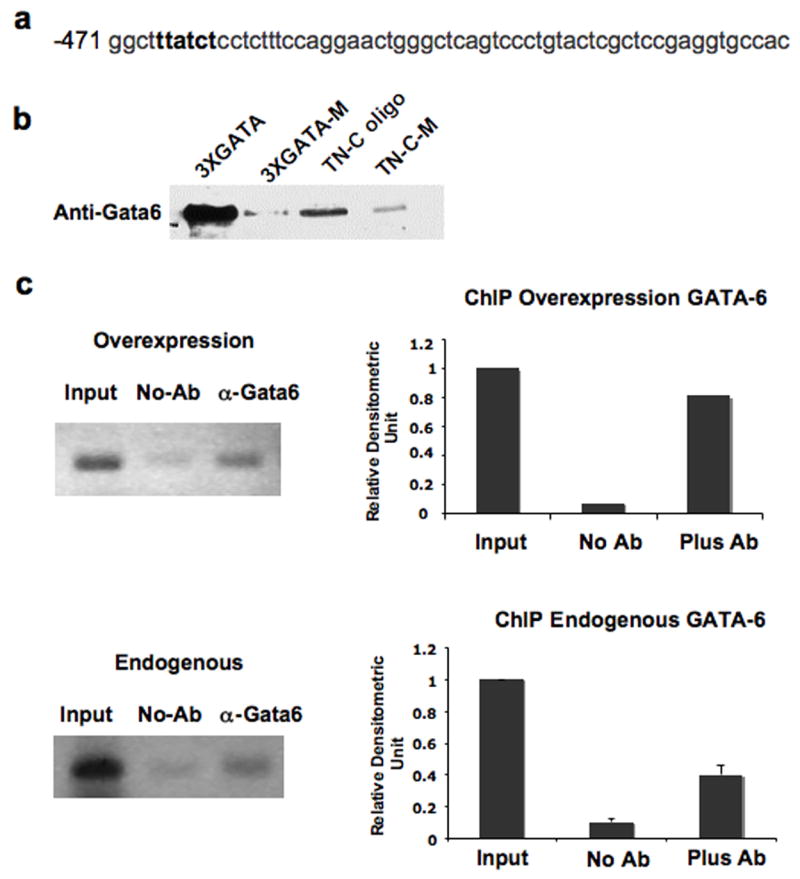
GATA-6 binds position −467~ −460 of the TN-C promoter in vitro and in vivo. (a) The TN-C promoter contains a consensus GATA binding element at position −467~ −460. (b) Cell lysates from 293T cells transfected with pcDNA3.GATA6 were incubated with biotin-labeled oligonucleotides as described in “Materials and Methods”. Protein-DNA complexes were isolated using streptavidin-agarose beads, and GATA-6 was detected by immunoblotting. (c) Fibroblasts were transduced with 10 MOI of Ad-GATA-6, immunoprecipitated with GATA-6 antibody and analyzed for enrichment by PCR (Overexpression). ChIP was also carried out with increased amounts of “Input” DNA from untransduced cells (Endogenous). Representative data of two independent experiments are shown for endogenous GATA-6 with quantitative representation obtained by densitometric analysis.
To further strengthen the hypothesis that GATA-6 binds to the endogenous TN-C promoter in vivo, we utilized chromatin immunoprecipitation (ChIP) assays. First we overexpressed GATA-6 in foreskin fibroblasts, immunoprecipitated with a GATA-6 antibody and analyzed enrichment of the TN-C promoter (position −467~ −460) by PCR (Figure 3c, top panel). Our results indicated that GATA-6, when overexpressed, occupied the TN-C promoter. Next, to examine whether basal levels of GATA-6 occupy the TN-C promoter, we carried out the ChIP assay with an increased amount of input DNA (Figure 3c, bottom panel). The results showed that endogenous GATA-6 is binding the TN-C promoter in vivo.
4. Discussion
In this study we show for the first time that GATA-6 is a repressor of TN-C gene expression. We demonstrated that forced expression of GATA-6 in dermal fibroblasts inhibited basal levels, as well as markedly decreased IL-4- and TGF-β-induced TN-C mRNA and protein levels. Serial 5′ deletions and the transient transfection analysis mapped a putative GATA-6 response element. The GATA-6 response element, located at −467 within the −516~ +75 bp construct, was verified by mutational analysis. In addition, using both DNA affinity precipitation assay and ChIP, we showed that GATA-6 binds this site both in vitro and in vivo. These findings demonstrate the existence of a novel GATA-6 response element in the TN-C promoter.
TN-C can be induced by a variety of cytokines and growth factors, as well as integrins and mechanical forces [1, 9]. Signaling pathways that lead to activation of various transcription factors, including TCF/LEF, NfκB and c-Jun, Ets and Sp1 and Prx-1, have been shown to induce the human TN-C promoter [21, 25–27]. While many activators of TN-C gene expression have been identified, very few repressors have been studied. The homeodomain protein, OTX2 was shown to bind with high affinity to the human TN-C promoter and repress its activity in transfected cells [28]. Interestingly, OTX2 binds the promoter within a cluster of regulatory elements found at −530, which is in close proximity of the validated −467 GATA site and only six base pairs away from another putative GATA site. It will be of interest to examine whether these two proteins act together as part of a repressor complex. It has been well documented that GATA factors interact with homedomain proteins. For example, GATA-4 has been shown to interact with Nkx2.5 in the heart to regulate expression of the atrial natriuretic factor and cardian α-actin promoter [29, 30].
GATA-6 has been shown to regulate a set of gene programs associated with the synthetic functions of VSMCs [19]. Transcriptional targets include genes involved in cell-cell signaling and cell-matrix interactions, such as vascular cell adhesion molecule-1 (VCAM1), angiotensin type1a (AT1a) receptor, and endothelin-1, as well as vascular extracellular matrix (ECM) components, such as matrillin precursor and nidogen.
Another GATA family member, GATA-4, has been shown to be a negative regulator of the ECM protein, α2(I) collagen (COL1A2) in murine fibroblasts [31, 32]. Overexpression of GATA-4 in NIH-3T3 fibroblasts resulted in downregulation of a −2.3kb promoter/reporter gene construct, as well as endogenous COL1A2 mRNA levels. In addition, Electrophoretic mobility shift assay (EMSA) studies demonstrated that GATA-4 from NIH-3T3 cells binds to a DNase I hypersensitive (HS) site located 2.3 kb from the transcriptional start site. Further support for the role of GATA-4 as a repressor of collagen gene expression was shown in mouse transgenic studies, where inhibition of the transgene requires the nuclear protein-binding sites within the intronic HS, including the GATA binding sites.
Interestingly, we found that in human dermal fibroblasts, GATA-6 was the only GATA factor detectable by microarray analysis, as well as qRT-PCR. While GATA-6 has not been previously studied in human fibroblasts, published reports show that both stromal cells in ovarian tissue and mesenchymal cells in embryonic day 17.5 mouse lung are positive for GATA-6 by immunostaining [15, 33]. In addition, we found that fibroblasts in both adult human skin and lung are also immunoreactive for GATA-6 (Ghatnekar & Trojanowska, unpublished data). Furthermore, we observed that GATA-6 overexpression significantly lowered basal mRNA levels of COL1A2 by 50% in human dermal fibroblasts (data not shown). Thus, in human and mouse fibroblasts, distinct GATA factors may play similar roles in regulating matrix-related genes. GATA factors are known to regulate tissue-specific gene expression across diverse cell types via unique interactions with other semi-restricted transcriptional factors or cofactors. A potential cofactor for GATA-6 is Fli-1, a known transcriptional repressor collagen gene expression in dermal fibroblasts, which has also been shown to physically interact with GATA factors in megakaryocytes [34, 35]. Therefore, the regulation of transcriptional repressors and their possible combinatorial interactions may play an important role in regulation of ECM remodeling in physiological and pathological conditions.
Acknowledgments
This work was funded by NIH grant AR42334.
Abbreviations
- TN-C
Tenascin-C
- CAT
chloramphenicol acetyltransferase
- ChIP
Chromatin Immunoprecipitation
- IL-4
Interleukin-4
- TGF-β
Transforming growth factor-β
- ECM
extracellular matrix
- COL1A2
α2(I) collagen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Ekblom P, Aufderheide E. Stimulation of tenascin expression in mesenchyme by epithelial-mesenchymal interactions. The International journal of developmental biology. 1989;33:71–79. [PubMed] [Google Scholar]
- 3.Chiquet-Ehrismann R, Hagios C, Schenk S. The complexity in regulating the expression of tenascins. Bioessays. 1995;17:873–878. doi: 10.1002/bies.950171009. [DOI] [PubMed] [Google Scholar]
- 4.Crossin KL. Tenascin: a multifunctional extracellular matrix protein with a restricted distribution in development and disease. Journal of cellular biochemistry. 1996;61:592–598. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C592::AID-JCB13%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Imanaka-Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, Sakakura T, Yoshida T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Laboratory investigation; a journal of technical methods and pathology. 2001;81:1015–1024. doi: 10.1038/labinvest.3780313. [DOI] [PubMed] [Google Scholar]
- 6.Mackie EJ. Molecules in focus: tenascin-C. The international journal of biochemistry & cell biology. 1997;29:1133–1137. doi: 10.1016/s1357-2725(97)00031-9. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer research. 2001;61:8586–8594. [PubMed] [Google Scholar]
- 8.Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. The Journal of pathology. 2003;200:488–499. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- 10.Midwood KS, Schwarzbauer JE. Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Molecular biology of the cell. 2002;13:3601–3613. doi: 10.1091/mbc.E02-05-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. The Journal of cell biology. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. The Journal of biological chemistry. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 13.Maeda M, Kubo K, Nishi T, Futai M. Roles of gastric GATA DNA-binding proteins. The Journal of experimental biology. 1996;199:513–520. doi: 10.1242/jeb.199.3.513. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki E, Evans T, Lowry J, Truong L, Bell DW, Testa JR, Walsh K. The human GATA-6 gene: structure, chromosomal location, and regulation of expression by tissue-specific and mitogen-responsive signals. Genomics. 1996;38:283–290. doi: 10.1006/geno.1996.0630. [DOI] [PubMed] [Google Scholar]
- 15.Capo-chichi CD, Roland IH, Vanderveer L, Bao R, Yamagata T, Hirai H, Cohen C, Hamilton TC, Godwin AK, Xu XX. Anomalous expression of epithelial differentiation-determining GATA factors in ovarian tumorigenesis. Cancer research. 2003;63:4967–4977. [PubMed] [Google Scholar]
- 16.Perlman H, Suzuki E, Simonson M, Smith RC, Walsh K. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. The Journal of biological chemistry. 1998;273:13713–13718. doi: 10.1074/jbc.273.22.13713. [DOI] [PubMed] [Google Scholar]
- 17.Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss JF., 3rd The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. The Journal of biological chemistry. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 18.Mano T, Luo Z, Malendowicz SL, Evans T, Walsh K. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circulation research. 1999;84:647–654. doi: 10.1161/01.res.84.6.647. [DOI] [PubMed] [Google Scholar]
- 19.Lepore JJ, Cappola TP, Mericko PA, Morrisey EE, Parmacek MS. GATA-6 regulates genes promoting synthetic functions in vascular smooth muscle cells. Arteriosclerosis, thrombosis and vascular biology. 2005;25:309–314. doi: 10.1161/01.ATV.0000152725.76020.3c. [DOI] [PubMed] [Google Scholar]
- 20.Nakerakanti SS, Kapanadze B, Yamasaki M, Markiewicz M, Trojanowska M. Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. The Journal of biological chemistry. 2006;281:25259–25269. doi: 10.1074/jbc.M600466200. [DOI] [PubMed] [Google Scholar]
- 21.Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Tenascin-C upregulation by transforming growth factor-beta in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene. 2004;23:1656–1667. doi: 10.1038/sj.onc.1207064. [DOI] [PubMed] [Google Scholar]
- 22.Makhluf HA, Stepniakowska J, Hoffman S, Smith E, LeRoy EC, Trojanowska M. IL-4 upregulates tenascin synthesis in scleroderma and healthy skin fibroblasts. The Journal of investigative dermatology. 1996;107:856–859. doi: 10.1111/1523-1747.ep12331160. [DOI] [PubMed] [Google Scholar]
- 23.Rettig WJ, Erickson HP, Albino AP, Garin-Chesa P. Induction of human tenascin (neuronectin) by growth factors and cytokines: cell type-specific signals and signalling pathways. Journal of cell science. 1994;107(Pt 2):487–497. [PubMed] [Google Scholar]
- 24.Sakai Y, Nakagawa R, Sato R, Maeda M. Selection of DNA binding sites for human transcriptional regulator GATA-6. Biochemical and biophysical research communications. 1998;250:682–688. doi: 10.1006/bbrc.1998.9374. [DOI] [PubMed] [Google Scholar]
- 25.Beiter K, Hiendlmeyer E, Brabletz T, Hlubek F, Haynl A, Knoll C, Kirchner T, Jung A. beta-Catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene. 2005;24:8200–8204. doi: 10.1038/sj.onc.1208960. [DOI] [PubMed] [Google Scholar]
- 26.McKean DM, Sisbarro L, Ilic D, Kaplan-Alburquerque N, Nemenoff R, Weiser-Evans M, Kern MJ, Jones PL. FAK induces expression of Prx1 to promote tenascin-C-dependent fibroblast migration. The Journal of cell biology. 2003;161:393–402. doi: 10.1083/jcb.jcb.200302126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mettouchi A, Cabon F, Montreau N, Dejong V, Vernier P, Gherzi R, Mercier G, Binetruy B. The c-Jun-induced transformation process involves complex regulation of tenascin-C expression. Molecular and cellular biology. 1997;17:3202–3209. doi: 10.1128/mcb.17.6.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherzi R, Briata P, Boncinelli E, Ponassi M, Querze G, Viti F, Corte G, Zardi L. The human homeodomain protein OTX2 binds to the human tenascin-C promoter and trans-represses its activity in transfected cells. DNA and cell biology. 1997;16:559–567. doi: 10.1089/dna.1997.16.559. [DOI] [PubMed] [Google Scholar]
- 29.Durocher D, Charron F, Warren R, Schwartz RJ, Nemer M. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. The EMBO journal. 1997;16:5687–5696. doi: 10.1093/emboj/16.18.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepulveda JL, Belaguli N, Nigam V, Chen CY, Nemer M, Schwartz RJ. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: role for regulating early cardiac gene expression. Molecular and cellular biology. 1998;18:3405–3415. doi: 10.1128/mcb.18.6.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antoniv TT, Tanaka S, Sudan B, De Val S, Liu K, Wang L, Wells DJ, Bou-Gharios G, Ramirez F. Identification of a repressor in the first intron of the human alpha2(I) collagen gene (COL1A2) The Journal of biological chemistry. 2005;280:35417–35423. doi: 10.1074/jbc.M502681200. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Tanaka S, Ramirez F. GATA-4 binds to an upstream element of the human alpha2(I) collagen gene (COL1A2) and inhibits transcription in fibroblasts. Matrix Biol. 2005;24:333–340. doi: 10.1016/j.matbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Nemer G, Nemer M. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Developmental biology. 2003;254:131–148. doi: 10.1016/s0012-1606(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 34.Eisbacher M, Holmes ML, Newton A, Hogg PJ, Khachigian LM, Crossley M, Chong BH. Protein-protein interaction between Fli-1 and GATA-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative DNA binding. Molecular and cellular biology. 2003;23:3427–3441. doi: 10.1128/MCB.23.10.3427-3441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubo M, Czuwara-Ladykowska J, Moussa O, Markiewicz M, Smith E, Silver RM, Jablonska S, Blaszczyk M, Watson DK, Trojanowska M. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. The American journal of pathology. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


