Abstract
This experimental study was designed to compare two different fluoroscopy-based stereotactic surgical techniques for transcutaneous cervical pedicle screw (CPS) placement in the subaxial human cervical spine: (1) a custom-made aiming frame (AF) in combination with conventional fluoroscopy versus (2) a targeting device in combination with a computer-assisted image guidance system [i.e. virtual fluoroscopy (VF)]. Surgery was carried out on six preserved human total body specimens in a laboratory setting. Sixty pedicles (levels C3–C7) were measured in a multislice computed tomography (CT) image data set prior to surgery. Two groups consisting of three specimens and 30 pedicles each were defined according to the surgical technique. The AF consisted of radiolucent components with a fully adjustable arm for carrying the instruments necessary for placing the screws. The arm was angled according to the cervical pedicle axis, as determined by the preoperative CT scans and intraoperative lateral fluoroscopy. For VF, a targeting device was combined with a computer-assisted image-guided surgery unit. For both stereotactic techniques, 3.5 mm screws made of carbon fibre polyetheretherketone (ECF-PEEK) were inserted transcutaneously through stab incisions. Screw placement was assessed using a four-point grading system ranging from ideal (I) to unacceptable (III) where I = screw centred in pedicle, IIa = perforation of pedicle wall is less than one-fourth of the screw diameter, IIb = perforation of the pedicle wall is more than one-fourth of the screw diameter without contact to neurovascular structures, and III = CPS in contact with neurovascular structures. Fifty-eight pedicle screws could be evaluated without interfering metal artefacts according to the same CT protocol that was used preoperatively. The AF technique achieved a significantly smaller number of screws in contact with neurovascular structures compared with the VF technique (P = 0.021; Fisher’s exact test) (Grade I n = 15; 64.3% AF vs. n = 13; 43.3% VF and Grade III n = 2; 7.1% AF vs. n = 10; 33.3% VF). Although neither of the two techniques was capable of completely preventing CPS perforations, transcutaneous CPS placement with a conventional fluoroscopy-based stereotactic AF can be considered a less expensive alternative to VF. This AF technique is able to reduce the number and severity of lateral pedicle wall violations compared to screw placement via the wide standard posterior open midline approach to the subaxial cervical spine. The results of this study are discussed in context with those obtained from different published modifications, since the first technical description of this surgical technique in 1994 by Abumi and co-workers.
Keywords: Cervical spine, Morphometry, Cervical pedicle screws, Frame-based stereotaxis, Computer-assisted fluoroscopic navigation
Introduction
Cervical pedicle screws (CPS) provide superior strengths of fixation compared to standard posterior stabilisation procedures involving wiring or lateral mass screws [20]. During the last decade, CPS have been used in the treatment of degenerative disorders [2], as well as in trauma surgery [3, 11]. Since 1994, several attempts have been made to enhance the safety and accuracy of CPS placement. Based on current experimental and clinical studies [3, 10, 23, 26, 36, 44] computer-assisted surgery systems (CAS) are suggested to be the safest procedures for CPS placement. Appealing clinical results were achieved with CPS [3, 36]. However, in laboratory studies pedicle perforation could not be completely prevented with any technique [10, 23, 34, 38].
In a previous study, CPS placement was examined using a handheld aiming device and newly designed aiming frame (AF) [34]. The present study represents a further attempt to improve the technical feasibility and safety of CPS placement. Therefore, the aims were (a) to test the accuracy of two different fluoroscopy-based stereotactic surgical techniques for CPS placement: a conventional fluoroscopy-based stereotactic AF, as well as a targeting device in combination with computer-assisted fluoroscopic navigation (i.e. “virtual fluoroscopy”) (VF) and (b) to compare the results with published data. As opposed to our earlier study, when the CPS were inserted through an open standard posterior approach [34], for this successive study, a less-invasive transcutaneous approach through stab incisions was applied for screw insertion in both methods.
Materials and methods
Specimens
Six (two males and four females) preserved and randomly chosen human total body specimens were obtained from the Department of Human Anatomy. All cervical spines were examined with standard radiographs and CT scans including multiplanar reconstruction to exclude anomalies, tumors or severe multi-segmental changes. All cervical spines had various degrees of osteoporosis and degenerative changes as expected for an average age of 81 years (range 56–95 years). The specimens were divided into two groups with three specimens each (30 pedicles), so that the pedicle dimensions of the groups were evenly distributed.
Pedicle morphometry
Preoperative CT scans (C3–C7) were acquired using a standard algorithm with a slice thickness of 1.0 mm. Multiplanar two-dimensional reconstructions were performed with Advantage Windows 4.2 (GE LightSpeed QX/I, Milwaukee, USA).
Direct digital CT measurement of four linear parameters and the pedicle axes was performed for all sixty pedicles and are listed below:
Measured pedicle dimensions and angles (Fig. 1)
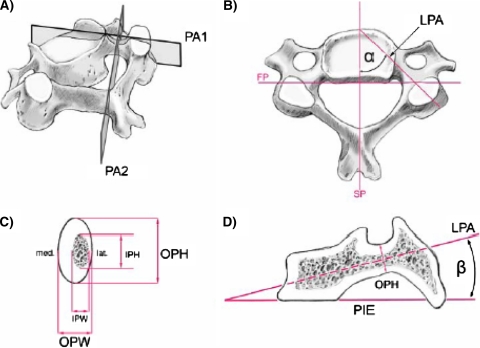
Fig. 1.
Mid-cervical vertebra showing cuts and lines used for CT measurements: a PA 1 designates the vertical cut through the longitudinal pedicle axis (LPA) and PA 2 is the vertical cut through the isthmus of the pedicle perpendicular to PA 1. b Superior view showing the sagittal plane (SP), frontal plane (FP), longitudinal pedicle axis (LPA), and pedicle transverse angle α (PTA) between PA 1 and SP. c A PA 2 slice through the pedicle isthmus and its associated measurements: outer pedicle height (OPH), outer pedicle width (OPW), inner pedicle height (IPH), inner pedicle width (IPW). Note the thinner lateral wall thickness compared to its medial counterpart. d A PA 1 cut and its associated measurements: plane of the inferior vertebral endplate (PIE) and the pedicle sagittal angle β (PSA) between PIE and the longitudinal pedicle axis (LPA)
Outer pedicle width (OPW) [mm]: outer medio-lateral diameter or width of the isthmus.
Outer pedicle height (OPH) [mm]: outer supero-inferior diameter or height of the isthmus.
Inner pedicle width (IPW) [mm]: inner medio-lateral diameter of the pedicle core.
Inner pedicle height (IPH) [mm]: inner supero-inferior diameter of the pedicle core.
Pedicle transverse angle α (PTA): angle between the sagittal plane and longitudinal pedicle axis (LPA).
Pedicle sagittal angle β (PSA): Angle between the inferior vertebral endplate (PIE) and LPA.
All paired structures of the vertebrae were measured individually for the left side and right side. Summary measurements such as the mean and standard deviation were then calculated at each vertebral level.
Implants
Endless carbon-fiber polyetheretherketone (ECF-PEEK) pointed screws1 (core diameter 2.8 mm, outer diameter 3.5 mm) were used for every cervical pedicle and both techniques. These screws were chosen because their material does not create significant artifacts in CT images, and allows for precise postoperative determination of the screw position.
Stereotactic surgical techniques
Surgery was performed in a laboratory operating setting by two surgeons (FM and MR) with the following identical procedures in both techniques. The bodies were placed in a prone position onto a radiolucent table with a halo ring attached to the head. The halo was fixed to a fully adjustable head fixation device [5]. The neck was placed in mild flexion so that the cervical spine was parallel to the floor. Abnormal axial rotation of the cervical spine was corrected by rotating the head, prior to application of the PTA at the exposed vertebra.
A straight posterior midline skin incision was used for exposure of the C3–C7 posterior vertebral elements. As opposed to our initial study [34], the screws were transcutaneously inserted through stab incisions. Therefore, the retraction of muscles was minimized to such an extent that allowed for adequate identification of the screw entry points.
AF
The device consists of an AF mounted onto a radiolucent plate (Fig. 2a–f), with a fully adjustable radiolucent arm to carry the instruments necessary for the implantation procedure.
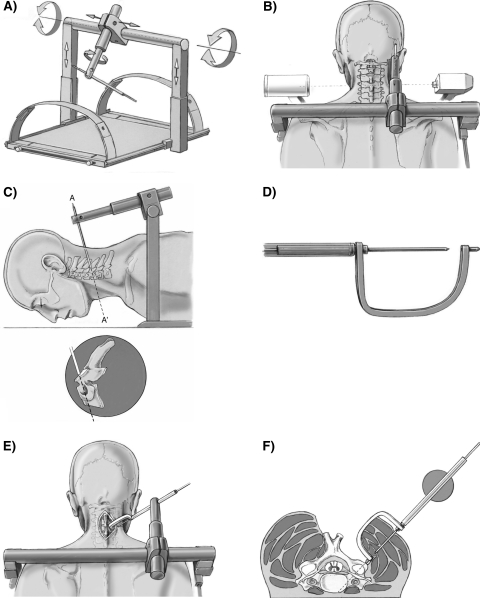
Fig. 2.
Description and stepwise adjustment procedure of the aiming frame (AF) for transcutaneous CPS placement with lateral fluoroscopic guidance
AF adjustment
The stepwise adjustment procedure of the AF for transcutaneous CPS placement begins by initially inserting the sleeve into a tissue protection sleeve together with a long K-wire. Lateral fluoroscopy is centered onto the respective vertebra such that the two pedicles, as well as the facet joints appear congruent with each other (Fig. 2b). The K-wire lying in the sagittal plane and positioned lateral to the neck, is then brought into alignment with the pedicle axis according to the PSA (Fig. 2c). Adjustment of the K-wire according to the predetermined individual PTA is achieved by rotating the radiolucent arm in the plane of the PSA. A special curved sleeve is then attached to the K-wire (Fig. 2d). With this curved sleeve inserted into the approach, the radiolucent arm can be shifted along the transverse bar until the tip of the curved sleeve is positioned on the entrance point of the screw. The axis of the tissue protection sleeve is aligned with the pedicle axis, and the entrance point is determined according to the method of Karaikovic and co-workers [15]. The components of the aiming frame are finally locked and all further manipulations are carried out through the adjusted tissue protection sleeve.
The K-wire is advanced to the skin. After a stab incision, further advancement of the K-wire through the muscles is followed by the pulling back of the K-wire such that the curved sleeve can be removed. The K-wire is again advanced to the entry point together with its sleeve and the screw hole is drilled whilst being monitored by lateral fluoroscopy (Fig. 2e, f). After the removal of the K-wire and its sleeve, the tissue protection sleeve is advanced to the surface of the bone and the screw is finally inserted also under lateral fluoroscopic control.
VF
Fluoroscopic navigation was performed with the commercially available FluoroNav® system (Stealth Station Treon plus, Orthopedics Trauma Version 3.0.3, Medtronic Inc., Louisville, USA), which was connected to a C-arm fluoroscope (OEC 9600, GE OEC Medical Systems, Lindon, USA). A calibration target equipped with infrared diodes was mounted on the C-arm. The position of the C-arm in relation to the specimen was measured by an optoelectric camera. VF allows for both planning of the CPS trajectory, as well as interactive tracking of the surgical instruments. In order to account for the initial learning curve for this particular procedure, a representative from the manufacturer introduced the system and supervised the first screw applications prior to the beginning this study.
Three basic steps were repeated for every vertebral level:
Referencing and image acquisition:
A spine clamp [dynamic reference array (DRA)] with passive reflectors is fixed to the spinous process of the specific vertebral level requiring instrumentation. After acquisition of two fluoroscopic images (i.e. lateral and oblique views in the axis of the pedicle), the computer then calibrates the obtained images and calculates the spatial relations among the acquired images, DRA, C-arm and real anatomy.
Determination of the screw trajectory:
The trajectory is determined on the basis of plans derived from the acquired fluoroscopic images, and follows a central pathway within the pedicle (Fig. 1).
Targeting and screw insertion:
An adjustable mechanical arm of a targeting device2 [4] is used to hold the drill sleeve, which is tracked by the navigation system. A real-time overlay of the virtual drill is initially generated onto the calibrated fluoroscopic images to determine the actual position of the instrument with respect to the plan. The targeting device is then adjusted according to the predefined screw trajectory (Fig. 3). Thereafter, the drill sleeve is transcutaneously advanced through a stab incision and rigidly locked in the defined position by the mechanical arm of the targeting device. The screw holes are finally drilled with a 2.7 mm drill bit through the drill sleeve, followed by insertion of the 3.5 mm ECF-PEEK screw.
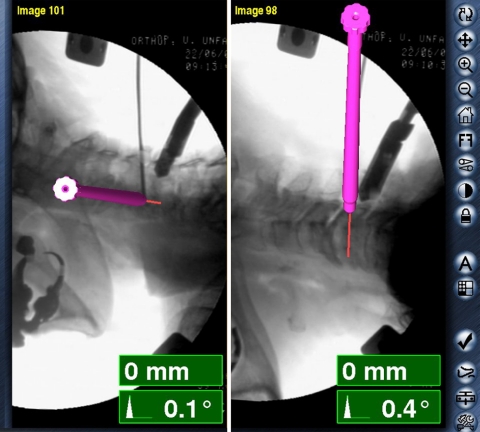
Fig. 3.
Virtual fluoroscopy. Computer images of real-time guidance for CPS (level C5) with simultaneous display of the planned screw trajectory in lateral and oblique fluoroscopic views
Grading of the CPS position and final data analysis
After CPS placement, a multislice CT with the original identical parameters was again carried out. The screw position was independently evaluated by two surgeons (MR, FM) with regard to the extent of pedicle wall violation using the following grading system:
Grade I: screw is centered in the pedicle with only plastic deformation of the pedicle cortex.
Grade IIa: screw threads or less than one-quarter of the screw cross-section penetrates the cortex. No contact of the screw with neurovascular structures.
Grade IIb: More than one-quarter of the screw cross-section penetrates the cortex but there is no contact with neurovascular structures.
Grade III: CPS in contact with neurovascular structures.
The means and SD were calculated for all linear and angular pedicle measurements. Large significance values (P > 0.05) from the one-sample Kolmogorov–Smirnov test indicated a normal distribution of the measured data. To compare the CT measurements between right and left pedicles, an independent sample t test procedure with the significance set at a 95% confidence level was performed. The Fisher’s exact test was used to compare screw positioning and accuracy between surgical techniques with regard to pedicle dimensions and vertebral level. The software SPSS 13.0 for Windows© (SPSS Inc., Chicago, USA) was used for statistical analysis.
Results
Pedicle measures
Preoperative linear and angular parameters of all 60 pedicles were obtained and are presented in Table 1. Overall, there were no significant differences in the dimensions and angular parameters as determined for the left and right pedicles, as well as for the two treatment groups (i.e. AF vs. VF) (P > 0.05; independent samples t test). For this reason, the values are presented without referring to either the pedicle side or treatment group.
Table 1.
Mean pedicle CT measurements as determined for the various vertebral levels, where the linear parameters (i.e. OPW, OPH, IPW, IPH) were measured at the isthmus of the pedicle and angular parameters (i.e. PTA, PSA) characterize the pedicle axis
| Pedicle | OPW (mm) | OPH (mm) | IPW (mm) | IPH (mm) | PTA (°) | PSA (°) |
|---|---|---|---|---|---|---|
| C3 | ||||||
| Mean ± SD | 5.1 ± 0.7 | 7.8 ± 1.0 | 1.9 ± 0.5 | 3.1 ± 0.5 | 46.0 ± 4.2 | 20.9 ± 7.6 |
| Range | 3.7–6.1 | 5.7–8.9 | 1.2–2.8 | 2.0–3.8 | 37.0–52.0 | 10.0–40.0 |
| C4 | ||||||
| Mean ± SD | 5.8 ± 0.6 | 7.8 ± 0.8 | 2.4 ± 0.4 | 3.4 ± 0.6 | 48.8 ± 4.5 | 10.5 ± 8.9 |
| Range | 4.8–6.8 | 6.6–9.3 | 1.9–3.5 | 2.2–4.3 | 40.0–56.0 | −6.0 to 26.0 |
| C5 | ||||||
| Mean ± SD | 5.7 ± 1.3 | 7.1 ± 1.0 | 2.9 ± 1.1 | 3.1 ± 0.4 | 48.1 ± 6.6 | −3.6 ± 6.6 |
| Range | 2.3–7.0 | 5.6–8.6 | 1.5–5.8 | 2.6–3.6 | 36.0–57.0 | −14.0 to 7.0 |
| C6 | ||||||
| Mean ± SD | 6.0 ± 0.9 | 6.7 ± 1.1 | 2.4 ± 0.5 | 2.7 ± 0.5 | 41.0 ± 3.7 | −12.2 ± 7.4 |
| Range | 4.6–7.2 | 4.4–8.3 | 1.9–3.2 | 1.9–3.5 | 35.0–46.0 | −23.0 to −2.0 |
| C7 | ||||||
| Mean ± SD | 7.1 ± 0.4 | 7.7 ± 1.5 | 3.3 ± 0.6 | 3.6 ± 0.7 | 40.3 ± 2.7 | −3.5 ± 12.4 |
| Range | 6.7–8.0 | 5.8–11.1 | 2.0–4.6 | 2.5–4.8 | 36.0–45.0 | −16.0 to 22.0 |
Pedicle measurements and dimensions confirm previous observations by our group, where outer and inner pedicle widths showed an increase from the cranial to caudal orientation. The pedicle heights were also found to be similar in all subaxial vertebrae [34].
The average internal pedicle dimensions (IPW and IPH) varied from 1.9 mm at the C3 level up to 3.6 mm at the C7 level. The average PTA was 45° with the range of 35°–57°. The smallest PTA was observed at C7, with the largest at C4. Pedicles were directed in a slightly caudal fashion with respect to the lower endplate at C7 and C6; they were also cranially directed at the C3 and C4 levels.
CPS position
A total of 58 pedicles were instrumented with CPS. Two pedicles were omitted because of severe changes at the articular massif that did not allow for exact identification of the entrance point of the screw. Table 2 displays the numbers and incidence of pedicle perforations in relation to the vertebral level (C3–C7) and surgical technique (AF vs. VF).
Table 2.
Accuracy of CPS positioning and the incidence of pedicle wall violations, in relation to vertebral level and surgical technique
| Pedicle | Technique | n (instrumented) | Violation of pedicle cortex | |||
|---|---|---|---|---|---|---|
| n (Grade I) | n (Grade IIa) | n (Grade IIb) | n (Grade III) | |||
| C3 | AF | 5 | 3 | 0 | 2 | 0 |
| VF | 6 | 5 | 0 | 0 | 1 | |
| C4 | AF | 6 | 4 | 1 | 0 | 1 |
| VF | 6 | 2 | 0 | 2 | 2 | |
| C5 | AF | 6 | 5 | 0 | 1 | 0 |
| VF | 6 | 2 | 2 | 0 | 2 | |
| C6 | AF | 6 | 4 | 0 | 2 | 0 |
| VF | 6 | 2 | 2 | 1 | 1 | |
| C7 | AF | 5 | 2 | 0 | 2 | 1 |
| VF | 6 | 2 | 0 | 0 | 4 | |
| AF (total) | 28 | 18 | 1 | 7 | 2 | |
| VF (total) | 30 | 13 | 4 | 3 | 10 | |
| AF (total %) | 64 | 4 | 25 | 7 | ||
| VF (total %) | 43 | 13 | 10 | 33 | ||
| 58 | 53% | 9% | 17% | 21% | ||
Cortical perforations were compared with regard to the right (30 screws) and left (28 screws) sides, where no significant difference was found (P = 0.544; Fisher’s exact test). Postoperative CT analysis showed an ideal position for 31 CPS (Grade I). Only five CPS caused Grade IIa perforations with 10 and 12 pedicle perforations graded under the category of IIb and III, respectively.
With regard to technique, statistical significance was found in the number, as well as severity of pedicle wall violations occurring between the two methods (P = 0.021; Fisher’s exact test). Apparent differences were particularly observed between the Grade I and Grade III perforations (Table 2). The latter perforations (i.e. Grade III) were significantly less frequent whilst employing the AF technique. At the same time, a higher rate of Grade I positioned screws were also found for the AF group (AF n = 18; 64.3% vs. VF n = 13; 43.3%). Hence, safer screw positioning was accomplished with the AF technique.
Although significant correlations existed between the vertebral level and linear pedicle measurements (IPW, OPW, IPH), as well as for both angular measurements (PTA, PSA) (P < 0.001; ANOVA), no statistically significant relationship was detected between the vertebral level and CPS position with either of the surgical techniques (AF P = 0.241; VF P = 0.634) (P = 0.608; Fisher’s exact test) (Tables 1, 2)
Summary of published data
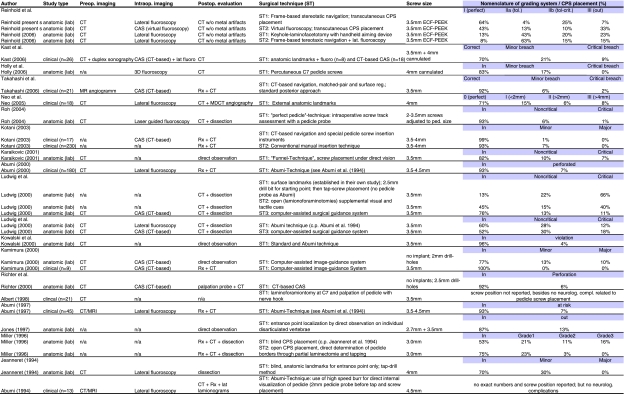
Table 3 represents the summary of results from the published literature regarding the different surgical techniques for subaxial CPS placement. The publications are listed in a chronological sequence from bottom to top beginning with Abumi and co-workers up to this day. The table lists study type, pre- as well as intraoperative imaging modalities, postoperative evaluation methods, type of implant, brief description of the surgical technique, applied grading systems, and the obtained results.
Table 3.
Summary of published data
One will find surgical techniques guided by morphological data or outside anatomic landmarks only that will partly result in severe misplaced screws and high perforation rates. Other surgical techniques depend on a microsurgical approach to the spinal canal/pedicle for direct detection of pedicle wall or introitus usually in conjunction with conventional intraoperative fluoroscopy. These techniques have been proven clinically successful with tolerable but still high potential for cortical violations. Most recently surgical techniques applying computerized image-guided technology have been published with promising results mostly obtained under laboratory conditions and precise CPS positioning.
Discussion
Biomechanical data confirm the outstanding stability of CPS fixation [12, 19–21]. Nevertheless, this technique has not yet replaced the traditional posterior fixation techniques, since it is technically demanding and burdened with the potential risk of severe complications [3, 7, 17, 26, 45]. CPS fixation is therefore only recommended for special indications, particularly in cases with pronounced osteoporosis or multilevel instability with defects of the articular massif [12, 19, 20, 43]. Abumi and co-workers report on a relatively low risk of complications associated with CPS misplacement [3]. On the other hand, laboratory studies revealed a significantly high rate of CPS misplacement [22–24, 38, 45, 46].
The aim of this study was to evaluate two new fluoroscopy-based stereotactic techniques for subaxial CPS placement: a frame-based stereotactic technique (AF) and a computer-assisted image-guided technique (“virtual fluoroscopy”). The results of this study were compared to a former study carried out by our group [34], as well as all pertinent literature (refer to Table 3). The two techniques were applied on three randomly allocated conserved total body specimens, each in a realistic operating setting. The anatomic features of the pedicles essential for placing the CPS were determined prior to surgery using CT measurements.
The dimensions and orientation of cervical pedicles have been thoroughly studied in the past [8, 30, 34, 41, 42, 47]. In general, pedicle measurements presented in this study are consistent with those observed in earlier publications such that no significant differences between the right and left sides could be detected [6, 8, 14, 29, 31, 39, 42, 45]. A slight cranial to caudal increase in pedicle height, as well as a more pronounced increase of pedicle width in a caudal direction was found. This was matched by a commensurate widening of the pedicle canal. The PTA decreased from a cranial to caudal orientation and the PSA was found to be directed cranially in the upper part of the cervical spine and caudally in the lower part. Cervical segments with very small inner diameters of the pedicle or without medullary canal should be excluded from the procedure. For instance, in an Asian population with generally smaller sized pedicle dimensions than in non-Asians, 3.5 mm screws may render cervical pedicles in as high as 54.2% of male or 73.3% of female patients not feasible for this procedure [47].
Since all measurements can vary significantly between individuals, preoperative CT evaluation is mandatory for precise planning of the surgical procedure [25, 35, 39, 47]. This is regarded as considerably safer, compared to defining screw trajectories by anatomic landmarks and averaged angular dimensions only [9].
Several modifications of CPS fixation as first described by Abumi et al. 1994 [1] have been proposed to facilitate surgery and increase the safety of the technique.
These techniques may be classified into four types and include: (1) techniques relying on anatomical landmarks and averaged angular dimensions [11, 15]; (2) techniques with direct exposure of the pedicle, e.g. by lamino-/foraminotomy [1, 16]; (3) CT-based CAS, and (4) fluoroscopy-based CAS techniques.
In our initial study [34], CPS were inserted through a standard midline approach. This approach has two major disadvantages. First, the insertion space needs to be unacceptably wide in order to allow for adequate screw angulations toward the sagittal plane (PTA), and second, the resultant counterpressure of the nuchal muscles may deviate the instruments medially. Hence, the screws tend to perforate the lateral wall of the pedicle. In order to avoid these problems, Jeanneret and co-workers proposed the transcutaneous insertion of CPS through stab incisions [11].
Our second study focused on the further development of the AF to allow transcutaneous screw insertion. Furthermore, the new AF technique required a comparison to another targeting device [4] and consequently, a state-of-the-art intraoperative fluoroscopy-based computer-assisted navigation system (VF) was employed for this purpose. To our knowledge, current literature has not yet addressed the use of VF with a targeting device for transpedicular screw placement at the subaxial cervical spine.
VF was chosen for comparison with the AF technique because most spine surgeons are familiar with fluoroscopy as an integral part of their daily work and thus, will familiarize themselves faster with this technique. Intraoperative image acquisition with the patient in a definite surgical position is fast and requires no surgeon-derived registration step, once the system is calibrated.
For this study, a realistic laboratory set up was considered essential for evaluating the new surgical techniques, instead of using isolated spine specimens or synthetic bone models. The type of implant was also an important prerequisite to obtain results with high informational value. The material of the screws used in this study consisted only of carbon fibers and PEEK. Unlike metal, these materials do not create artifacts that would inevitably hinder exact postoperative CT evaluation of the screw position [1, 26]. Upon reviewing the literature, the comparison of results obtained with the various surgical techniques remains difficult because of the different study designs, as well as the lack of a standardized method for rating the degree of screw perforations (Table 3). Several publications confirmed low pedicle perforation rates as obtained by CAS [13, 18, 23, 28, 37, 44. Nevertheless, in this study, as well as a number of other more recent studies [17, 22, 33], the use of a CAS system neither prevented screw perforations [44] nor significantly reduced critical screw perforation rates.
No matter what navigational system is used, the results and accuracy will also always depend on the size of the surgical object, that is, there is certainly a threshold with regard to the pedicle size. Once below this limit, the rate of malpositioned screws will eventually increase [22]. As anticipated, accuracy requirements are greatest where the relevant screw diameter (3.5 mm) approximates the internal dimensions of the pedicles, as demonstrated in this study. Mathematical models on accuracy requirements for image-guided pedicle screw placement [32], and clinically relevant precision obtained with current optoelectric computer navigation systems ranging from 1 to 1.7 mm [27, 40] indicate that cervical pedicles can be regarded as objects of marginal size for CAS systems.
The AF technique also has potential sources of error. Since preoperatively determined PTA refers to the sagittal plane (Fig. 1b), abnormal rotation of the cervical spine during surgery would lead to an incorrect screw PTA. Therefore, any detectable degree of rotation was eliminated prior to surgery and the AF was adjusted according to the PTA. Furthermore, the AF technique is not capable of keeping track “online” of unintentional intraoperative displacement of cervical vertebrae, e.g., due to pressure resulting from screw hole drilling. Finally, the AF technique requires exposure of the screw entrance point, while screw implantation can be strictly carried out percutaneously with the VF technique.
Perforations of the lateral pedicle wall remain a point of concern due to the fact that the lateral pedicle wall is thinner and therefore less resistant [12, 14, 16, 26, 31]. A second reason is that the flat angle of the PTA in the cervical spine can hardly be accomplished through a standard midline approach. As already mentioned, when using this approach the counter pressure of the nuchal muscles may deviate instruments medially, thus leading to a higher tendency for lateral perforation of the pedicle wall.
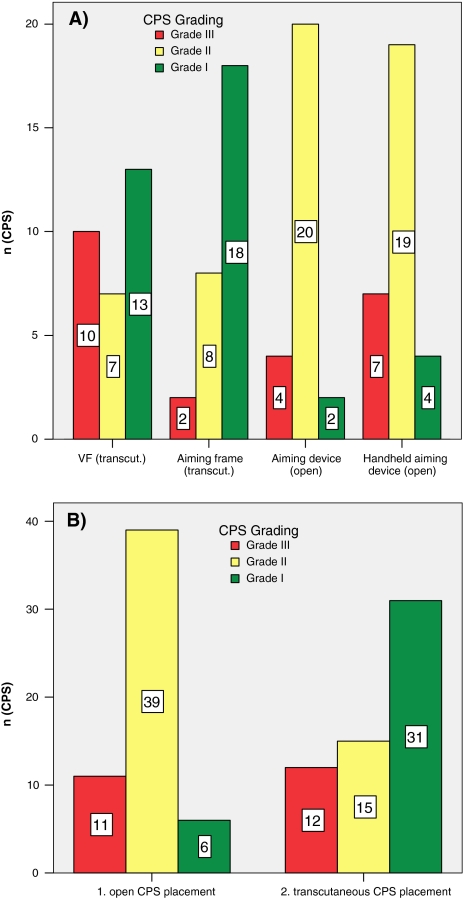
The results obtained from two consecutive studies concerning four different surgical techniques for CPS placement with identical operative setup, evaluation methods, and grading system for CPS position were compared [34] (Fig. 4a). Transcutaneous CPS screw placement showed favorable and significantly better results compared to CPS application via a standard midline approach (P < 0.01; Fisher’s exact test). A higher number of Grade I positions (transcutaneous n = 31 vs. standard approach n = 6) in conjunction with a lower number of Grade II positions (transcutaneous n = 15 vs. standard approach n = 39) occurred after transcutaneous screw implantation (Fig. 4b). Therefore, the higher tendency for a lateral pedicle wall perforation can be successfully obviated by transcutaneous screw placement.
Fig. 4.
a Results obtained from two anatomical studies comparing four different surgical techniques for CPS placement. b Transcutaneous versus open CPS placement
Conclusion
The positioning of pedicle screws in the subaxial cervical spine is a technically demanding surgical method, which is burdened with potential risks. Although data obtained in clinical studies reveal relatively low risk of complications, results achieved under laboratory conditions are less favourable. Pedicle perforations occur whilst employing any pedicle screw placement technique, even in association with CAS navigation. With regard to perforation of the pedicle lateral wall, transcutaneous screw application through a stab incision is apparently safer than screw application through a midline approach. Pedicle perforations occurred with both the frame-based stereotactic procedure (i.e. AF) and computer-assisted fluoroscopic navigation (i.e. VF) technique tested in this study. Even though considerably less critical pedicle perforations occurred with the AF method, it does not seem highly appropriate to clearly favor this technique at this time. Both techniques have their specific advantages and disadvantages. For the VF procedure, it may be considered an advantage that the screws can be inserted percutaneously prior to opening the neck for completing pedicle fixation. Alternately, advantages of the AF are its price and the fact that it can be accomplished with any standard fluoroscope.
Acknowledgments
Implants were provided by Icotec AG, Altstaetten, Switzerland
Footnotes
ICOTEC AG, Altstaetten, Switzerland
EasyTaxis PHILIPS Medical Systems, Wien, Austria
References
- 1.Abumi K, Ito M, Taneichi H, Kaneda K. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: description of the techniques and preliminary report. J Spinal Disord. 1994;7:19–28. doi: 10.1097/00002517-199407010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Abumi K, Kaneda K. Pedicle screw fixation for nontraumatic lesions of the cervical spine. Spine. 1997;22(16):1853–1863. doi: 10.1097/00007632-199708150-00010. [DOI] [PubMed] [Google Scholar]
- 3.Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962–969. doi: 10.1097/00007632-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Bale RJ, Hoser C, Rosenberger R, Rieger M, Benedetto KP, Fink C. Osteochondral lesions of the talus: computer-assisted retrograde drilling-feasibility and accuracy in initial experiences. Radiology. 2001;218(1):278–282. doi: 10.1148/radiology.218.1.r01ja18278. [DOI] [PubMed] [Google Scholar]
- 5.Blauth M, Duschek R, Schmidt U. Gerät zur Reposition und intraoperativen Lagerung instabiler Verletzungen der Halswirbelsäule. Operat Orthop Traumatol. 1994;6(4):285–289. doi: 10.1007/BF02511336. [DOI] [Google Scholar]
- 6.Bozbuga M, Ozturk A, Ari Z, Sahinoglu K, Bayraktar B, Cecen A. Morphometric evaluation of subaxial cervical vertebrae for surgical application of transpedicular screw fixation. Spine. 2004;29(17):1876–1880. doi: 10.1097/01.brs.0000137065.62516.01. [DOI] [PubMed] [Google Scholar]
- 7.Carbone JJ, Tortolani PJ, Quartararo LG. Fluoroscopically assisted pedicle screw fixation for thoracic and thoracolumbar injuries: technique and short-term complications. Spine. 2003;28(1):91–97. doi: 10.1097/00007632-200301010-00021. [DOI] [PubMed] [Google Scholar]
- 8.Ebraheim NA, Xu R, Knight T, Yeasting RA. Morphometric evaluation of lower cervical pedicle and its projection. Spine. 1997;22(1):1–6. doi: 10.1097/00007632-199701010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Holly LT, Foley KT. Intraoperative spinal navigation. Spine. 2003;28(15 suppl):S54–S61. doi: 10.1097/00007632-200308011-00010. [DOI] [PubMed] [Google Scholar]
- 10.Holly LT, Foley KT. Percutaneous placement of posterior cervical screws using three-dimensional fluoroscopy. Spine. 2006;31(5):536–540. doi: 10.1097/01.brs.0000201297.83920.a1. [DOI] [PubMed] [Google Scholar]
- 11.Jeanneret B, Gebhard JS, Magerl F. Transpedicular screw fixation of articular mass fracture-separation: results of an anatomical study and operative technique. J Spinal Disord. 1994;7(3):222–229. doi: 10.1097/00002517-199407030-00004. [DOI] [PubMed] [Google Scholar]
- 12.Jones EL, Heller JG, Silcox DH, Hutton WC. Cervical pedicle screws versus lateral mass screws. Anatomic feasibility and biomechanical comparison. Spine. 1997;22(9):977–982. doi: 10.1097/00007632-199705010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kamimura M, Ebara S, Itoh H, Tateiwa Y, Kinoshita T, Takaoka K. Cervical pedicle screw insertion: assessment of safety and accuracy with computer-assisted image guidance. J Spinal Disord. 2000;13(3):218–224. doi: 10.1097/00002517-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Karaikovic EE, Daubs MD, Madsen RW, Gaines RW., Jr Morphologic characteristics of human cervical pedicles. Spine. 1997;22(5):493–500. doi: 10.1097/00007632-199703010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Karaikovic EE, Kunakornsawat S, Daubs MD, Madsen TW, Gaines RW., Jr Surgical anatomy of the cervical pedicles: landmarks for posterior cervical pedicle entrance localization. J Spinal Disord. 2000;13(1):63–72. doi: 10.1097/00002517-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Karaikovic EE, Yingsakmongkol W, Gaines RW., Jr Accuracy of cervical pedicle screw placement using the funnel technique. Spine. 2001;26(22):2456–2462. doi: 10.1097/00007632-200111150-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kast E, Mohr K, Richter HP, Borm W. Complications of transpedicular screw fixation in the cervical spine. Eur Spine J. 2006;15(3):327–334. doi: 10.1007/s00586-004-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotani Y, Abumi K, Ito M, Minami A. Improved accuracy of computer-assisted cervical pedicle screw insertion. J Neurosurg. 2003;99(3 suppl):257–263. doi: 10.3171/spi.2003.99.3.0257. [DOI] [PubMed] [Google Scholar]
- 19.Kotani Y, Cunningham BW, Abumi K, McAfee PC. Biomechanical analysis of cervical stabilization systems. An assessment of transpedicular screw fixation in the cervical spine. Spine. 1994;19(22):2529–2539. doi: 10.1097/00007632-199411001-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kothe R, Ruther W, Schneider E, Linke B. Biomechanical analysis of transpedicular screw fixation in the subaxial cervical spine. Spine. 2004;29(17):1869–1875. doi: 10.1097/01.brs.0000137287.67388.0b. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski JM, Ludwig SC, Hutton WC, Heller JG. Cervical spine pedicle screws: a biomechanical comparison of two insertion techniques. Spine. 2000;25(22):2865–2867. doi: 10.1097/00007632-200011150-00005. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig SC, Kowalski JM, Edwards CC, Heller JG. Cervical pedicle screws: comparative accuracy of two insertion techniques. Spine. 2000;25(20):2675–2681. doi: 10.1097/00007632-200010150-00022. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig SC, Kramer DL, Balderston RA, Vaccaro AR, Foley KF, Albert TJ. Placement of pedicle screws in the human cadaveric cervical spine: comparative accuracy of three techniques. Spine. 2000;25(13):1655–1667. doi: 10.1097/00007632-200007010-00009. [DOI] [PubMed] [Google Scholar]
- 24.Miller RM, Ebraheim NA, Xu R, Yeasting RA. Anatomic consideration of transpedicular screw placement in the cervical spine. An analysis of two approaches. Spine. 1996;21(20):2317–2322. doi: 10.1097/00007632-199610150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Misenhimer GR, Peek RD, Wiltse LL, Rothman SL, Widell EH., Jr Anatomic analysis of pedicle cortical and cancellous diameter as related to screw size. Spine. 1989;14(4):367–372. doi: 10.1097/00007632-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Neo M, Sakamoto T, Fujibayashi S, Nakamura T. The clinical risk of vertebral artery injury from cervical pedicle screws inserted in degenerative vertebrae. Spine. 2005;30(24):2800–2805. doi: 10.1097/01.brs.0000192297.07709.5d. [DOI] [PubMed] [Google Scholar]
- 27.Nolte LP, Zamorano L, Visarius H, Berlemann U, et al. Clinical evaluation of a system for precision enhancement in spine surgery. Clin Biomech (Bristol, Avon) 1995;10(6):293–303. doi: 10.1016/0268-0033(95)00004-5. [DOI] [PubMed] [Google Scholar]
- 28.Nolte LP, Zamorano LJ, Jiang Z, Wang Q, Langlotz F, Berlemann U. Image-guided insertion of transpedicular screws. A laboratory set-up. Spine. 1995;20(4):497–500. doi: 10.1097/00007632-199502001-00016. [DOI] [PubMed] [Google Scholar]
- 29.Panjabi MM, Duranceau J, Goel V, Oxland T, Takata K. Cervical human vertebrae. Quantitative three-dimensional anatomy of the middle and lower regions. Spine. 1991;16(8):861–869. doi: 10.1097/00007632-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Panjabi MM, Goel V, Oxland T, Takata K, et al. Human lumbar vertebrae. Quantitative three-dimensional anatomy. Spine. 1992;17(3):299–306. doi: 10.1097/00007632-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Panjabi MM, Shin EK, Chen NC, Wang JL. Internal morphology of human cervical pedicles. Spine. 2000;25(10):1197–1205. doi: 10.1097/00007632-200005150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Rampersaud YR, Simon DA, Foley KT. Accuracy requirements for image-guided spinal pedicle screw placement. Spine. 2001;26(4):352–359. doi: 10.1097/00007632-200102150-00010. [DOI] [PubMed] [Google Scholar]
- 33.Reichle E, Sellenschloh K, Morlock M, Eggers C. Placement of pedicle screws using different navigation systems. A laboratory trial with 12 spinal preparations. Orthopade. 2002;31(4):368–371. doi: 10.1007/s00132-001-0277-6. [DOI] [PubMed] [Google Scholar]
- 34.Reinhold M, Magerl F, Rieger M, Blauth M (2007) Cervical pedicle screw placement: feasibility and accuracy of two new insertion techniques based on morphometric data. Eur Spine J 16(1):47–56. Epub 21 April 2006 [DOI] [PMC free article] [PubMed]
- 35.Rezcallah AT, Xu R, Ebraheim NA, Jackson T. Axial computed tomography of the pedicle in the lower cervical spine. Am J Orthop. 2001;30(1):59–61. doi: 10.1007/s001320050574. [DOI] [PubMed] [Google Scholar]
- 36.Richter M, Cakir B, Schmidt R. Cervical pedicle screws: conventional versus computer-assisted placement of cannulated screws. Spine. 2005;30(20):2280–2287. doi: 10.1097/01.brs.0000182275.31425.cd. [DOI] [PubMed] [Google Scholar]
- 37.Richter M, Mattes T, Cakir B. Computer-assisted posterior instrumentation of the cervical and cervico-thoracic spine. Eur Spine J. 2004;13(1):50–59. doi: 10.1007/s00586-003-0604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roh JS, Teng AL, Rice JA et al Cervical Spine Research Society, editor (2004) Paper #16: Accurate pedicle screw placement using laser-guided fluoroscopy: The “Perfect Pedicle” Technique. Boston, MA. Cervical Spine Research Society, 16. p 69, 32nd Annual Meeting. C_Ped III
- 39.Sakamoto T, Neo M, Nakamura T. Transpedicular screw placement evaluated by axial computed tomography of the cervical pedicle. Spine. 2004;29(22):2510–2514. doi: 10.1097/01.brs.0000144404.68486.85. [DOI] [PubMed] [Google Scholar]
- 40.Schlenzka D, Laine T, Lund T. Computer-assisted spine surgery: principles, technique, results and perspectives. Orthopade. 2000;29(7):658–669. doi: 10.1007/s001320050508. [DOI] [PubMed] [Google Scholar]
- 41.Shin EK, Panjabi MM, Chen NC, Wang JL. The anatomic variability of human cervical pedicles: considerations for transpedicular screw fixation in the middle and lower cervical spine. Eur Spine J. 2000;9(1):61–66. doi: 10.1007/s005860050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanescu S, Ebraheim NA, Yeasting R, Bailey AS, Jackson WT. Morphometric evaluation of the cervico-thoracic junction. Practical considerations for posterior fixation of the spine. Spine. 1994;19(18):2082–2088. doi: 10.1097/00007632-199409150-00014. [DOI] [PubMed] [Google Scholar]
- 43.Sutterlin CE, III, McAfee PC, Warden KE, Rey RM, Jr, Farey ID. A biomechanical evaluation of cervical spinal stabilization methods in a bovine model. Static and cyclical loading. Spine. 1988;13(7):795–802. doi: 10.1097/00007632-198807000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi J, Shono Y, Nakamura I, Hirabayashi H et al (2006) Computer-assisted screw insertion for cervical disorders in rheumatoid arthritis. Eur Spine J 16(4):485–494. Epub 6 October 2006 [DOI] [PMC free article] [PubMed]
- 45.Ugur HC, Attar A, Uz A, Tekdemir I, et al. Surgical anatomic evaluation of the cervical pedicle and adjacent neural structures. Neurosurgery. 2000;47(5):1162–1168. doi: 10.1097/00006123-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Xu R, Kang A, Ebraheim NA, Yeasting RA. Anatomic relation between the cervical pedicle and the adjacent neural structures. Spine. 1999;24(5):451–454. doi: 10.1097/00007632-199903010-00008. [DOI] [PubMed] [Google Scholar]
- 47.Yusof MI, Ming LK, Abdullah MS, Yusof AH. Computerized tomographic measurement of the cervical pedicles diameter in a Malaysian population and the feasibility for transpedicular fixation. Spine. 2006;31(8):E221–E224. doi: 10.1097/01.brs.0000210263.87578.65. [DOI] [PubMed] [Google Scholar]