Abstract
Study of complex I (NADH:ubiquinone oxidoreductase) activity in Parkinson’s disease (PD) brain has identified loss of activity only in substantia nigra although loss of activity of this enzyme has been identified in a number of non-brain tissues. We investigated this paradox by studying complex I and other complexes of the mitochondrial electron transport chain in frontal cortex from PD and aged control brain using a variety of assay conditions and tissue preparations. We found increasingly significant losses of complex I activity in PD frontal cortex as increasingly pure mitochondria were studied. Complexes II, III, and IV were comparable in PD and controls. Inclusion of bovine serum albumin in the assay increased enzyme activity but lessened discrimination between PD and controls. Complex I deficiency in PD brain is not confined to substantia nigra. Methodological issues are critical in demonstrating this loss of activity.
Keywords: Parkinson’s disease, complex I, mitochondria, assay, frontal lobe
Introduction
Loss of activity of complex I (NADH:ubiquinone oxidoreductase) has been an important finding in Parkinson’s disease (PD) because inhibition of it by the neurotoxin, MPTP (1-methyl-4-phenyltetrahydropyridine), produces parkinsonism in humans and animals(Langston et al., 1983; Vyas et al., 1986). This relationship between loss of complex I activity and PD gave particular significance to the findings that complex I is deficient in PD platelets and in PD substantia nigra(Parker et al., 1989a; Schapira et al., 1989). The significance of this relationship is reinforced by the fact that related electron transport chain defects underlie other focal degenerations of the central nervous system, e.g. Leber’s hereditary optic neuropathy(Howell et al., 1990; Parker et al., 1989b; Singh et al., 1989). A direct pathogenic link is suggested by mounting evidence that mitochondrial dysfunction plays an important role in initiation of apoptotic cell death(Budd et al., 2000; Miller1998). The novel finding by our group in 1996, and subsequent confirmation by Gu et al, that complex I deficiency in PD arises from mitochondrial DNA raises the possibility that complex I deficiency may be one of the primary genetic factors in the pathogenesis of sporadic PD as we first postulated in 1989(Gu et al., 1998; Parker et al., 1989a; Swerdlow et al., 1996). In-depth sequencing studies of all 7 mitochondrial genes encoding complex I subunits in PD and control frontal cortex suggests that idiopathic PD can be segregated from control on the basis of low abundance mutations in a very narrow region of the mitochondrial complex I gene, ND5 (Parker et al., 2005; Smigrodzki et al., 2004a; Smigrodzki et al., 2004b). It is impossible to say with certainty whether or not these ND5 mutations are responsible for the loss of complex when activity but they were the only consistent abnormality seen in an extremely in-depth study of 28 PD and control brains. We hypothesize on the basis of their very strong correlation with PD that they are responsible for the loss of complex I activity in PD. Since its initial discovery, the question of complex I deficiency in PD has faced two puzzling issues. First, the biochemical lesion has been difficult for some groups to demonstrate. Second, it has seemed paradoxical that the complex I defect has been found in multiple non-CNS tissues but only in substantia nigra in brain(Schapira et al., 1990). We undertook this study of complex I in PD frontal cortex in order to evaluate both of these issues and to determine whether or not methodological issues might account for conflicting findings among various investigators.
Results
Assay of genomic DNA from all 10 brains confirmed that none of our subjects had the α-synuclein form of familial PD (data not shown).
Four of the five PD samples and all four of the control samples were previously evaluated for the presence of microheteroplasmic mutations in the previously reported, relevant region of the complex I gene, ND5. All four PD samples were positive (codons 133, 145 and 136, 148, 145) while none of the control samples had mutations in this region. These data were part of the previously reported series (Parker et al., 2005; (Parker et al., 2005; Smigrodzki et al., 2004a; Smigrodzki et al., 2004b) Smigrodzki et al., 2004b)
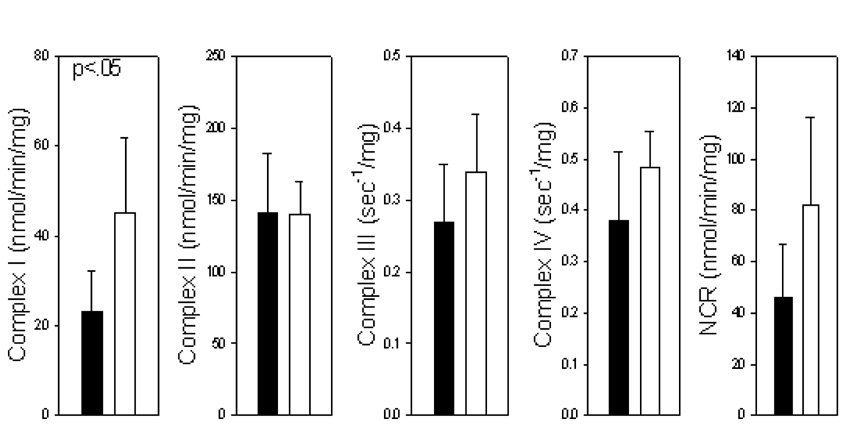
Assay of electron transport chain complexes I, III, IV, and of complexes I/III together (NADH:cytochrome c reductase, NCR) in purified frontal cortex mitochondria revealed a significant loss of complex I activity in PD samples as compared to controls (Fig 1). There was a tendency for some loss of activity of all electron transport chain complexes but this loss was significant only for complex I.
Figure 1.
Activities of electron transport chain complexes in purified frontal cortex mitochondria in PD (solid) and control (open) samples. NCR, NADH:cytochrome c oxidoreductase. Complex I is significantly (p<0.05) reduced in PD frontal cortex.
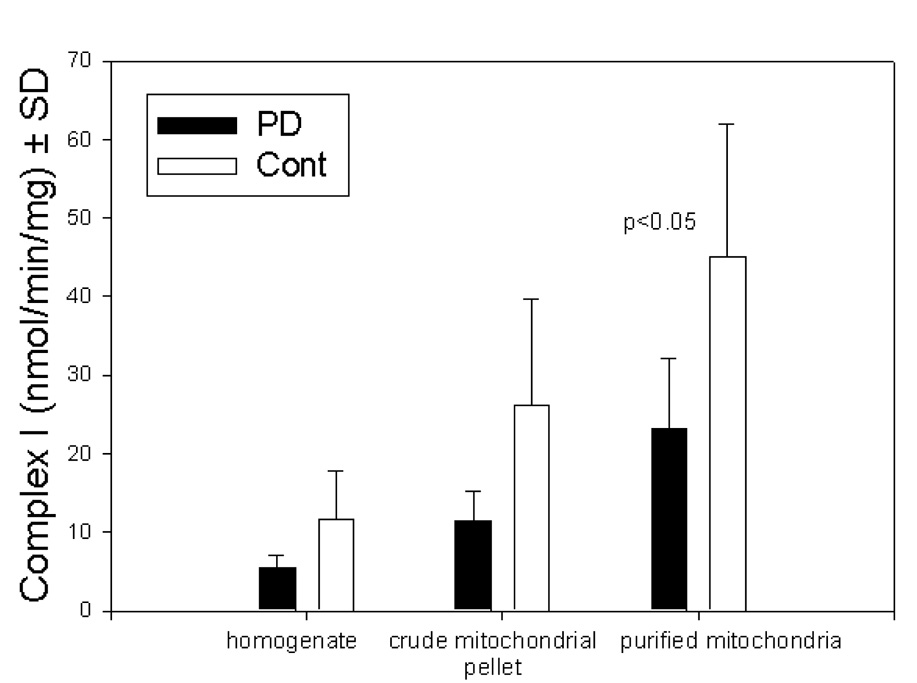
In order to evaluate the effects of mitochondrial purification on measurements of complex I activity, we assayed this enzyme in at various points during the mitochondria purification process: homogenized tissue, crude mitochondrial pellet, and purified mitochondria. As expected, specific activity of complex I increased approximately 4 fold during purification. Complex I activity was lower in PD samples at all stages of purification but reached significance only in purified mitochondria (Fig 2).
Figure 2.
Complex I activities in frontal cortex mitochondria at various stages of purification. As increasingly pure preparations are assayed, discrimination between PD and controls decreases.
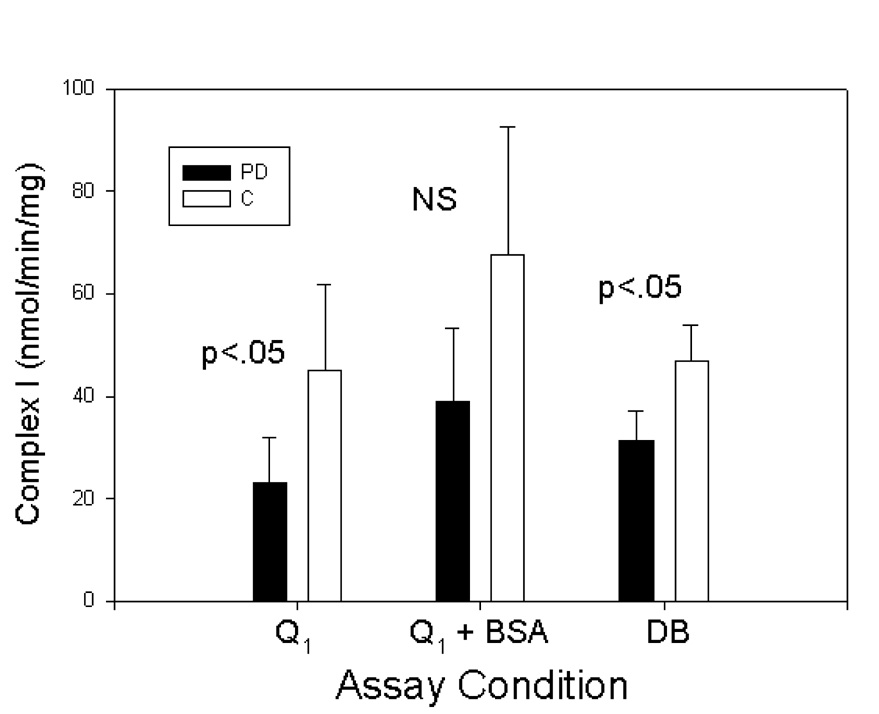
Finally, we evaluated the effects of different electron acceptors (Q1 or DB (decylubiquinone)) on complex I activity. We also evaluated the effects of inclusion of bovine serum albumin (BSA) in the assay mixture by conducting parallel assays on the final purified mitochondrial pellets (Fig 3). PD complex I activity was lower than control complex I activity under all 3 assay conditions but failed to reach significance when BSA was included. BSA tends to increase measured activity in complex I assays and is often included in assays for that reason. It appears, however, to lessen the discrimination between PD and controls.
Figure 3.
Comparison of various assay conditions on complex I activity in purified frontal cortex mitochondria. Inclusion of BSA increases complex I activity but lessens discrimination between PD and control samples.
Discussion
Understanding the anatomic extent of the complex I lesion in PD is important because of its impact on understanding the pathogenesis of PD. Cybrid experiments in which mtDNA is transferred PD subjects or controls into recipient cells (cybrids) indicate that the complex I lesion of PD is genetic and arises from mtDNA. This provides a rational basis for an anatomically widespread biochemical lesion. In keeping with its genetic origin, a loss of activity of complex I of the mitochondrial electron transport chain has been observed in many different PD tissues including platelets, brain, muscle, and lymphocytes(Benecke et al., 1993; Haas et al., 1995; Parker et al., 1989a; Shoffner et al., 1991; Yoshino et al., 1992). Immunoblot studies of complex I suggested abnormalities in PD striatum(Mizuno et al., 1989). Impaired pyruvate oxidation, consistent with an electron transport chain defect is present in PD fibroblasts(Mytilineau et al., 1994). Resolution of this paradox is critical.
Factors related to assay conditions may provide the resolution. Assay of ETC activities in tissue homogenates is rendered difficult by other nonspecific activities present in many tissues, which act to generate a very high background signal that makes identification of the relevant enzymatic rate difficult. This is a particular problem in the case of complex I because of the presence of a very high background rate in many tissues. Complex I catalyzes a redox reaction in which electrons are passed from NADH to ubiquinone (coenzyme Q). This activity is specifically inhibitable by rotenone and complex I activity is typically reported as a rotenone sensitive rate of NADH oxidation or of ubiquinone reduction. In theory, subtraction of a rotenone inhibitable rate from the overall rate permits the investigator to subtract out the nonspecific activity and identify a true complex I rate. The present study demonstrates the difficulty in identifying a small change in a very large signal. The difficulty tends to bias the results toward not finding significant changes; in fact, PD complex I activity was lower than control complex I activity at each step of the assay but the differences only reached statistical signficance in purified mitochondria (Figure 2). Our use of a more purified preparation probably explains the discrepancy between this study and the previous finding of Schapira et al(Mann et al., 1992). Similar issues may explain the original failure of Mann et al to identify a complex I lesion in PD platelet homogenates since they were subsequently able to confirm platelet complex I deficiency in a more purified preparation(Krige et al., 1992; Mann et al., 1992).
Bovine serum albumin (BSA) is often included in assays of complex I because its inclusion results in increased activity as is demonstrated by the present study (Figure 3). However, BSA also tends to decrease the difference between PD and controls and inclusion resulted in loss of signficance. This may be because BSA is intensely charged and causes major distortion of the inner mitochondrial membrane and of complex I. Whatever the explanation, inclusion or non-inclusion of BSA may also explain the conflicting results from different investigators.
The factors we have identified tend to bias data in a negative direction. Data should be carefully evaluated with regard to sample preparation and assay technique before a study is conclusively regarded as negative. Indeed, Swerdlow and Kish recently reviewed data addressing the question of reduced cytochrome c oxidase activity in Alzheimer’s disease brain, and found that studies utilizing purified mitochondrial preparations were almost uniformly successful in demonstrating enzyme abnormalities while studies of homogenized tissue yielded erratic and inconsistent results(Swerdlow et al., 2002).
Complex I is an extremely complicated enzyme of approximately 50 subunits under control of both nuclear and mitochondrial genomes. There will prove to be many different modes of failure of this system with a number of different phenotypes produced. This will be a fruitful area for further investigation.
We conclude that methodological issues continue to be critical in this arena and that neither the loss of complex I activity in PD nor the associated ND5 mutations are confined to substantia nigra (Parker et al., 2005; Smigrodzki et al., 2004a; Smigrodzki et al., 2004b).
Experimental Procedure
This project was approved by the University of Virginia Human Subjects Committee. Brain fragments of approximately 2–3 grams were obtained from frozen, banked brain tissue. PD brains (n=5) and control brains (n=4) were obtained from the University of Virginia Brain Bank. Mean age at death was 71.2 years for controls and 79.6 years for PD (not significant). Although the control group was slightly younger than the PD group the difference was minor and very unlikely to account for the observed differences. Diagnosis of PD or control status was based on both premortem clinical information and on neuropathological evaluation. Anatomically equivalent fragments of cortical gray matter from the frontal pole were obtained in each instance. The postmortem interval for PD samples was 11.35 hours (3.7 s.d.) and 14.25 hours (7.5 s.d.) (n.s.). The final yield of mitochondria after purification was 1.78 mgm mito protein/gram wet wt for PD samples and was 1.95 mgm mito protein/gram wet wt (n.s.).
Unless otherwise noted, all reagents were obtained from Sigma. Q1 was a gift from Eisai. Brain tissue was homogenized and mitochondria were purified using a multi-step procedure on a Ficoll gradient(Parker et al., 1994; Partridge et al., 1994). Aliquots were removed at each step of the purification process and saved for assay of electron transport chain activities. Enzyme assays were performed as previously described (Parker et al., 1994). Briefly, an aliquot of the material to be assayed was sonicated using a cuphorn sonicator. Complex I was then assayed by following the oxidation of added NADH spectrophotometrically. Unless otherwise indicated Q1 served as the electron acceptor. A second identical reaction was run in the presence of rotenone and the two values were subtracted yielding a rotenone sensitive rate which was reported as complex I activity. All assays were performed blind.
Accidental inclusion of cases of the α-synuclein form of familial PD was excluded by assay of the G209A mutation of α-synuclein. In order to determine whether our PD brains carried the G209A α-synuclein Contursi mutation, 25 mg of tissue was isolated from each brain and genomic DNA was extracted using a Qiagen tissue DNA extraction protocol (Qiagen). As a positive control, a similar procedure was used to extract genomic DNA from 200 µl of blood obtained from a PD-affected member of the Contursi kindred. For each sample, PCR amplification of the portion of the α-synuclein gene flanking nucleotide 209 was performed with the primers described by Polymeropolous et al., 100 ng of genomic DNA per reaction, and Taq/Pwo polymerase (Boehringer-Mannheim)(Polymeropoulos et al., 1997). This reaction generated a 216 bp segment that was then incubated with Tsp 45 I (New England Biolabs). The G209A mutation creates a new restriction site for Tsp 45 I, so that mutant but not wild type products are cut into 88 and 128 bp fragments from the original 216 bp segment. To visualize the results of the Tsp 45 I digestion, samples were electrophoresed for two hours through a 2% agarose gel containing ethidium bromide.
Statistical analysis was performed by ANOVA (SigmaStat).
Acknowledgements
This work was supported by grants from the National Institutes of Health, the Dana Foundation, and APDA. The authors are grateful to Dr. Fred Wooten for review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- Benecke R, Str□mper P, Weiss H. Electron transfer complexes I and IV of platelets are abnormal in Parkinson's disease but normal in Parkinson-plus syndromes. Brain. 1993:1451–1463. doi: 10.1093/brain/116.6.1451. [DOI] [PubMed] [Google Scholar]
- Budd SL, Tenneti L, Lishnak T, Lipton SA. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proceedings of the National Academy of Science of the U.S.A. 2000:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Cooper JM, Taanman JW, Schapira AHV. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann.Neurol. 1998:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol. 1995:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- Howell N, McCullough D. An example of Leber hereditary optic neuropathy not involving a mutation in the mitochondrial ND4 gene. Am.J Hum.Genet. 1990:629–634. [PMC free article] [PubMed] [Google Scholar]
- Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AHV. Platelet mitochondrial function in Parkinson's disease. Ann Neurol. 1992:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Mann VM, Cooper JM, Krige D, Daniel SE, Schapira AHV, Marsden CD. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson disease. Brain. 1992:333–342. doi: 10.1093/brain/115.2.333. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Mitochondria - the Kraken awakes. Trends in Neuroscience. 1998:95–97. doi: 10.1016/s0166-2236(97)01206-x. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989:1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Mytilineau C, Werner P, Molinari S, Di Rocco A, Cohen G, Yahr MD. Impaired oxidative decarboxylation of pyruvate in fibroblasts from patients with Parkinson's disease. J Neural Transm [P-D Sect] 1994:223–228. doi: 10.1007/BF02260943. [DOI] [PubMed] [Google Scholar]
- Parker WD, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Annals of Neurology. 1989a:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker WD, Oley CA, Parks JK. Deficient NADH:coenzyme Q oxidoreductase in Leber's hereditary optic neuropathy. New England Journal of Medicine. 1989b:1331–1333. doi: 10.1056/NEJM198905183202007. [DOI] [PubMed] [Google Scholar]
- Parker WD, Parks JK. Mitochondrial ND5 mutations in idiopathic Parkinson's disease. Biochem Biophys Res Commun. 2005:667–669. doi: 10.1016/j.bbrc.2004.11.093. [DOI] [PubMed] [Google Scholar]
- Parker WD, Parks JK, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- Partridge RS, Munroe SM, Parks JK, Johnson K, Parker WD, Eaton GR, Eaton SS. Spin trapping of azidyl and hydroxyl radicals in azide-inhibited rat brain submitochondrial particles. Arch Biochem Biophys. 1994:210–217. doi: 10.1006/abbi.1994.1159. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekhrappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Shoffner JM, Watts RL, Juncos JL, Torroni A, Wallace DC. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991:332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Singh G, Lott MT, Wallace DC. A mitochondrial DNA mutation as a cause of Leber's hereditary optic neuropathy. New England Journal of Medicine. 1989:1300–1305. doi: 10.1056/NEJM198905183202002. [DOI] [PubMed] [Google Scholar]
- Smigrodzki R, Goertzel B, Pennachin C, Coelho L, Prosdocimi F, Parker WD. Genetic Algorithm for Analysis of Mutations in Parkinson's Disease. Artificial Intelligence in Medicine. 2004a doi: 10.1016/j.artmed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Smigrodzki R, Parks JK, Parker WD. High frequency mitochondrial DNA mutations in Parkinson's disease brain. Neurobiology Aging. 2004b:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int.Rev.Neurobiol. 2002:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson's disease. Ann.Neurol. 1996:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Vyas I, Heikkila RE, Nicklas WJ. Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine: inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-pyridinium. J Neurochem. 1986:1501–1507. doi: 10.1111/j.1471-4159.1986.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Nakagawa-Hattori Y, Kondo T, Mizuno Y. Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson's disease. J Neural Transm [P-D Sect] 1992:27–34. doi: 10.1007/BF02257619. [DOI] [PubMed] [Google Scholar]