Abstract
En bloc spondylectomy is a technique that enables wide or marginal resection of malignant lesions of the spine. Both all posterior techniques as well as combined approaches are reported. Aim of the present study was to analyse the results of 21 patients with malignant lesions of the spine, all treated with en bloc excision in a combined posteroanterior (n = 19) or all posterior approach (n = 2). Twenty-one consecutive patients, operated between 1997 and 2005, were included into this retrospective study. Thirteen patients had primary malignant lesions, eight patients had solitary metastases, all located in the thoracolumbar spine. There were 16 single level, three two-level, one three-level and one four-level spondylectomy. The patients were followed clinically and radiographically (including CT studies) with an average follow-up of 4 years. Out of 11 patients with primary Ewing or osteosarcoma seven patients are alive without any evidence of disease. One patient died after 5 years from other causes and three are alive with evidence of disease. Latter had either a poor histologic response to the preoperative chemotherapy (n = 2) or an intralesional resection (n = 1). All three patients with solitary spinal metastases of Ewing or osteosarcoma died of the disease. Five patients with solitary metastases of mainly hypernephroma are alive. In total, six resections were intralesional, mainly due to large intraspinal tumor masses, with two patients having had previous surgery. In the remaining cases, wide (n = 10) or marginal (n = 5) resection was accomplished. There were one pseudarthrosis requiring extension of the fusion and two cases with local recurrences and repeated excisional surgery. At follow-up CT studies, all cages were fused. Health related quality of life analysis (SF-36) revealed only slightly decreased physical component and normal mental component scores compared to normals in those patients with no evidence of disease. En bloc spondylectomy enables wide or marginal resection of malignant lesions of the spine in most cases with acceptable morbidity. Intralesional resection, poor histologic response, and solitary spinal metastases of Ewing and osteosarcoma are associated with a poor prognosis.
Keywords: Spondylectomy, Vertebrectomy, En bloc resection, Malignant tumors, Metastases, Spine
Introduction
The first case of a total en bloc spondylectomy was published by Bertil Stener in 1971 in a case of chondrosarcoma of T6–T8 in a 49-year-old farmer [20]. Roy-Camille further standardized and popularized the technique [14–17]. Larger series were published by Tomita et al. and Fidler in 1994 [7, 22]. While Tomita’s technique was an all posterior procedure, Fidler preferred a combined simultaneous posteroanterior approach. Fidler’s series consisted of ten patients with mainly Giant cell tumors, whereas Tomita et al. reported on 20 patients with solitary metastases of the spine. In a later publication Tomita et al. reported on five patients with primary malignant tumors of the spine [23]. Further authors adopted and modified these techniques and published their results with series of between seven and 29 patients [2, 3, 8, 10, 11].
The rationale of en bloc spondylectomy is to allow a resection of the tumor in one piece together with a layer of healthy tissue (marginal or wide resection) and thus to reduce local recurrence rate and to improve long-term survival of the patients [4, 6, 8]. While Tomita’s technique involves cutting through both pedicles to release the dural tube and thus potential tumor spread in case of tumor involvement of one or both pedicles (two-piece spondylectomy), the techniques described by other authors enable a true extralesional resection without violating the tumor margins (one-piece spondylectomy) [4, 7, 8, 10, 11, 17]. Latter is in correspondance with Enneking’s principles of musculoskeletal tumor resection [6].
In 1997, we performed our first en bloc spondylectomy applying an all posterior approach for the first two cases. However, to increase the safety of the procedure we then further modified our technique to a simultaneous posterior–anterior resection and instrumentation. Adding the anterior approach to the procedure enables a dissection of the tumor from the anterior elements under direct visual control, which is believed to be safer. Furthermore, completing the discectomies, resection of the anterior longitudinal ligament and anterior column reconstruction is easier. During the very stage of the spondylectomy, simultaneous anterior and posterior approach enables full control of both the anterior visceral structures and the posterior neural elements.
Aim of the present study is to report our preliminary results on a consecutive series of 21 en bloc spondylectomies in primary malignant bone tumors (n = 13) and solitary metastases (n = 8), operated by two surgeons (U.L. and H.H.). To the best knowledge of the authors, this is one of the largest series published, so far.
Materials and methods
Between 1997 and 2005 we performed 21 en bloc spondylectomies with 13 patients having primary malignant tumors of the spine and eight patients having solitary metastases (Table 1, 2). Average age of the patients at index operation was 30 years (9–68 years) with an average follow-up of 46 months (range: 8–114 months). There were nine female and 12 male patients.
Table 1.
Data on patients with primary malignant tumors of the spine (n = 13)
| Initials | Diagnosis | Age (in years) | Levels resected | Fusion length | OR-time (in min) | Intraop. blood loss (in milliliters) | Resection | Histological response | Status | Frankel grade | Follow-up (in months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | N.K. | Ewing sarcoma | 20 | T12 | T10–L2 | 360 | 2.500 | Wide | III | NED | E | 18 |
| 2 | T.P. | Ewing sarcoma | 29 | L1 | T11–L3 | 420 | 1.500 | Intralesional | I | AWD | E | 42 |
| 3 | E.K. | Ewing sarcoma | 10 | T6–T7 | T4–9 | 465 | 2.600 | Wide | I | NED | E | 10 |
| 4 | L.G. | Ewing sarcoma | 9 | L3 | L2–4 | 375 | 1.800 | Wide | I | NED | E | 12 |
| 5 | J.B. | Ewing sarcoma | 10 | L4 | L3–5 | 390 | 1.500 | Wide | II | DOCa | E | 58 |
| 6 | S.S. | Ewing sarcoma | 16 | L2 | L1–3 | 390 | 2.200 | Wide | I | NED | E | 36 |
| 7 | A.R. | Ewing sarcoma | 24 | T12 | T10–L1 | 600 | 4.600 | Marginal | I | NED | E | 96 |
| 8 | D.H. | Osteosarcoma | 31 | T9–12 | T6–L3 | 720 | 4.500 | Marginal | IV | AWD | D | 75 |
| 9 | W.M. | Osteosarcoma | 38 | T6 | T4–8 | 420 | 3.400 | Marginal | I | NED | E | 36 |
| 10 | M.P. | Osteosarcoma | 16 | L4 | L3–5 | 480 | 2.200 | Wide | IV | AWD | E | 18 |
| 11 | D.G. | Osteosarcoma | 31 | L1 | T11–L3 | 450 | 4.000 | Wide | – | NED | E | 8 |
| 12 | M.M. | Desmoplastic fibroma | 14 | T9–11 | T7–L1 | 390 | 2.300 | Marginal | – | NED | E | 84 |
| 13 | M.F. | Malignant fibrous histiocytoma | 59 | T7–8 | T5–10 | 580 | 6.000 | Intralesional | – | DOD | Cb | 24 |
aDied 5 years post index operation of generalised aspergillosis after bone marrow transplantation secondary to acute myeloic leukaemia
bRadiation induced myelopathy
Table 2.
Data on patients with solitary metastases of the spine (n = 8)
| Initials | Diagnosis | Age (in years) | Levels resected | Fusion length | OR-time (in min) | Intraop. blood loss (in milliliters) | Resection | Histological response | Status | Frankel grade | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | H.K. | Solitary metastasis Ewing sarcoma | 34 | T6 | T4–9 | 360 | 3.400 | Wide | IV | DOD | E | 12 |
| 15 | M.S. | Solitary metastasis osteosarcoma | 18 | L4 | L3–S1 | 450 | 2.000 | Intralesional | V | DOD | E | 8 |
| 16 | B.T. | Solitary metastasis osteosarcoma | 29 | L4 | L3–5 | – | – | Intralesional | V | DOD | D | 36 |
| 17 | H.K. | Metastases chondrosarcoma | 68 | T9, L2 | T7–L4 | 470 (135) | 4.300 (700) | Marginal | – | AWD | E | 36 |
| 18 | N.J. | Solitary metastasis paraganglioma | 16 | L1 | T12–L2 | 390 | 6.500 | Wide | VI | NED | E | 114 |
| 19 | B.M. | Solitary metastasis hypernephroma | 50 | T11 | T9–L1 | 420 | 3.100 | Intralesional | – | AWD | E | 42 |
| 20 | C.B. | Solitary metastasis hypernephroma | 66 | T12 | T10–L2 | 390 | 2.800 | Intralesional | – | AWD | E | 12 |
| 21 | B.D. | Solitary metastasis hypernephroma | 44 | L3 | L1–4 | 345 | 3.000 | Wide | – | NED | E | 84 |
Data of second procedure in brackets
All charts and X-rays were retrospectively reviewed by an independent observer not being involved in the treatment of the patients (T.L.). Histological diagnosis, details of the surgical procedure including operating time and blood loss were recorded as well as intra- and postoperative complications. In one patient (number 16), the anaesthesia notes were missing, so blood loss and operating time could not be recorded. The postoperative histological report was reviewed with respect to surgical margins and histological response according to Salzer-Kuntschik in cases of preoperative chemotherapy [18]. In the follow-up evaluation, the oncological status was recorded differentiating between “no evidence of disease” (NED), “alive with disease” (AWD), “died of disease” (DOD) and “died of other causes” (DOC). Radiographs and CT scans including sagittal plane reconstruction were analysed with respect to cage subsidence, fusion of the bone-cage interfaces, fusion through the cage and bridging fusion masses around the cage. Furthermore, radiometric analysis of the sagittal profile and frontal plane was conducted. Health related quality of life (HRQOL) assessment was obtained in 15 patients applying the German version of the SF-36 questionnaire [5]. Five patients had died and one patient (patient number 11) had left the country eight months post index operation and was unable to contact.
Adjuvant therapy
All patients with primary Ewing sarcoma were treated according to the protocol of EURO-Ewing with pre- and postoperative chemotherapy [9]. Out of a total of seven patients with primary Ewing sarcoma three patients received preoperative radiotherapy either due to large soft tissue masses or because of prior surgery (emergency laminectomy in two cases). Four patients had postoperative radiotherapy [19]. All four patients with primary osteosarcoma were treated according to the protocol of the cooperative osteosarcoma study group with both pre- and postoperative chemotherapy [1].
Surgical technique (Figs. 1–4)
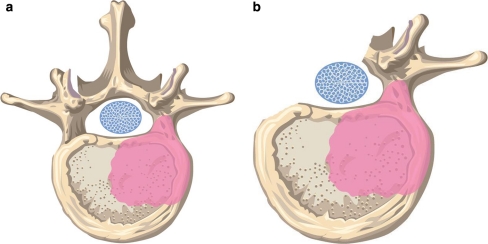
Fig. 1.
a Illustration of tumor involvement of one pedicle. b The non affected, healthy posterior elements are resected including the pedicle in order to create a corridor to release the dural tube
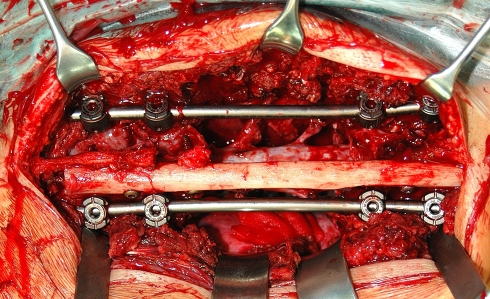
Fig. 4.
Intraoperative view of a two-level en bloc spondylectomy with posterior fixation of an allogenic fibula strut graft secured with titanium screws to the spinous processes
With the patient in a prone position the posterior aspect of the spine is exposed paying meticulous attention not to violate any potential soft tissue masses of the tumor. In one-level spondylectomies, harvesting of both cancellous and cortical iliac crest bone is performed prior to the posterior exposure, in order not to risk any tumor spread. This bone graft is mainly used for posterior fusion at the end of the procedure. In multilevel spondylectomies a fibula allograft is used for posterior fusion, thus iliac bone harvesting is not necessary. Pedicle screws are placed and controlled fluoroscopically. Typically, two levels above and below the resected vertebra(e) were instrumented, except for one case with a four-level spondylectomy which was instrumented three levels above and below (pt. number 8). In adolescents with lumbar lesions only one level above and below was instrumented in order to safe motion segments (n = 6).
Prerequisite for an extralesional resection is tumor involvement of no more than one side of the posterior structures, so that a corridor can be created through which the spinal cord is released during the spondylectomy (Fig. 1a, b). The posterior elements without tumor infiltration (lamina, spinous, articular and transverse processes, pedicle) are resected and the dura and nerve roots mobilised (Fig. 2). In case of multilevel involvement the nerve roots passing through the tumor need to be sacrificed. In thoracic lesions the ribs attached to the tumor vertebra(e) are cut and anterolateral soft-tissue attachments (i.e. parietal pleura, aorta) at the non-affected side of the vertebrae are released. In thoracolumbar lesions attachment of the diaphragm needs to be released and in lumbar lesions the psoas muscle. The discs including the posterior longitudinal ligament and the lateral parts of the annulus are incised and a temporary rod is inserted on the non-affected side.
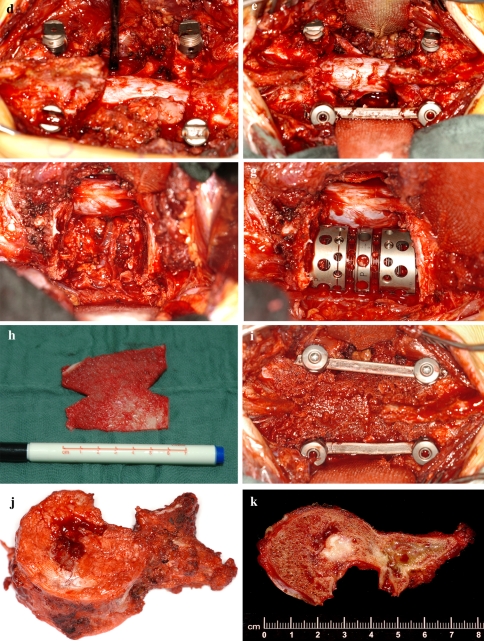
Fig. 2.
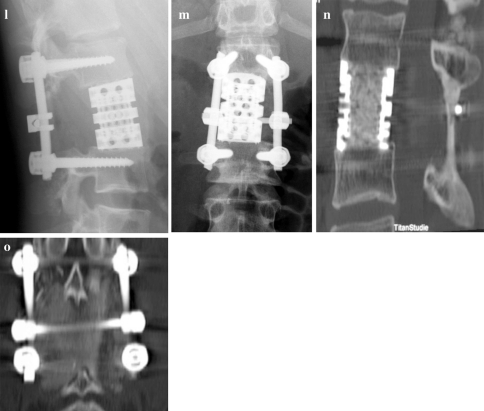
a–c 16-year-old male with Ewing’s sarcoma of L2 (patient number 6) and involvement of the left pedicle and transverse process. d Intraoperative view after resection of the healthy posterior structures to the base of the right pedicle with release of the right L2 nerve root. e and f Posterior and anterior view after en bloc spondylectomy. g Anterior column reconstruction with expandable titanium cage. h and i The cortical graft is trimmed and placed pressfit inbetween the spinous processes and covered with bone chips. j and k Macrospecimen and macrosection of L2 demonstrating clear margins. l and m Postoperative X-rays. n and o CT scans 3 years postop. showing bony fusion of both the cage and the posterior cortical graft
After temporary wound closure, the patient is placed in a lateral position with the affected side facing upwards. A standard anterior approach is accomplished (thoracotomy, thoracophrenolumbotomy, or lumbotomy) and the anterior elements are carefully dissected from the tumor including ligation of the segmental vessels. The disc release is completed including transection of the anterior longitudinal ligament.
Finally, the posterior wound is opened again and by simultaneous control of the visceral structures through the anterior approach and of the neural elements through the posterior approach the vertebra(e) is (are) rotated around the dural sac and removed en-bloc through the anterior approach. Then the second rod is placed and all the set screws are tightened. For anterior column reconstruction an expandable titanium ring cage (Vertebral body replacement cage, ulrich medical, Ulm, Germany), which has been filled with autologous bone either from a removed rib or from the iliac crest, is placed and slightly expanded in-situ until it is firmly seated between the adjacent vertebral endplates. In three- or four-level spondylectomies we additionally place an anterior single or dual rod depending on the size of the vertebrae to increase the stability of the construct. In two cases in the early series with a three- respectively four-level spondylectomy we used autologous fibula strut grafts to reconstruct the anterior column (Fig. 3). The anterior wound is closed in layers after insertion of drains.
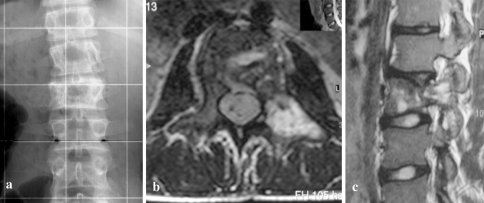
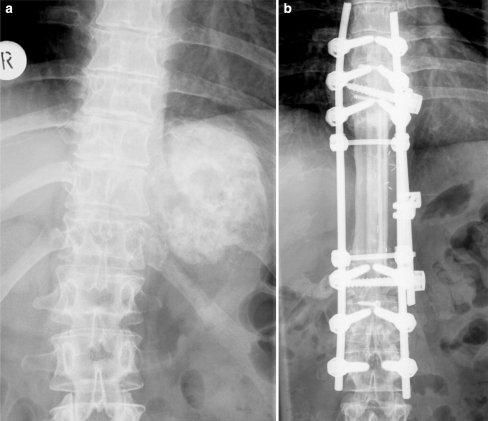
Fig. 3.
Preoperative and 6 years follow-up X-rays of patient number 8 with a large osteosarcoma involving T9–12. En bloc spondylectomy of four vertebrae with anterior fibula reconstruction and single rod instrumentation and posterior fibula fusion and instrumentation T6–L3
Posteriorly, the remaining elements are decorticated. For single-level spondylectomies the outer cortical table of the iliac crest is trimmed to fit inbetween the adjacent spinous processes with the smooth (cortical) side facing towards the dural sac. Bone chips are placed posteriorly over the entire instrumentation length and the wound is closed after insertion of a drain. In multi-level spondylectomies a fibular strut graft is secured with titanium screws to the remaining spinous processes to achieve a biologic posterior tethering and fusion (Fig. 4). In two early cases of this series (one three- and one four-level thoracic spondylectomy) an all-posterior procedure was performed. All cases but two were operated in one stage. One case of two distant level metastases of chondrosarcoma (patient number 17) was treated in a two-stage procedure with sequential resection of T9 and L2. In another case of a huge osteosarcoma metastasis (patient number 16) the vena cava was infiltrated and due to prolonged operating time the procedure was staged.
The patients were mobilised on the first postoperative days. In cases of short lumbar fixation, multilevel spondylectomies, or poor bone quality, a brace was prescribed for day time wear in the first three to six postoperative months.
Results
The details of all patients are given in Table 1 and 2. Average operative time was seven hours (6–12 h) with an average intraoperative blood loss of 3.210 ml (1.500–6.500 ml). In six out of 21 cases, the tumor had reached the surgical margins resulting in an, per definition, intralesional resection [6]. Two out of these six patients had had previous decompressive surgery. In the remaining four cases it were the intraspinal tumor masses that extended to the surgical margins. Dura resection was not performed in any of these cases. In the remaining cases wide (n = 10) or marginal (n = 5) resection was accomplished.
Seven out 11 patients with osteosarcoma or Ewing sarcoma had NED at follow-up. One patient with Ewing sarcoma died of a generalized aspergillosis after bone marrow transplantation and leukaemia 5 years after the index operation (pt. number 5). Three patients are alive but with evidence of metastatic disease. Two had a poor histologic response of grade IV, one was resected intralesionally (Table 1).
All patients with solitary metastases of either Ewing sarcoma or osteosarcoma had a poor histologic response (grade IV or V) and died of the disease 8–36 months postoperatively. Out of the five patients with solitary metastases two patients with a hypernephroma and paraganglioma, respectively, are alive without evidence of disease 7–10 years postoperatively. Three patients are alive with metastatic disease with a follow-up of between 1 and 3 years (Table 2).
Radiometric and CT analysis of the cages
In 17 patients the X-rays were complete for follow-up analysis. The segmental sagittal Cobb angle measured between the first cranial and caudal vertebra adjacent to the cage or fibula averaged 0.5° postoperatively (−28–26°) and 2,6° (−23−27°) at latest follow-up, resulting in an average increase of kyphosis of 2.2°. The mean sagittal Cobb angle of the entire instrumented area measured 1.1° (−43−30°) postoperatively and 3.2° (−41−32°) at follow-up, resulting in an average increase of kyphosis of 2.1°. In the frontal plane, the mean postoperative Cobb angle measured 3.1° (0–10°) and 3.4° (0–10°), at follow-up. Cage subsidence was measured as percentage of the anterior cage height to compensate for errors due to different magnification of the radiographs. Cage subsidence was minimal and averaged 1.7% (0–6.6%) of the cage height. Radiographically, both cases with anterior fibula interposition and all cases of titanium cage placement, except for two, appeared fused without any lucent lines at the bone-cage interfaces. There were no cases with radiographic signs of screw loosening, at follow-up.
In 12 out of 14 patients with cages (five out of 19 patients with cages had died), follow-up CT studies were available. In ten cases the upper and lower bone-cage interfaces appeared fused without any lucencies. In the remaining two cases (patients number 1 and 3) the interfaces still showed some lucencies after 18 respectively 10 months of follow-up. In all 12 cases there was bony growth through the cage with continuous bony bridging to the neighbouring vertebrae demonstrating bony ingrowth of the cages (Fig. 2n). In two cases, there was a continuous anterior bony bridging along the entire cage, in two cases the bridging was incomplete (Fig. 5). There were no cases of screw loosening.
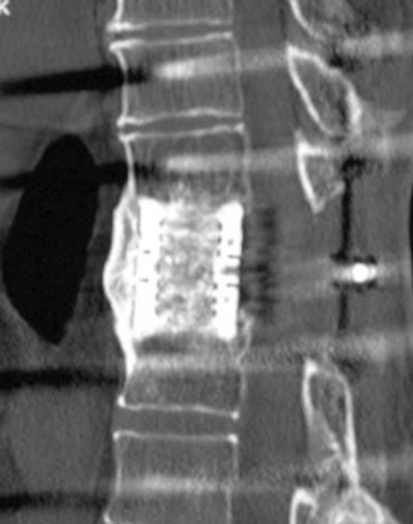
Fig. 5.
CT scan 42 months post en bloc spondylectomy T11 for solitary metastasis of a hypernephroma (patient number 19) demonstrating solid bony growth through the cage and continuous bony bridging anterior to the cage
Health related quality of life
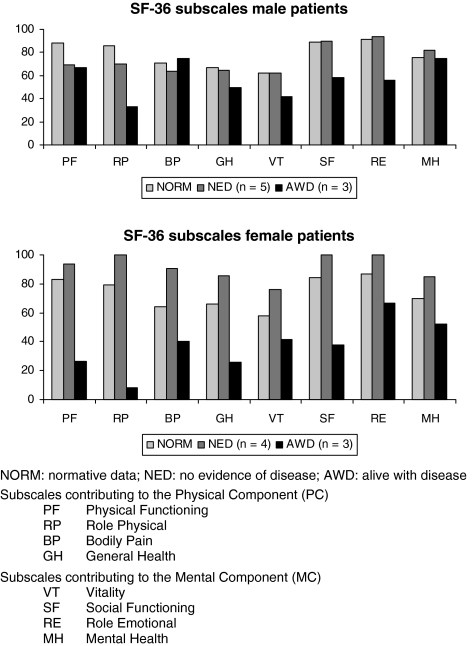
HRQOL data were obtained in nine out of ten patients without any evidence of tumor disease (NED) and in all six patients who were alive with tumor disease (AWD) at the latest follow-up. For the male patients (n = 8), the physical component summary averaged 66.80 in the NED and 56.09 in the AWD group (norm 77.90) and the mental component summary 81.75 in the NED and 57.59 in the AWD group (norm 79.50). For the female patients (n = 7), the physical component summary averaged 92.50 in the NED and 25.25 in the AWD group (norm 72.98) and the mental component summary 90.31 in the NED and 49.46 in the AWD group (norm 74.60). The different subscales are summarized in Fig. 6.
Fig. 6.
Health related quality of life data of our male and female patients
Complications
Postoperatively, one patient (patient number 9) with a thoracic one-level spondylectomy developed a chylothorax that resolved after prolonged parenteral nutrition. There was one complete paraplegia in a thoracic four-level spondylectomy which was attributed to a temporary vascular compromise due to the extensive dissection and division of three nerve roots. The patient recovered to Frankel D at latest follow-up (patient number 8). In total there were four revision surgeries. There were two local recurrences requiring repeated excision with both patients having had previous surgery before the index operation leading to an intralesional resection (patients number 2 and 13). There was one case of pseudarthrosis in a L4 spondylectomy with screw loosening and cage subsidence that was originally stabilized from L3 to L5, which required revision with extension of the posterior fusion (L2–S1) and bone grafting 18 months after index operation (patient number 5). In one patient (patient number 7), we resected a pseudobursa over one of the spinous processes six years post index operation without evidence of tumor recurrence. There were no deep infections, implant breakages, or graft respectively cage dislocations.
Discussion
En bloc resection of primary malignant bone tumors with negative surgical margins increases survival rate and is a crucial part of the treatment regime besides chemotherapy and radiation [21]. In solitary metastases en bloc resection is performed to improve local control, however, it remains unclear if long-term survival is affected by this procedure [24]. Different techniques of en bloc resection have been described [2, 7, 10, 11, 14, 15, 22]. We prefer a simultaneous combined approach which enables full control of both the posterior neural structures as well as the anterior visceral structures during the resection. In our series, mean operating time was seven hours with an intraoperative blood loss of 3.200 ml. Other authors reported an average operating time of between 10 and 18 h and an intraoperative blood loss of 3.900 ml [2, 8, 10]. Fidler even staged the procedure with an overnight stay in the intensive care unit in some cases [7].
The complications in our series included one pseudarthrosis, two cases with local recurences, and one patient with a complete paraplegia in a four level en bloc resection who recovered to Frankel D at latest follow-up. There were no deep infections. Krepler et al. published their series with seven patients and had three cases of wound necrosis requiring debridement and even pedicled latissimus dorsi flap in one patient. Three patients had to be revised because of implant failure [10]. In a large series of 29 patients, Boriani et al. [2] reported of one case with deep and persisting infection and three cases of mechanical implant failure requiring revision.
Typically, two levels above and below the lesion were instrumented in our series. However, in six adolescent cases with lumbar lesions, only one level above and below were instrumented in order to safe motion segments. In all but two cases expandable titanium ring cages filled with autologous bone were used for anterior column reconstruction. In all twelve patients with complete follow-up CT studies, the bone inside the cages was fused to the adjacent end plates (Figs. 2n, 5). In a biomechanical study using a vertebrectomy model, Oda et al. found out that a combination of anterior and posterior instrumentation in addition to an anterior cage provided the best stability [12]. For multilevel spondylectomies, we routinely add an anterior single or dual rod instrumentation to increase the stability of the construct (Fig. 3).
Health related quality of life analysis (SF-36) revealed only slightly decreased physical component and normal mental component scores compared to normals in those patients with NED. Fisher et al. reported on an acceptable morbidity with an average physical component summary of 38 and an average mental component summary of 52 [8]. Krepler et al. reported that all five surviving patients in their series were free from pain and had returned to their previous occupation at latest follow-up [10].
In total, six resections were intralesional, mainly due to large intraspinal tumor masses, with two patients having had previous surgery. In the remaining cases, wide (n = 10) or marginal (n = 5) resection was accomplished. In Fisher’s series, consisting of 26 patients with primary tumors of the spine, 15 resections were wide, four marginal, and seven intralesional [8]. Intralesional resection was associated with a risk of local recurrence of 33% (compared to 11% after wide or marginal resection). According to Talac et al. [21] local recurrence results in disease progression and death in 92% of the patients. In our series, both patients with intralesional resection of primary tumors (patient number 2 and 13) presented with local recurrence. Four out of six patients with intralesional resection had large intraspinal tumor masses in our series. A more aggressive approach with dural resection might improve sugical margins in these patients, as proposed by Fisher et al. [8]. In correspondance with other reports [13], we found intralesional resection, poor histologic response, and solitary spinal metastases of Ewing and osteosarcoma to be associated with a poor prognosis.
Conclusion
En bloc spondylectomy enables wide or marginal resection of malignant lesions of the spine in most cases with acceptable morbidity. Intralesional resection, poor histologic response, and solitary spinal metastases of Ewing and osteosarcoma are associated with a poor prognosis. In cases of extensive intraspinal tumor masses, dural resection might improve surgical margins.
References
- 1.Bielack SS, Kempf-Bielack B, Delling G, Exner G, Flege S, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;1:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 2.Boriani S, Biagini R, DeLure F. En bloc resections of bone tumors of the thoracolumbar spine. Spine. 1996;21:1927–1931. doi: 10.1097/00007632-199608150-00020. [DOI] [PubMed] [Google Scholar]
- 3.Boriani S, Chevally F, Weinstein J. Chordoma of the spine above the sacrum. Spine. 1996;21:1569–1577. doi: 10.1097/00007632-199607010-00017. [DOI] [PubMed] [Google Scholar]
- 4.Boriani S, Weinstein J, Biagini R. Primary bone tumors of the spine. Spine. 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 5.Ellert U, Bellach B. German national health survey 1998—description of an update random sampling test. Gesundheitswesen. 1999;61:S184–S190. [PubMed] [Google Scholar]
- 6.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop. 1980;204:9–24. [PubMed] [Google Scholar]
- 7.Fidler MW. Radical resection of vertebral body tumours. J Bone Joint Surg. 1994;76-B:765–772. [PubMed] [Google Scholar]
- 8.Fisher C, Keynan O, Boyd M, et al. The surgical management of primary tumors of the spine. Spine. 2005;30:1899–1908. doi: 10.1097/01.brs.0000174114.90657.74. [DOI] [PubMed] [Google Scholar]
- 9.Juergens C, Weston C, Lewis I, Whelan J, Paulussen M, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide in the treatment of Ewing tumors in the EURO-Ewing 99 clinical trial. Pediatr blood cancer. 2006;47:22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 10.Krepler P, Windhager R, Bretschneider W, et al. Total vertebrectomy for primary malignant tumours of the spine. J Bone Joint Surg. 2002;84-B:712–715. doi: 10.1302/0301-620X.84B5.12684. [DOI] [PubMed] [Google Scholar]
- 11.Mazel Ch, Grunenwald D, Laudrin P, Marmorat J. Radical excision in the management of thoracic and cervicothoracic tumors involving the spine: results in a series of 36 cases. Spine. 2003;28:782–792. doi: 10.1097/00007632-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 12.Oda I, Cunningham B, Abumi K, et al. The stability of reconstruction methods after thoracolumbar total spondylectomy. Spine. 1999;24:1634–1638. doi: 10.1097/00007632-199908150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the cooperative osteosarcoma study group. Cancer. 2002;15:1069–1077. doi: 10.1002/cncr.10258. [DOI] [PubMed] [Google Scholar]
- 14.Roy-Camille R, Mazel Ch, Sailant G, Lapresle Ph, et al. Treatment of malignant tumors of the spine with posterior instrumentation. In: Sundaresan N, Schmidek HH, Schiller AL, et al., editors. Tumors of the spine: diagnosis and clinical management. Philadelphia: Saunders; 1990. pp. 473–487. [Google Scholar]
- 15.Roy-Camille R, Mazel Ch. Vertebrectomy through an enlarged posterior approach for tumors and malunions. In: Bridwell KH, Wald RL, editors. The text book of spinal surgery. Philadelphia: Lippincott; 1991. pp. 1245–1256. [Google Scholar]
- 16.Roy-Camille R, Saillant G, Bisserie M, Judet T, Hautefort E, Mamoudy P. Total excision of thoracic vertebrae. Rev Chir Orthop Reparatrice Appar Mot. 1981;67:421–430. [PubMed] [Google Scholar]
- 17.Roy-Camille R, Saillant G, Mazel Ch, Monpierre H. Total vertebrectomy as treatment of malignant tumors of the spine. Chir Organi Mov. 1990;75:94–96. [PubMed] [Google Scholar]
- 18.Salzer-Kuntschik M, Brand G, Delling G. Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe. 1983;4:135–141. [PubMed] [Google Scholar]
- 19.Schuck A, Ahrens S, Schorlemer I, Kuhlen M, Paulussen M, et al. Radiotherapy in Ewing tumors of the vertebrae: treatment results and local relapse analysis of the CESS and EICESS trials. Int J Radiat Oncol Biol Phys. 2005;63:1562–1567. doi: 10.1016/j.ijrobp.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Stener B. Total spondylectomy in chondrosarcoma arising from the seventh thoracic vertebra. J Bone Joint Surg. 1971;53-B:288–295. [PubMed] [Google Scholar]
- 21.Talac R, Yaszemski M, Currier B, et al. Relationship between surgical margins and local recurence in sarcomas of the spine. Clin Orthop. 2002;397:127–132. doi: 10.1097/00003086-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy for solitary spinal metatases. Int Orthop. 1994;18:291–298. doi: 10.1007/BF00180229. [DOI] [PubMed] [Google Scholar]
- 23.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. Spine. 1997;22:324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 24.Tomita K, Kawahara N, Kobayashi T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]