Abstract
This study examines axonal changes in goat cervical facet joint capsules (FJC) subjected to low rate loading. Left C5–C6 FJC was subjected to a series of tensile tests from 2 mm to failure using a computer-controlled actuator. The FJC strain on the dorsal aspect was monitored by a stereo-imaging system. Stretched (n = 10) and unstretched (n = 7) capsules were harvested and serial sections were processed by a silver impregnation method. The mean peak actuator displacement was 21.3 mm (range: 12–30 mm). The average peak strain encompassing various regions of the capsule was 72.9 ± 7.1%. Complete failure of the capsule was observed in 70% of the stretched capsules. Silver impregnation of the sections revealed nerve fibers and bundles in all the regions of the capsule. A blinded analysis of digital photomicrographs of axons revealed a statistically significant number of swollen axons with non-uniform caliber in stretched FJCs. Axons with terminal retraction balls, with occasional beaded appearance or with vacuolations were also observed. Stretching the FJC beyond physiological range could result in altered axonal morphology that may be related to secondary or delayed axotomy changes similar to those seen in central nervous system injuries where axons are subjected to stretching and shearing. These may contribute to neuropathic pain and are potentially related to neck pain after whiplash events.
Keywords: Facet joint capsule, Stretch, Axons, Swelling, Silver staining
Introduction
Whiplash is a major cause of traumatic neck pain, carries tremendous socio-economic impact and is among the most common injuries associated with motor vehicle accidents. Previous kinematic studies addressing the mechanisms of whiplash injury offer a wealth of knowledge on the possible involvement of cervical facet joints (FJ) during a whiplash impact [11, 33, 35, 42]. The potential role of FJs as a major source of pain after whiplash injury has been demonstrated by studies involving local anesthetic blocks to FJ or its nerve supply [3, 26], percutaneous radio-frequency neurotomy to nerve innervating the FJ [25] and FJ distension with contrast medium [12].
The presence of nerve fibers associated with pain transmission in the cervical facet joint capsules (FJC) has been previously demonstrated [21, 30]. Facet distraction was shown to lead to allodynia in rats, with the level of allodynia varying according to the magnitude of distraction [22]. FJ distraction in anesthetized goats was also shown to elicit response of low and high threshold group III and IV afferents [28]. In the lumbar region pulling the FJ or mechanical probing of the capsule also elicited response from group III and IV units [36, 43]. Induced inflammation in the lumbar FJC resulted in increased multiunit discharge rate, sensitization to mechanical stimuli and recruitment of silent units [34].
Axonal abnormalities of the FJC as a consequence of FJ distraction have never been reported in the published literature. Some of the earliest descriptions of peripheral axonal injury come from the studies of Cajal on sciatic nerve injury [9]. In fact, there is a general lack of literature on axonal changes in mechanically injured peripheral soft tissue.
The most extensive knowledge on altered axonal cytoskeleton comes from studies aimed at understanding the mechanism(s) of traumatic brain injury (TBI) [13, 15, 19, 38]. It has been reported that an episode of TBI results in distinct phases of axonal injury termed primary axotomy; the disruption of axonal cylinder and a delayed secondary axotomy; progressive alterations of the axon cylinder [37]. Using anterograde tracers, it was shown that a traumatic episode triggers a perturbation the point of anterograde transport impairment within an hour. The impairment or focal block leads to further accumulation resulting in swelling and expansion of the axonal cylinder. This, over time leads to lobulation of the focal axonal swellings and subsequent disconnection of the axon at 6–12 h after injury [37]. In peripheral nerve injuries, altered axonal morphology, neuronal excitability and painful behavioral outcomes are reported [5, 10, 24, 44]. We hypothesize that severe joint sprains, including whiplash injury, may involve injury to nerve branches, with consequent peripheral neuropathy and chronic pain.
Consequently, the purpose of this study was to examine the presence of abnormal axons in cervical FJCs subjected to mechanical deformation. We hypothesize that stretching of the FJC beyond the physiologic range leads to cytoskeletal changes in the axons. The ensuing cascade of events following the axonal changes may be involved in the etiology of persistent pain in the event of a whiplash. More generally, such axonal changes may be involved in chronic pain after soft tissue injury and possible neuroma formation.
Materials and methods
Surgery
All surgical procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). Adult Lamancha or Alpine goats (33–55 kg) were anaesthetized by diazepam (0.5 mg/kg, i.m.), pentothal (15 mg/kg, i.v.), butorphanol (0.22 mg/kg, i.m.) and atropine (0.066 mg/kg, i.m.). The anaesthesia was maintained by inhalation of isoflurane (2.5–3%). A C2 to T2 midline incision was made and the spinal muscles were carefully retracted exposing the left C5/C6 FJC as any damage to FJC would compromise the experiment. The ventral rami of C4–C6 nerves were cut and a C4–C7 laminectomy was performed exposing the left C6 roots.
Surgical preparation of the C5/C6 FJC for biomechanical testing
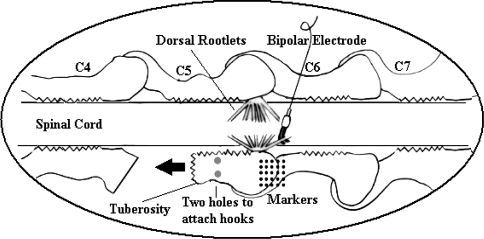
A total of ten goats that were part of a larger biomechanical and neurophysiological study were used for the purpose of this histochemical study. The left C5–C6 FJC in each goat was mechanically stretched using a method described by Lu et al. [27]. The right C5–C6 FJC was not stretched. As part of the preparation of left C5/C6 capsule for biomechanical testing, the left C5 superior articular process was removed. The C5 inferior articular process was freed very carefully without causing any damage to the capsule. Then the C5 inferior articular process was trimmed such that there was sufficient bony mass to drill two miniature holes (5 mm apart) for the insertion of hooks just rostral to the C5–C6 facet joint capsule (Fig. 1). The C5 inferior articular process was then carefully separated from the pedicle using a surgical chisel without disturbing the vertebral artery or the spinal nerves, yielding a freely movable C5–C6 facet joint capsule.
Fig. 1.
Diagram showing neurophysiology recording, and biomechanical testing of the cervical facet joint capsule. Arrow indicates the direction of stretch (modified from Lu et al. 2005)
The freed process was connected to an actuator by a stainless steel hook. Mechanical stretching of the C5–C6 FJ was achieved by a computer-controlled Gemini GV6 digital servo actuator (Parker Hannifin Corp., Roherk Park, CA, USA) attached with a 50 lb load cell (22.7 kg) (Entran, Fairfield, NJ, USA). The whole set-up included a testing frame, a spine fixture, and a stereo imaging system. A restraining strut attached from the test frame to the goat spine through the T1 spinous process was aimed at minimizing whole body motion. Additional spinal movement was also minimized by immobilizing the C6–C7 and C7–T1 joints. These steps enabled that maximum movement was limited to the freed C5–C6 FJC and not translated to the rest of the body or the contra lateral capsule.
The left FJ was then subjected to a series of low rate loading while simultaneously monitoring the afferent discharge. Neural activity of left C6 dorsal rootlets was recorded with a custom designed miniature bipolar electrode as described by Chen et al. [7]. In the biomechanical testing, each test had a trapezoidal loading pattern: a displacement ramp, a 10 s hold and a release ramp. A displacement rate of 0.5 mm/s was used in both ramps. The first test was a 2 mm displacement, with 2 mm increases in displacement for subsequent tests until the capsule ruptured. An array of tantalum spheres (diameter = 0.5 mm) were applied on the dorsal FJC to monitor its deformation. Utilizing these markers, the dorsal capsule (n = 7) was later evaluated at nine different locations for strain distribution using a finite element method [19]. The nine areas were on rostral (medial, middle, lateral), middle or joint gap (medial, middle, lateral) and caudal (medial, middle, lateral) aspects of the capsule.
Biomechanical testing and neurophysiological recordings lasted an average of 6 h after the initial surgical preparations that varied in duration from approximately 4 to 7 h. During the entire process of FJ preparation for biomechanical testing, the exposed left C5/C6 capsule was covered in 0.9% saline. The right FJC was left intact and was not exposed. These untested right FJC were harvested (n = 10) to serve as controls. The left C5/C6 FJC were also harvested after the conclusion of biomechanical testing and neurophysiological recordings.
Determination of strain
For three-dimensional 3D strain analysis, ImageExpress 3D Wizard (SAI, Utica, NY, USA) was used to track the displacements of the tantalum spheres to obtain their 3D coordinates at any time point. Linear membrane elements were developed using Hypermesh (Altair, Troy, MI, USA) by interpolating the array of markers to an element mesh. Digitized marker displacements were imposed on the nodes of the mesh to reconstruct the capsule deformation. LS-DYNA (LSTC, Livermore, CA, USA) was then used to perform strain analysis for the whole capsules. Maximum principal Lagrangian strain contour and principal strain vectors on the mesh could be obtained for any time point. The strain history of a mesh element, where the receptor field was located, was obtained for maximum principal Lagrangian strain [28, 29].
Histological study
At the conclusion of biomechanical testing and neurophysiological recordings, the animals were euthanized and death ensured by bilateral pneumothorax. Then stretched left C5-C6 (n = 10) and un-stretched right C5–C6 (n = 10) dorsal FJ capsules were harvested. The capsules were harvested enblock using a #10 scalpel blade. They were fixed in 10% neutral buffered formalin. Thirty five to 40 μm thick frozen longitudinal sections were cut (Leica CM 3050, Leica Microsystems Nussloch GmbH, Nussloch, Germany), mounted on super frosted slides and stored at −20°C until processed for silver impregnation.
Silver staining
The sections were air dried and glued to the slides using cyanoacrylate. The sections were stained by a silver impregnation technique [16] to visualize nerve fibers. The sections were thoroughly washed in distilled water and immersed for 3 min in pretreatment solution prepared with a mixture of equal volumes of 9% sodium hydroxide and 15% hydroxylamine. The sections were then thoroughly washed in 0.5% acetic acid for three times (3 min each). The sections were then incubated in an impregnation solution (5 mg/ml ferric nitrate and 100 mg/ml silver nitrate) for 30 min and then washed in 1% citric acid (4 × 2 min) followed by a wash in 0.5% acetic acid for 5 min. The sections were placed in developer solution until they turned pale gray. Once sufficiently developed they were removed and washed in 0.5% acetic acid (3 × 10 min). Finally, they were rinsed in distilled water, dehydrated in graded alcohol, cleared in xylene and cover-slipped using Permount. The sections were observed under a Leica light microscope (Leica DMLB, Leica Microsystems Ltd, Heerburg, Switzerland) for stained fibers that were photographed by a digital camera system (Diagnostic Instruments Inc, Sterling Heights, MI, USA) attached to the microscope.
Abnormal axon quantification
The digital photomicrographs of the stained axons were screened on a computer monitor. They were then evaluated by four blinded investigators. The axonal abnormalities were categorized as: swollen axons, beaded axons (round retraction balls), axonal vacuolations, and any other non-specific changes. Axonal swellings and retraction balls are hallmarks of diffuse axonal injury (DAI) in studies of TBI. Each photomicrograph was assigned as having one of these described abnormalities or no abnormality. An abnormality was counted valid only when judged abnormal by at least three of the four investigators. All the observations were tabulated and compared using Chi-square testing to compare stretched versus unstretched capsules.
Results
Mechanical testing
The actuator displacement ranged from 12 to 30 mm with an average displacement of 21.3 mm in ten stretched capsules (Table 1). Seven of the ten stretched capsules were analyzed for peak tensile strain, which ranged from 60.2 to 82.4% and averaged 72.9%. No effort was made to correlate the number of damaged axons to regional strain distribution. The detailed results of the relationship between strain and nerve discharge are reported elsewhere [27–29].
Table 1.
Maximum actuator displacement, average strain from 9 designated areas of the capsule in the preceding pull prior to rupture, and capsular injury in 10 capsules subjected to low rate loading
| Goat | Actuator displacement (mm) | Strain (%) | Capsule injury |
|---|---|---|---|
| 1 | 22 | NA | Partial tear |
| 2 | 20 | NA | Rupture |
| 3 | 18 | 77.8 | Partial tear |
| 4 | 30 | 71.4 | Rupture |
| 5 | 26 | 71.7 | Rupture |
| 6 | 26 | 69.8 | Partial tear |
| 7 | 26 | 82.4 | Rupture |
| 8 | 25 | 60.2 | Rupture |
| 9 | 18 | 76.7 | Rupture |
| 10 | 16 | NA | Rupture |
Abnormal axons
Axonal observations were made from ten stretched and seven out of the ten unstretched FJCs that were harvested. Both stretched and unstretched FJCs demonstrated extensive innervation in the superficial dense collagenous layer as well as in the deep adipose-like layer. Nerve fibers could be seen traversing the capsule as short segments, single fibers or in groups of 2 or 3 fibers. They were also seen as thick bundles.
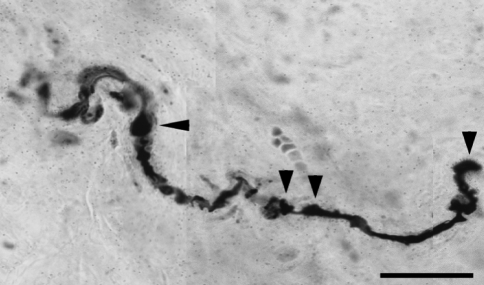
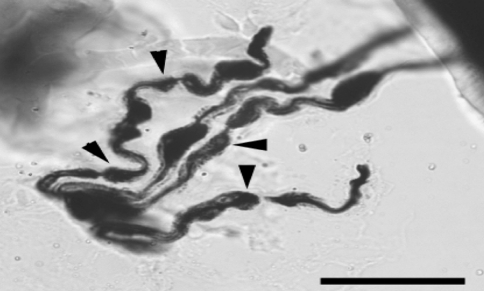
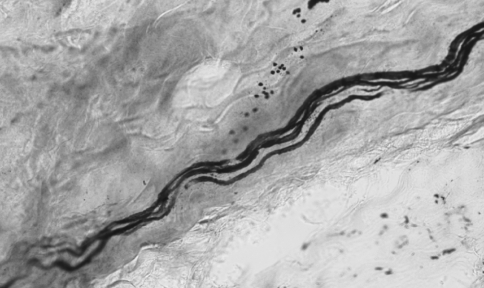
In the stretched FJCs a significantly larger number of abnormal axons were observed compared to un-stretched capsules (Table 2; P < 0.05 Chi-square). Abnormal axons were observed in 94 of 280 photographs from stretched capsule. The most common abnormalities were axons with swellings (Fig. 2) and non-uniform caliber (Fig. 3). Occasionally the swellings appeared to be fusiform. The swollen region could be confined to either one or multiple locations in the course of the axonal path (Fig. 4). Axonal swellings could be best distinguished in single axons or in groups of 2 or 3. For axons in a bundle, the abnormality was either limited to one or more or in some cases to none of the axons.
Table 2.
Total photomicrographs showing normal and abnormal axons in the stretched (n = 10) and unstretched capsules (n = 7)
| Unstretched capsules | Stretched capsules | |
|---|---|---|
| Normal axons | 108 | 186 |
| Abnormal axons | 29 | 94 |
Fig. 2.
A single axon with repeated swellings and terminal retraction balls (bar = 20 μm, ×100)
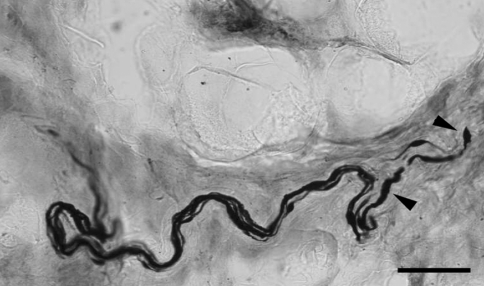
Fig. 3.
Group of axons in a bundle with one of them showing swellings and terminal retraction ball (bar = 50 μm; ×40)
Fig. 4.
Group of axons in a capsule with multiple swollen regions (bar = 20 μm; ×100)
Terminal ball like structures were also seen alone or in association with axons that were swollen (Fig. 3). Although not very common, there were some axons presenting ball-like structures as a chain of beads or as knotty thickenings. Some axons appeared to be excessively wrinkled and distorted. However, not all axons in the stretched capsule were abnormal.
In unstretched FJCs, only 29 of 137 photomicrographs displayed abnormal axons. Normal axons were of uniform caliber with no apparent changes (Fig. 5).
Fig. 5.
Axons with normal appearance in an unstretched capsule (bar = 50 μm; ×40)
Discussion
The primary purpose of this study was to compare the morphological characteristics of axons in stretched facet joint capsules to those in un-stretched capsules in a goat model. As this was a series of tests in which the capsules were pulled beyond the physiologic limits, the study can only address whether such stretch can produce axonal pathology, but does not provide the threshold levels at which these occur.
The goat model has been previously used in several spine studies as a surrogate for human spine including studies involving disk healing and spine fusion [4, 6]. The cervical musculoskeletal structures of goat are similar to their human counterparts in morphology and alignment of facet joints [4]. Hence, findings from these in vivo studies can potentially be related to signs and symptoms in humans such as those in whiplash associated disorders.
The dorsal FJC is a tough collagenous structure covered by multifidus muscle and tendinous insertions. In this study, the capsules were subjected to repeated actuator displacements in increments of 2 mm until rupture. Based on our experimental observations, no gross capsular changes were observed from 2 to 12 mm displacements with the exception of one experiment in which capsular rupture was observed at 12 mm. Tears and rupture were apparent from stretches of 16 mm and above. These numbers represent the displacement of the actuator whereas the displacement across the joint was less than the actuator displacement due to the excursion of the cervical spine during stretch. The response of the tissue is better represented by the tissue strain.
The average peak strain in the ten capsules was 72.9 ± 7.1% (from preceding pull before rupture) with an average actuator displacement of 22.14 mm. In comparison, studies using human cadaver specimens revealed that the C5–C6 facet joints experienced average strains of 60% in moderate speed rear impacts and suggested that the capsular ligaments may undergo stretch levels exceeding their tolerance limits [11]. However, alterations in properties of tissues ex vivo as well as lack of muscle tone cannot be ignored and should be considered cautiously when applying to in vivo biological systems. Using cineradiography technique on human volunteers Ono et al. [33] demonstrated that lower cervical vertebrae (C3–C6) move beyond physiologic range under moderate speed (4, 6, and 8 km/h) rear impacts [33].
A manifestation of the capsular stretch was the presence of nerve fibers with altered morphology. However, it is not possible to predict the strains at which axonal alterations were initiated as this was not the intent of the original study. Additionally, our observations indicate that these axonal changes were not limited to any specific area or the point of rupture of the capsule as they were observed throughout the capsule. Thus, the axonal injuries occurred at areas of capsular stretch that did not necessarily have complete rupture.
These abnormal axonal changes have a close resemblance to those observed in previous studies of TBI [20, 32, 37]. Similar changes were also seen in cultured axons subjected to dynamic stretch with tensile strains between 58 and 77% that resulted in undulating distortions and multiple swellings immunoreactive to neurofilament proteins [40]. The neurofilament immunoreactivity has been suggested as indication of impaired axonal transport or local disassembly of cytoskeleton [40]. However, some abnormal axons were also found in some sections of unstretched FJC. This may be similar to the presence of an occasional degenerating area in normal control brain material in studies of axonal degeneration due to minor head injury as reported by Jane et al. [20]. One limitation of this study was that capsules were harvested 6–12 h following the first stretch of the capsule. The protocol involved repeated actuator displacements until the capsule ruptured. Hence, these acute axonal alterations observed could be related to multiple capsular stretch tests over a period of time. However, to our knowledge, this is the first study in which such axonal changes have been reported.
It has been postulated that reactive changes (secondary axotomy or progressive alterations of the axonal caliber) may occur independent of primary axotomy, which is severing of axons at the time of injury [37]. Bain and Meany [2] reported that optic nerves stretched to 4 mm did not exhibit any injury where as nerves subjected to stretch levels above 6 mm demonstrated injury in the form of axonal swellings and retraction balls. The corresponding nerve strains were in the range of 10 and 50%. Taken together, these studies suggest that axonal changes may occur at various strains starting as low as 10% [2]. The process of axonal expansion (swelling) and disconnection may be slower in some axons than in others due to their caliber, location and other factors [8] and may not be observed in all the axons.
Some of the foremost descriptions of axonal injury come from Cajal [9] who demonstrated various phases of axonal degeneration in injured peripheral stumps of sciatic nerve. Specific axonic masses or neurobiones were reported as the seat of degenerative changes where living units are dislocated and congregate in certain places to form colonies or fusiform masses. These fusiform masses could be similar to the swollen axons that were observed following the capsular stretch in our study. The axonal swellings were thought to be focal accumulations of membranous organelles at presumed sites of interrupted axonal transport [31]. Not all axons would degenerate at the same time and it is possible that degenerating axons could exist amidst some normal appearing axons. Cajal [9] also reported that some axons that degenerate early could appear granular, vacuolated and broken. Although not very common, some axons with vacuolations were also observed in the current study.
What would be the potential functional significance of these axonal changes? Evidence for altered functional changes comes from studies by Bain et al. [1] who showed that ocular displacements (strain rate ∼30 to 60/s, duration 60 ms) greater than 5 mm produced electrophysiological impairment and demonstrated that less stretch was required to elicit electrophysiological changes (5.5 mm) than morphological signs of damage (6.8 mm). Singh et al. [39] studied affects of strain on dorsal nerve roots. They demonstrated that conduction velocity and compound action potentials decreased with increasing strain (<10%, 10–20% and >20%) and displacement (0.01 and 15 mm/s) rate. They further showed increased spacing, tearing and impaired axonal transport that were also strain and displacement rate dependent. Additionally, simple stretching of axons was also shown to lead to changes in membrane potential [14] and transient depolarization [41]. It was also demonstrated that injury to axons leads to increase in intracellular calcium levels due to altered sodium channel structure and subsequent proteolysis of sodium channel alpha subunit. Degradation of the alpha-subunit has been thought to promote persistent elevations in intracellular calcium, fueling additional pathologic changes [18].
Can abnormal axonal morphological changes observed in the stretched facet joint capsules be related to altered behavioral and functional changes? The neurophysiology studies performed on these same goats demonstrated that high threshold mechanoreceptor activation, saturation of the neural discharge and after discharges occurred at peak strains of 47.2, 44, and 45%, respectively. However, determination of capsule threshold strains for axonal injury would require that capsules be harvested at lower strains, perhaps in the range seen for after discharges in this study. Support for potential behavioral changes comes from studies by Lee et al. [23], who investigated cervical FJ distraction and associated behavioral changes in rats. Subjecting the C6/C7 FJ and its capsule to sub-catastrophic vertebral distractions by the translation of C6 spinous process in rats resulted in immediate and sustained behavioral sensitivity as measured by allodynia. Tissue injury, peripheral axonal injury, and/or altered central changes due to sub-catastrophic vertebral distractions may contribute to the behavioral changes.
Nerve injury can lead to formation of neuroma, which can consist of regenerative nerve sprouts and be the source of abnormal excitability and discharge characteristics with pronounced erratic mechanical sensitivity [44]. Altered axonal morphology can also lead to accumulation of sodium channels [10] that may be related to enhanced excitability and spontaneous activity. Additionally, these altered axons can also become sensitive to various mediators released such as cytokines. Recently, Igarashi et al. [17] have shown the release of inflammatory cytokine interleukin 1ß from degenerating lumbar FJ tissue that was shown to be correlated to higher visual analog scores for leg pain. It likely such degenerative changes in cervical FJC tissue may contribute to painful conditions in the neck following a whiplash injury.
Loeser [24] has opined that it is not needed to transect a nerve and create a neuroma to elicit altered sensitivity. It is widely accepted that injury to nerve tissue both in the periphery and central nervous system is capable of causing acute or chronic pain. It is possible that nonphysiological movements of neck as in a whiplash event can potentially lead to axonal changes besides triggering degenerative changes that can ultimately lead to production of various chemical mediators that can sensitize both normal and abnormal axons in the surrounding peripheral tissue.
The findings of this study may have some clinical relevance. The presence of axonal changes in the present study provides credence to the onset of neuropathic changes in FJCs stretched beyond physiologic limits. We propose that such affects may lead to alterations in sensory input and other pathological changes leading perhaps to peripheral neuropathy. This may have particular relevance in cases where the FJ have been damaged/or stretched beyond physiological limits and may be related to persistent pain after a whiplash event.
Conclusions
This is the first known study to demonstrate that nonphysiological stretch of a peripheral tissue such as FJC leads to morphological changes in the axons. These morphological changes may lead to altered functional states in the damaged axons.
Acknowledgments
This research was supported by CDC grants # R49-CCR519751 and R49-CE000455.
References
- 1.Bain AC, Raghupathi R, Meaney DF. Dynamic stretch correlates to both morphological abnormalities and electrophysiological impairment in a model of traumatic axonal injury. J Neurotrauma. 2001;18(5):499–511. doi: 10.1089/089771501300227305. [DOI] [PubMed] [Google Scholar]
- 2.Bain AC, Meaney DF. Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J Biomech Eng. 2000;122:615–622. doi: 10.1115/1.1324667. [DOI] [PubMed] [Google Scholar]
- 3.Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20:20–25. doi: 10.1097/00007632-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Baisden J, Voo LM, Cusick JF, et al. Evaluation of cervical laminectomy and laminoplasty. A longitudinal study in the goat model. Spine. 1999;24:1283–1288. doi: 10.1097/00007632-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 6.Brantigan JW, McAfee PC, Cunningham BW, et al. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone. An investigational study in the Spanish goat. Spine. 1994;19:1436–1444. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chen CY, Lu Y, Cavanaugh JM, Kallakuri S, Patwardhan A. Recording of neural activity from goat cervical facet joint capsule using custom-designed miniature electrodes. Spine. 2005;30(12):1367–1372. doi: 10.1097/01.brs.0000166193.39389.21. [DOI] [PubMed] [Google Scholar]
- 8.Christman CW, Grady MS, Walket CA, Halloway KL, Povlishock JT. Ultrastructural studies of diffuse axonal injuries in humans. J Neurotrauma. 1994;11:173–186. doi: 10.1089/neu.1994.11.173. [DOI] [PubMed] [Google Scholar]
- 9.DeFelipe J, Jones EG (ed) (1991) Cajal’s degeneration and regeneration of the nervous system, History of neuroscience No 5. Oxford University Press, Oxford
- 10.Devor M. The pathophysiology of damaged peripheral nerves. In: Wall PD, Melzack R, editors. Textbook of pain. 3rd edn. Edinburgh: Churchill Livingstone; 1994. pp. 79–101. [Google Scholar]
- 11.Deng B, Begeman PC, Yang KH, Tashman S, King AI (2000) Kinematics of human cadaver cervical spine during low speed rear-end impacts. In: Proceedings of 44th stapp car crash conference, pp 171–188 [DOI] [PubMed]
- 12.Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns. I: A study in normal volunteers. Spine. 1990;15:453–457. doi: 10.1097/00007632-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Graham DI, Raghupathi R, Saatman KE, Meaney D, McIntosh TK. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. 2000;99(2):117–124. doi: 10.1007/PL00007414. [DOI] [PubMed] [Google Scholar]
- 14.Galbraith JA, Thibault LE, Matteson RA. Mechanical and electrical response of the squid giant axon to simple elongation. J Biomech Eng. 1993;115:13–22. doi: 10.1115/1.2895464. [DOI] [PubMed] [Google Scholar]
- 15.Gennarelli TA, Thibault LE, Tipperman R, Tomei G, Sergot R, Brown M, Maxwell WL, Graham DI, Adams JH, Irvine A. Axonal injury in the optic nerve: a model simulating DAI in the brain. J Neurosurg. 1989;71(2):244–253. doi: 10.3171/jns.1989.71.2.0244. [DOI] [PubMed] [Google Scholar]
- 16.Gallyas F, Wolff JR, Bottcher H, Zaborszky L. A reliable method for demonstrating axonal degeneration shortly after axotomy. Stain Tech. 1980;55(5):291–229. doi: 10.3109/10520298009067257. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi A, Kikuchi S, Konno S. Correlation between inflammatory cytokines released from the lumbar facet joint tissue and symptoms in degenerative lumbar spinal disorders. J Orthop Sci. 2007;12(2):154–160. doi: 10.1007/s00776-006-1105-y. [DOI] [PubMed] [Google Scholar]
- 18.Iwata A, Stys PK, Wolf Chen JA XH, Taylor AG, Meaney DF, Smith DH. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci. 2004;24(19):4605–4603. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafari SS, Maxwell WL, Neilson M, Graham DI. Axonal cytoskeletal changes after non-disruptive axonal injury. J Neurocytol. 1997;26(4):207–221. doi: 10.1023/A:1018588114648. [DOI] [PubMed] [Google Scholar]
- 20.Jane JA, Steward O, Gennarelli TA. Axonal degeneration induced by experimental noninvasive minor head injury. J Neurosurg. 1985;62:96–100. doi: 10.3171/jns.1985.62.1.0096. [DOI] [PubMed] [Google Scholar]
- 21.Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical FJCs. Spine. 2004;29:1182–1186. doi: 10.1097/00007632-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Lee KE, Thinnes JH, Gokhin DS, Winkelstein BA. A novel rodent neck pain model of facet mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods. 2004;137(2):151–159. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In Vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373–395. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 24.Loeser JD. Pain due to nerve injury. Spine. 1985;10(3):232–235. doi: 10.1097/00007632-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Lord SM, Barnsley L, Wallis McDonald BJ GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 26.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophyseal joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21:1737–1744. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Chen CY, Kallakuri S, Patwardhan A, Cavanaugh JM. Development of an in vivo method to investigate biomechanical and neurophysiological properties of spine facet joint capsules. Er Spine J. 2005;14(6):565–72. doi: 10.1007/s00586-004-0835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Chen CY, Kallakuri S, Patwardhan A, Cavanaugh JM. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005;23:779–787. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Chen CY, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- 30.McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19:495–501. doi: 10.1097/00007632-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell WL, Graham DI. Loss of axonal microtubules and neurofilaments after stretch-injury to guinea pig optic nerve fibers. J Neurotrauma. 1997;14(9):603–614. doi: 10.1089/neu.1997.14.603. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of non-disruptive axonal injury: a review. J Neurotrauma. 1997;14(7):419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 33.Ono K, Kaneoka K, Wittek A, Kajzer J (1997) Cervical injury mechanism based on the analysis of human cervical vertebral motion and head–neck-torso kinematics during low-speed rear impacts. In: Proceedings of 41st stapp car crash conference, pp 339–356
- 34.Ozaktay AC, Cavanaugh JM, Blagoev DC, Getchell TV, King AI. Effects of a carrageenan-induced inflammation in rabbit lumbar FJC and adjacent tissues. Neurosci Res. 1994;20(4):355–64. doi: 10.1016/0168-0102(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 35.Pearson AM, Ivancic PC, Ito S, Panjabi MM. FJ kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29:390–397. doi: 10.1097/01.BRS.0000090836.50508.F7. [DOI] [PubMed] [Google Scholar]
- 36.Pickar JG, McLain RF. Responses of mechanosensitive afferents to manipulation of the lumbar facet in the cat. Spine. 1995;20:2379–2385. doi: 10.1097/00007632-199511001-00002. [DOI] [PubMed] [Google Scholar]
- 37.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12(4):555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 38.Povlishock JT, Becker DP. Fate of reactive axonal swellings induced by head injury. Lab Invest. 1985;52(5):540–552. [PubMed] [Google Scholar]
- 39.Singh A, Lu Y, Chen CY, et al. A new model of traumatic axonal injury to determine the effects of strain and displacement rates. Stapp Car Crash Jr. 2006;50:601–623. doi: 10.4271/2006-22-0023. [DOI] [PubMed] [Google Scholar]
- 40.Smith DH, Wolf JA, Lusardi TA, Lee VMY, Meaney DF. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J Neurosci. 1999;19(11):4263–4269. doi: 10.1523/JNEUROSCI.19-11-04263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomei G, Spagnoli D, Ducati Landi A, Villani R, Fumagalli G, Sala C, Gennarelli T. Morphology and neurophysiology of focal axonal injury experiments induced in the guinea pig optic nerve, Acta Neuropathol. 1990;80:506–513. doi: 10.1007/BF00294611. [DOI] [PubMed] [Google Scholar]
- 42.Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine. 2000;25:1238–1246. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita T, Cavanaugh JM, El-Bohy AA, Getchell TA, King AI. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg. 1990;72A(6):865–870. [PubMed] [Google Scholar]
- 44.Zimmermann M. Functional characteristics of sensory fibers in regenerating cutaneous nerves. In: Delwaide PJ, Gorio A, editors. Clinical neurophysiology in peripehral neuropathies. Amsterdam: Elsevier; 1985. pp. 41–56. [Google Scholar]