Abstract
Intentional self-poisonings with seeds from the yellow oleander tree (Thevetia peruviana) are widely reported. Activated charcoal has been suggested to benefit patients with yellow oleander poisoning by reducing absorption and/or facilitating elimination. Two recent randomised controlled trials (RCTs) assessing the efficacy of activated charcoal reported conflicting outcomes in terms of mortality. The effect of activated charcoal on the pharmacokinetics of Thevetia cardenolides has not been assessed. This information may be useful for determining whether further studies are necessary. Serial blood samples were obtained from patients enrolled in a RCT assessing the relative efficacy of single dose (SDAC) and multiple doses (MDAC) of activated charcoal compared to no activated charcoal (NoAC). The concentration of Thevetia cardenolides was estimated using a digoxin immunoassay. The effect of activated charcoal on cardenolide pharmacokinetics was compared between treatment groups using the AUC24, the 24h Mean Residence Time (MRT24), and regression lines obtained from serial concentration points adjusted for exposure. Erratic and prolonged absorption patterns were noted in each patient group. The apparent terminal half-life was highly variable, with a median time of 42.9h. There was a reduction in MRT24 and the apparent terminal half-life estimated from linear regression in patients administered activated charcoal compared to the control group (NoAC). This effect was approximately equal in patients administered MDAC or SDAC. Activated charcoal appears to favourably influence the pharmacokinetic profile of Thevetia cardenolides in patients with acute self-poisoning, which may have clinical benefits. Given the conflicting clinical outcomes noted in previous RCTs, this mechanistic data supports the need for further studies to determine whether a subgroup of patients (eg. those presenting soon after poisoning) will benefit from activated charcoal.
Keywords: Thevetia, oleander, pharmacokinetic, poisoning, activated charcoal
Introduction
The yellow oleander tree (Thevetia peruviana, previously T. neriifolia) is common through much of the tropics and subtropics.(1) Poisonings are reported widely, but it is only in South Asia that ingestion of oleander seeds has become a popular means of self harm.(2-5) Each year there are tens of thousands of yellow oleander poisoning cases in South Asia and probably hundreds of deaths, given the mortality of 4-10%.(6)
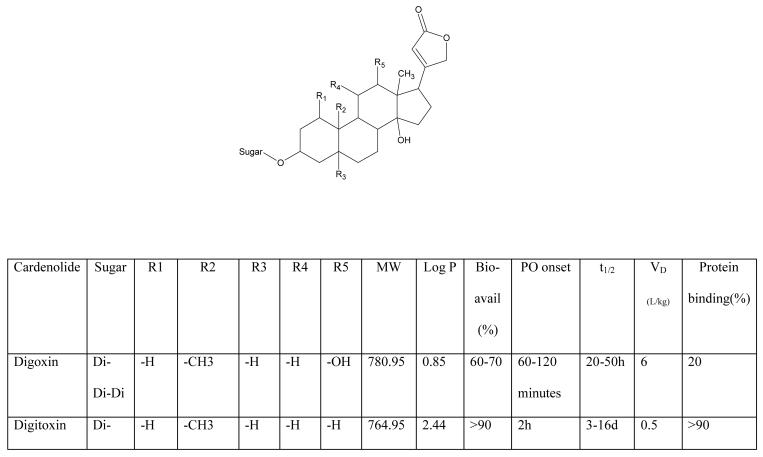
Many cardenolides have been identified in yellow oleander, predominantly thevetin A and B, but also peruvoside, neriifolin, thevetoxin, ruvoside, and theveridoside. These cardenolides are structurally similar to the digitalis cardenolides derived from foxglove (Figure 1).(1;7-9) Despite this similarity, individual cardenolides vary widely in their pharmacokinetic properties (Figure 1).
Figure 1.
Structures of selected cardenolides and their known physicochemical and human pharmacokinetic properties.(8;13-17;48-52)
Earlier studies assessed the clinical role of Thevetia cardenolides in the management of congestive cardiac failure,(10-14) and pharmacokinetic studies were conducted in animals, volunteers and patients with cardiac failure.(8;14-16) The outcomes of some of the pharmacokinetic studies conflicted in certain respects, for example the extent to which enterohepatic recycling occurs.(15;17;18) How these outcomes relate to patients with acute poisoning with plant material has not been defined.
Digitalis cardenolides appear to undergo significant enterohepatic recycling, which is why human and animal studies have shown increased clearance of digitalis glycosides with administration of multiple doses of activated charcoal (MDAC).(19-23) In one study the mean digoxin clearance increased by 47% in volunteers administered therapeutic doses of intravenous digoxin and oral MDAC compared to volunteers administered intravenous digoxin alone.(19).
Because of the structural similarity, it was hypothesised that Thevetia cardenolides also undergo enterohepatic recirculation, and that MDAC would increase clearance, producing clinical benefits.(24) Two randomised controlled trials (RCTs) subsequently assessed the efficacy of MDAC on mortality from yellow oleander poisoning (n=401(25)and n=1515 (at the final interim analysis) (26)), but the results were inconsistent.(6;27) . Further assessment of the effect of MDAC on the pharmacokinetics of Thevetia cardenolides may clarify the effect of this treatment and guide decisions regarding whether further clinical trials are required.
The objective of this study is to describe the pharmacokinetics of Thevetia cardenolides in patients with acute intentional self-poisoning with yellow oleander. The effect of activated charcoal (MDAC or a single dose of activated charcoal (SDAC)) on plasma cardenolide concentrations is also assessed.
Materials and methods
Clinical
This study was part of a large RCT (ISRCTN02920054) evaluating the efficacy of oral superactivated charcoal (Carbomix BP, Norit, NL; 2000m2/g) in Sri Lanka. All patients with a history of acute poisoning were eligible for inclusion in this RCT, except for those under the age of 14 years, known to be pregnant, or who reported ingestion of hydrocarbons alone or corrosives. Patients with acute yellow oleander poisoning were identified by on-site study doctors on presentation to study hospitals. Written informed consent was obtained prior to enrolment. Ethics approval was obtained from the Universities of Colombo (Sri Lanka) and Oxford (United Kingdom).
History of exposure (including the number of seeds) and clinical details were obtained on presentation and regularly throughout the admission until discharge or death. Patients were randomised into one of three groups: (1) to receive MDAC (50g dispersed in 300mL water every 4h for 6 doses); (2) SDAC (50g dispersed in 300mL water on admission) or (3) no activated charcoal (NoAC). All patients received supportive care, while atropine (usually 0.3-0.6mg/h) and intravenous fluids were administered as needed to maintain a heart rate above 70 and systolic BP above 80mmHg. Other treatments were determined by the treating medical team irrespective of their allocation in the RCT. Patients with severe cardiotoxicity were either administered anti-digoxin Fab antitoxin (when available) or transferred to other hospitals for placement of a temporary pacing wire because this service was not available in two of the study hospitals.
Serial blood samples were collected from a sample of patients admitted to Anuradhapura General Hospital and Kurunegala Teaching Hospital. A blood sample was obtained on admission, then at 1, 4, 12, and 24 hours after administration of the first charcoal dose, then once daily until discharge or death, as allowed by clinical factors. Samples were centrifuged and the plasma or serum was removed and immediately frozen at −23°C until analysis.
Laboratory
Assays developed for use in digoxin therapeutics cross-react with Thevetia cardenolides because of the structural similarity.(7;28;29) This cross-reactivity is a convenient means for approximating the concentration of Thevetia cardenolides in the plasma of patients who have been exposed to this plant.(6) Concentrations of digoxin cross-reacting substances (DXS) were quantified by fluorescence polarisation immunoassay (FPIA) on an Abbott TDx utilising cross reactivity in the Digoxin II assay (Abbott laboratories, IL, USA). The assay performance was monitored with Abbott digoxin controls and the results were as follows (mean ± SD); low: 0.78 μg/L ± 0.1, CV=12.5% n=104; medium: 1.53 μg/L ± 0.13, CV=8.7%, n=105; and high: 3.66 μg/L ± 0.23, CV=6.3% n=105. Six patient samples were tested five times each to determine interassay precision of DXS. Patient results had a CV% of 7-13 % with a mean ± SD of (1) 0.50 ± 0.04; (2) 0.31 ± 0.02; (3) 0.26 ± 0.04; (4) 0.43 ± 0.07; (5) 1.41 ± 0.09; (6) 1.42 ± 0.15. These values can be converted to molar concentrations (nmol/L) by multiplying the μg/L concentration by 1.28.
The sensitivity of the assay was 0.2μg/L, defined as the lowest concentration of the cardenolide that had a CV% of 25%. A similar limit of 0.2μg/L (0.26 nmol/L) for Thevetin B was reported by Uber-Bucek et al for the digoxin assay.(30)
The baseline (endogenous) concentration of digoxin-like immunoreactive substances (DLIS) was determined on plasma samples obtained from 197 controls. These were patients with acute poisoning with substances other than plants (eg. pesticides, medicines) who presented at the same hospitals. The median DLIS concentration was <0.2μg/L (range 0-0.30μg/L), so the lower level of discrimination for DXS concentration for this study was taken to be 0.3μg/L.
To identify sampling errors, such as accessing a vein proximal to an intravenous infusion, samples were identified where the DXS concentration was very low (generally undetectable) compared to that of both adjacent serial samples. Sampling from an infusion arm has been noted to occur in up to 8% of samples obtained by the same study doctors in other studies conducted by our Collaboration. Samples where this was noted were not considered further.
Pharmacokinetic Analysis
The pharmacokinetics of Thevetia cardenolides were described using serial DXS concentrations during hospital admission. The relatively sparse sampling that was possible in these patients with acute self-poisoning limits discussion to a general description of the basic pharmacokinetics. Log-linear decline in three consecutive concentration-time points (allowing ∼10% variation of the middle point) on visual inspection indicated that a patient was in the elimination phase. It was not possible to determine the pharmacokinetics of individual compounds because the immunoassay measures the total concentration of cross-reacting cardenolides in each sample.
Novel methods were used to describe the effect of MDAC and SDAC on the pharmacokinetics of Thevetia cardenolides. Pharmacokinetic research in clinical toxicology is a challenge when the dose consumed is not accurately known and patients present at variable times post-ingestion. It is further complicated when the poison is a natural product, such as yellow oleander, composed of multiple potentially active ingredients with unknown and individual pharmacokinetic properties. Two techniques were used to quantify the effect of activated charcoal during the first 24h post-admission (see below). A study period of 24h was chosen as this was the intended duration of treatment with MDAC. Where a blood sample was not obtained on (or very close to) 24h post-admission, the DXS concentration at this time was calculated by logarithmic interpolation of the two data points either side of the 24h time point.
In the first approach, we standardised for exposure by determining the area-under-the-curve (AUC) for each patient in the 24h period post-admission using the trapezoidal method (AUC24).(31) Each DXS concentration during that period was divided by the AUC24, generating a ‘fractional’ DXS concentration (concentration/AUC24). This adjustment is effectively correcting for differences in the fractional dose absorbed (F.D) and clearance (CL) between individual patients (AUC0-∞ =F.D/CL) – see Box 1 for more details. These results were pooled on the basis of their allocation in the randomised controlled trial and presented graphically. Trend lines of these three groups were generated by non-linear (first-order exponential decay) and linear regression using GraphPad Prism (Version 3.02 for Windows, San Diego, USA). These regression lines were compared using the F-Test to determine the equation which best fits the data.
Box 1.
The effect of dividing concentration by area-under-the-curve (concentration/AUC) is shown mathematically using single-compartment pharmacokinetics.(31)
Then:
Changes in the concentration/AUC with respect to time shown graphically will demonstrate the net balance of ka and ke, reflecting the rates of absorption and elimination. This will be independent of dose and the effects of charcoal on F. Since charcoal is not considered to alter ka (assuming that adsorption is an instantaneous and irreversible process, it will decrease F which is independent of ka), the overall trend in concentration/AUC noted for each group will be a reflection of ke. As ke increases, the gradient of the trend line will move further from the horizontal. Both ka and ke are first order rate constants.
This principle will also hold true if Thevetia cardenolides display multi-compartment pharmacokinetics.
[ka = absorption constant, ke = elimination constant, t = time, F = bioavailability, V = volume of distribution, CL = clearance]
In the second approach, the mean residence time (MRT) was calculated for each patient during the same 24h period (MRT24). MRT is a dose-independent non-compartmental method for quantifying the time course of a drug through the body. It reflects the rate of drug dissolution, absorption from the gut and elimination in an individual.(31;32) MRT can therefore be used to evaluate the effect of interventions such as activated charcoal which may alter these pharmacokinetic parameters. Changes in pharmacokinetics from an intervention can be measured by comparing the MRT of two or more groups in whom treatment was otherwise standardised.(31) Even when specific information about the dose and/or pharmacokinetic parameters for the poison are limited, as is the case for Thevetia cardenolides, the relative effect of this intervention can be quantified.
The MRT24 was calculated by dividing the area-under- the-moment-curve (AUMC24) by the AUC24.(32) The AUMC24 was determined using the trapezoidal method for each patient from concentration-time points obtained during the first 24h of admission.(31) Because MRT24 was determined from data over a 24h period only, the values calculated for each treatment arm are lower than an MRT calculated using a complete data set (t=0 to infinity). As such, times determined from this 24h data are only useful for comparing the relative MRT24 between treatment arms.
Statistical Analysis
Statistics were applied to compare the three groups enrolled in the randomised controlled trial. Continuous variables were compared using the Mann-Whitney test and categorical variables were compared by the Fisher's exact test. When comparing the differences in MRT24 and the gradients of the concentration/AUC24 regression lines between treatments, the Student t-test was used if the results were normally distributed. Normality was determined by visual inspection, and confirmed using the Kolmogorov-Smirnov test (GraphPad Prism) where appropriate. A P<0.05 was taken as statistically significant.
Results
There were 254 patients with acute yellow oleander poisoning who provided multiple blood samples. Of these, 104 patients were eligible for inclusion in this study after excluding those who provided samples for less than 24h (eg. consent was subsequently withdrawn, or the patient was transferred to another hospital for insertion of a temporary pacemaker, or they died), those with DXS concentrations mostly below the level defined as DLIS (consistent with minimal or no exposure), and those administered the anti-digoxin Fab antitoxin. Demographics and basic clinical details of patients who provided serial samples are listed in Table 1. 15 samples (3% of all samples) suspected of being collected proximal to an intravenous infusion were excluded from the analysis.
Table 1.
Summary of demographics of patients who provided serial samples within the randomised controlled trial.
| Patients satisfying inclusion criteria | ||||
|---|---|---|---|---|
| NoAC | SDAC | MDAC | P-value | |
| N= | 40 | 28 | 36 | |
| Gender (M:F) | 20:20 | 8:20§ | 22:14§ | 0.012§ |
| Age (median, IQR; y) | 21.5 (17.5-28.5) | 22 (18.0-33.0) | 22.5 (17.5-28.0) | N/S |
| Time to presentation (median, IQR; h) | 6.5 (3.8-12.3) | 5.5 (4.0-8.3) | 5.0 (3.8-12.5) | N/S |
| No. of seeds (median, IQR)* | 3.0 (3.0-5.0; n=34) | 3.5 (2.0-5.0; n=26) | 3.0 (2.0-4.5; n=28) | N/S |
| Patients not satisfying inclusion criteria, and excluded from pharmacokinetic analysis † | ||||
| NoAC | SDAC | MDAC | P-value | |
| N= | 33 | 27 | 26 | |
| Gender (M:F) | 18:15 | 13:14 | 13:13 | N/S |
| Age (median, IQR; y) | 22.0 (18.0-27.5) | 22.0 (17.0-31.0) | 20 (17.5-32.0) | N/S |
| Time to presentation (median, IQR; h) | 5.0 (4.0-9.0) | 7.0 (5.0-12.5) | 7.5 (3.25-12.0) | N/S |
| No. of seeds (median, IQR)* | 4.5 (3.0-6.75; n=30) | 4.0 (2.0-5.0; n=25) | 5.0 (2.75-6.25; n=24) | N/S |
| Selected reasons for not satisfying criteria: | ||||
| Death within 24h of admission: | 2 | 3 | 2 | N/S |
| Administration of Fab anti-toxin | 6 | 4 | 5 | N/S |
| Insertion of temporary pacing wire | 6 | 5 | 7 | N/S |
The number of seeds consumed by some patients was not known
Results which are significantly different and the corresponding P-value
N/S Non-significant
An additional 54 patients provided serial samples, but the DXS concentrations were mostly below the level of discrimination, consistent with minimal or no exposure. These patients were mostly asymptomatic, although some reported mild symptoms.
Absorption
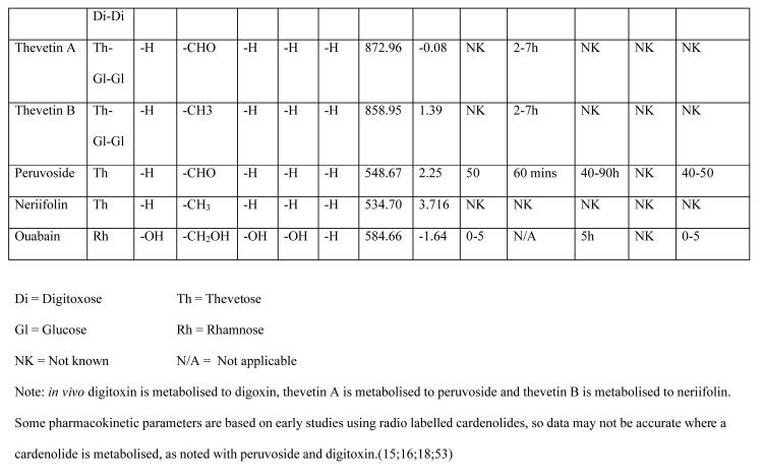
Unpredictable absorption patterns (both intra- and inter-individual) were noted in each patient group. Ongoing absorption was commonly observed, extending beyond 50h post-ingestion in some (Figure 2a). Erratic absorption was also noted in each patient group, with two DXS peak concentrations occurring in a number of the concentration-time profiles. In 57% of patients a single peak DXS concentration (Cmax) was noted at admission (examples, Figure 2b), which may have been preceded by the true Cmax. In others, DXS concentrations did not increase above the level of discrimination until more than 6h post-ingestion (Figure 2c).
Figure 2.
Examples of pharmacokinetic profiles of individual patients with acute yellow oleander poisoning.*
2a. Prolonged and erratic absorption
2b. Apparent rapid disposition post-admission; possibility of ongoing absorption
2c. Delayed onset of absorption with probable ongoing absorption
* level of discrimination represented by dashed line at DXS concentration of 0.3μg/L
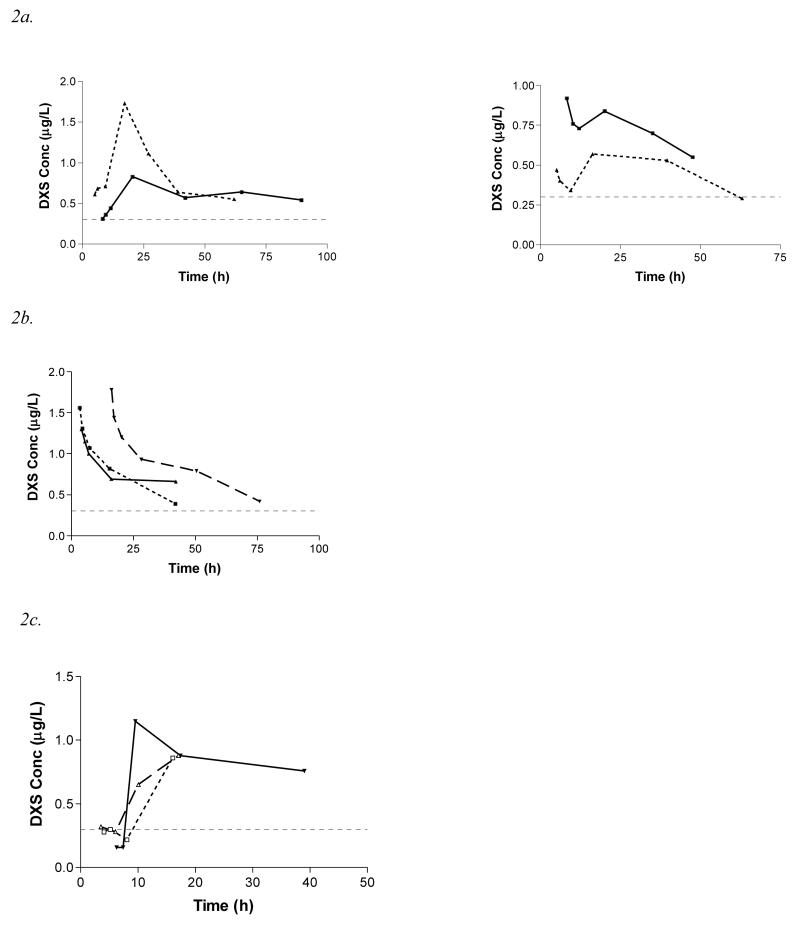
The relationship between AUC24 and the number of seeds ingested is shown in Figure 3. There is a marked variability in AUC24 between patients who reported ingesting the same number of seeds. There is no clear trend towards increased AUC24 with larger ingestions, suggesting saturable (zero order) absorption, although other factors may have influenced this effect, as discussed later.
Figure 3.
AUC24 vs number of seeds ingested (n=88; the number of seeds consumed by some patients was not known). Regression line for AUC24 = [Vm×No. of seeds]/[Km+ No. of seeds]
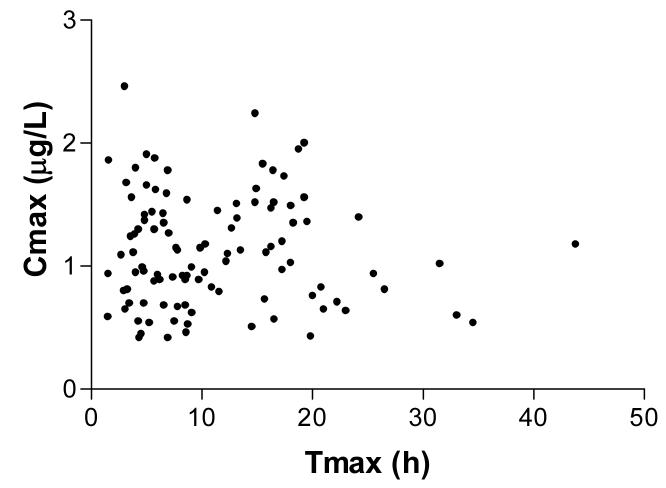
The time corresponding to the Cmax (Tmax) varied widely, occurring in some patients beyond 24h and at a high concentration (eg. more than 1.5μg/L, see Figure 4). The random distribution of concentrations in Figure 4 suggests that absorption is not first order, and that factors unrelated to dose or the absorption coefficient were influencing the observed absorption kinetics. One such likely factor was co-administration of atropine, but a clear relationship between administration of atropine and changes in DXS concentrations was not observed (data not shown).
Figure 4.
Relationship between peak concentration (Cmax) and the time when this occurs post-ingestion (Tmax) for all patients (n=104).
Distribution, metabolism and excretion
More rapid disposition is noted in some patients (Figure 2b). The duration of this apparent disposition phase appears in the order of 6-10h, which is prolonged compared to ∼2h for oral digoxin, depending on the formulation.(33;34) Volume of distribution, metabolism or route of elimination could not be estimated from these data. Metabolites of Thevetia cardenolides may also cross react with the digoxin assay and contribute to the DXS concentration. The pharmacokinetics of the metabolites will differ from the parent compound; for example, they are likely to be more polar which decreases the volume of distribution (VD). Where bioactivation decreases the VD there is an increase in DXS concentration compared to the same dose of the parent compound. Depending on the time-delay for bioconversion, this effect may contribute to the double peaks observed in some patients (Figure 2a).
Terminal half-life
Log-linear declines in concentration were noted in some patients during the sampling period. Only 14 (34%) of patients randomised to NoAC entered an apparent elimination phase during admission. In these patients, the median time to onset of the log-linear decline was 15.8h, and the median terminal half-life was 42.9h (range 13.27-94.24h).
The effect of activated charcoal on Thevetia cardenolide pharmacokinetics
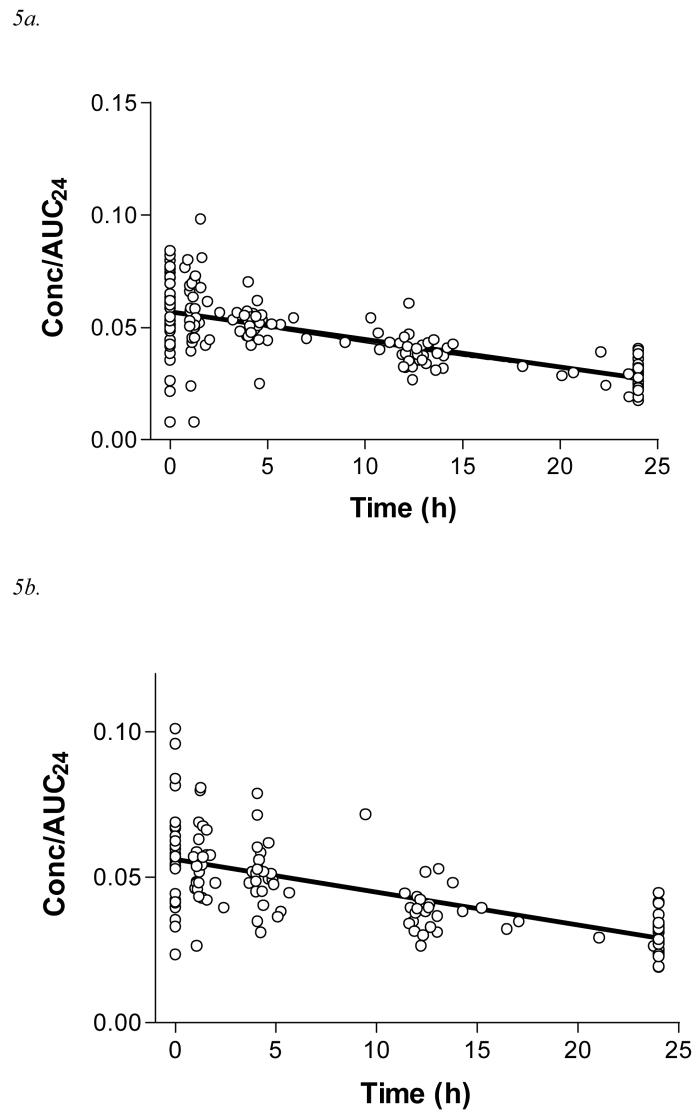
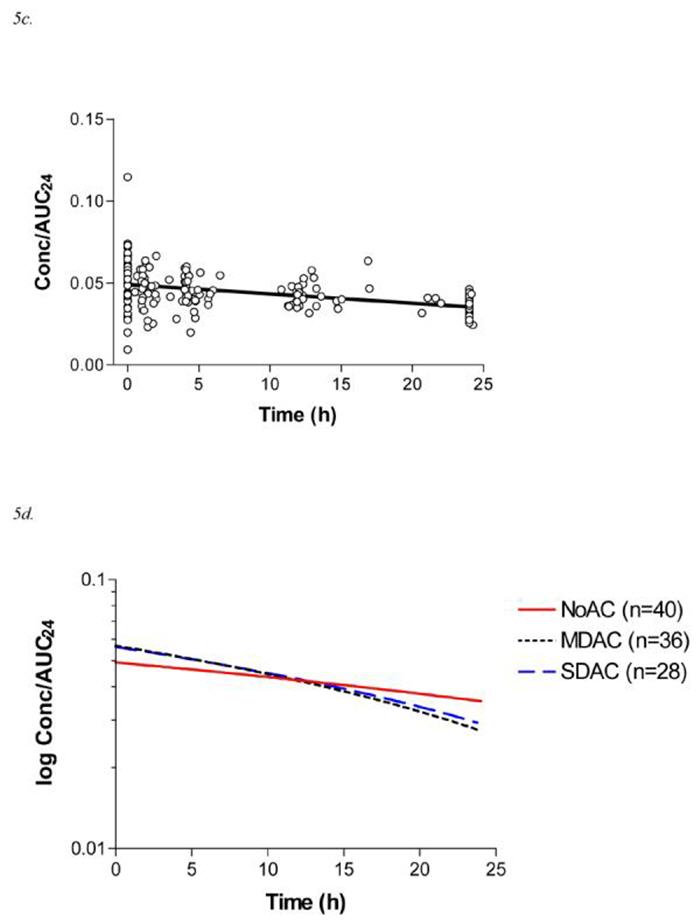
A summary of the pharmacokinetic parameters are shown in Table 2. The baseline characteristics of each treatment arm were similar, except for gender; all patients in these groups survived to discharge. Further, the demographics of the included patients did not differ significantly from those who were excluded. The rate of change in concentration/AUC24 with time was greater for patients randomised to MDAC and SDAC, compared to NoAC, consistent with increased elimination of DXS (Figure 5). Linear regression best described the data in each treatment arm, the gradient of which reflects the relative values of ke (Table 2). This gradient differed significantly for NoAC compared to MDAC and SDAC. The regression lines were approximately log-linear (Figure 5d), allowing the terminal half-life of these pooled samples to be estimated (Table 2).
Table 2.
Pharmacokinetic parameters of patients who fulfilled inclusion criteria
| NoAC | SDAC | MDAC | P-value | |
|---|---|---|---|---|
| Cmax (median, IQR; μg/L) | 1.05 (0.75-1.40) | 0.98 (0.72-1.50) | 1.13 (0.86-1.47) | N/S |
| Tmax (median, IQR; h) | 12.1 (5.4-17.4) | 7.2 (5.7-13.8) | 8.3 (4.8-15.0) | N/S |
| AUC24 (median, IQR; μg.h L−1) | 19.0, 13.7-24.3 | 17.7, 11.1-21.8 | 17.3, 12.8-21.7 | N/S |
| Atropine administered (% of patients) | 90.0 | 85.7 | 94.4 | N/S |
| Diarrhoea present (% of patients) | 20.0 | 25.0 | 19.4 | N/S |
| Gradient of linear regression line (m ×10−4 h−1; 95% CI)† |
−5.8 (−4.0 to −7.5) | −11.3 (−9.1 to −13.4) | −12.2 (−10.4 to −14.1) | <0.0001(M), 0.0001(S) |
| Apparent terminal half-life (t1/2; h) # | 62.9 | 33.9 | 32.3 | |
| MRT24 (mean, SD, h) † | 11.21 +/− 1.55 | 10.36 +/− 1.14 | 10.20 +/− 0.99 | 0.001(M), 0.015(S) |
usually t1/2=0.693/ke,(31) where ke is the gradient of a concentration-time graph. Because the gradient (m) of the regression line in this study was determined using concentration/AUC24 data points, ke=m.AUC24.
Comparison with NoAC, where respective P-values are shown as S=SDAC and M=MDAC
N/S Non-significant
Figure 5.
5a. Data points for Concentration/AUC24 vs time and linear trend line for the MDAC arm of the randomised controlled trial (n=36, 178 measurements; y = −0.0012x+ 0.057; r2=0.48)
5b. Data points for Concentration/AUC24 vs time and linear trend line for the SDAC arm of the randomised controlled trial (n=28, 134 measurements; y = −0.0011x+ 0.056; r2=0.44)
5c. Data points for Concentration/AUC24 vs time and linear trend line for the NoAC arm of the randomised controlled trial (n=40, 189 measurements; y = −0.00058x+ 0.049; r2 = 0.17)
5d. Comparative trend lines of log Concentration/AUC24 vs time for each arm of the randomised controlled trial.
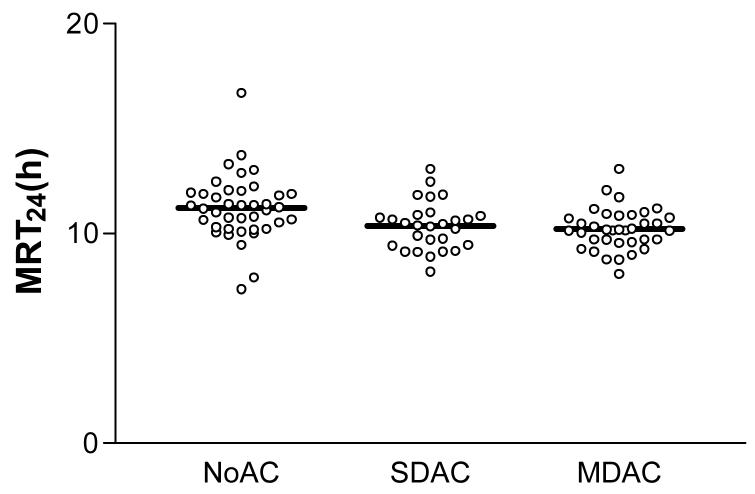
Similar results were noted using the MRT24 method, where a significantly shorter MRT24 was noted for SDAC and MDAC compared to NOAC (Table 2, Figure 6).
Figure 6.
MRT24 for each patient according to the treatment arm, including mean values
Discussion
To our knowledge, this is the first description of the pharmacokinetics of Thevetia cardenolides in acute poisoning. These data were provided by patients enrolled in a randomised controlled trial assessing the efficacy of activated charcoal. Unpredictable absorption was noted commonly and this dominated the pharmacokinetic profile. An apparent increase in clearance of Thevetia cardenolides was observed in patients administered either SDAC or MDAC.
Ingestion of pharmaceutical-grade cardenolides, such as digoxin, produces a standard concentration-time curve with a Cmax, distribution phase and log-linear elimination phase.(33) This was not noted generally in our patients, for which ongoing cardenolide absorption appears the most likely explanation, although enterohepatic recirculation may have also contributed. Log-linear elimination was noted in some patients in this study, suggesting that absorption was completed earlier in some patients than others.
While slow and variable absorption of cardenolides has been noted previously,(35;36) the reason for the markedly variable and erratic absorption noted here (Figure 2) is not clear from data obtained in this study. Processes which alter bowel motility probably contributed, in particular the effect of intravenous atropine given that anticholinergic drugs slow gut motility and prolong the absorption phase.(37-43). Other potential contributors include the slow release of cardenolides from the cellulose matrix of intact particles in the oleander seeds, which may relate also to the extent to which the seeds were ground up or chewed prior to ingestion. This has been noted with digoxin formulations, where bioavailability and AUC decrease as particle size increases.(34) Further, poorly chewed seeds might not pass through the gastric pylorus easily. Inhibition of intestinal Na+-K+ ATPase by cardenolides may cause smooth muscle spasm,(10;13;36;44) manifesting as colicky abdominal pain and diarrhoea,(10) reducing the amount of cardenolides absorbed. The relative influence of each of these factors is not known.
The poor correlation between AUC24 and the number of seeds ingested (Figure 3) has been noted clinically with regards to the severity of cardiotoxicity. Death has occurred after ingestion of one or two seeds despite admission to a referral hospital experienced in the management of oleander poisoning.(24;25) In contrast, patients have been discharged from hospital in good health without requiring pacing or antitoxin after reporting consumption of ten or more seeds.(5) This poor correlation may relate to variability in cardenolide contents, capacity-limited absorption from the gut (as suggested in animal studies(36)), or perhaps more vigorous methods of decontamination by clinical staff in patients who report very large exposures. Additionally, the force and frequency of spontaneous vomiting may increase as the exposure increases, improving the efficiency of self-decontamination. Persistent vomiting is noted with severe poisonings,(6) as well as being an important side effect which limited the therapeutic use of Thevetia in cardiac failure.(10;13)
SDAC and MDAC appear to increase DXS elimination to a similar extent (Figures 5d and 6). It is not clear why this is the case since MDAC is thought to increase clearance by interruption of enterohepatic recirculation of long-acting cardenolides. But clinical benefits (mortality, secondary outcomes are yet to be reported) were not noted in patients administered MDAC or SDAC in the RCT from which these samples were obtained, compared to those not administered activated charcoal.(26) In the other RCT, MDAC was more effective than SDAC in decreasing mortality, but this trial did not have a control arm (a group where no activated charcoal was administered) and pharmacokinetic analyses were not conducted to correlate the clinical outcomes with alterations in the rate of elimination.(25) Nevertheless, outcomes in the latter RCT are unexpected given that MDAC and SDAC alter cardenolide pharmacokinetics equally. Of course, if the absorption process is saturable as the initial modelling of the AUC24 versus no. of seeds ingested suggested, the effect of charcoal on AUC24 would be expected to be minimal if a large number of seeds had been ingested.
Atropine may prolong gut transit time to an extent that SDAC is retained long enough to interrupt enterohepatic recirculation. Alternatively, SDAC and the first dose of MDAC may sit with the seed fragments as they pass slowly through the gut, reducing absorption. If charcoal promotes the dialysis of Thevetia cardenolides across the gut membrane, as noted with other drugs,(45) then prolonging the gut transit time of SDAC may increase its effectiveness, if the dose is sufficient. Similarly, co-administration of atropine has been noted to increase the efficacy of SDAC in volunteer studies.(37)
The effect noted in Figures 5d and 6 might also be due to a change in cardenolide absorption kinetics. Drug absorption first requires disintegration of the seed, followed by dissolution of the drug, and then absorption through the gut wall. The overall rate of this process is dependent on the rate-limiting step. Since drug dissolution is usually rapid (thevetin is water soluble (46)), absorption kinetics depend on absorption through the gut wall.(31) If this process is slower than the rate of elimination, absorption kinetics will dominate the pharmacokinetic profile and prolong the apparent rate of elimination. Administration of charcoal reversibly adsorbs drugs, after which point the drug is unavailable for absorption. If the affinity of the drug for charcoal is sufficiently strong, the drug is considered to be removed from the system and the amount available for absorption is reduced. In the event that there is saturable absorption of Thevetia cardenolides, as suggested in Figure 3 and by others previously,(35;36) their removal from the system will decrease the duration over which drug absorption will occur. Consequently, the MRT24 will shorten and the gradient of the concentration/AUC24 graph will increase, suggesting a favourable effect from activated charcoal.
The effects of SDAC and MDAC on cardenolide pharmacokinetics that were noted in this study may not be readily applicable to all patients with yellow oleander poisoning. Because serial blood samples could not be obtained in all patients with severe poisoning (and therefore higher exposures) it is not known whether differences in mortality would be noted if more patients with severe poisoning were included. While pharmacokinetic data presented here suggests that SDAC and MDAC are equally effective, this may require treatments such as atropine to be administered. This is an issue for centres where atropine is not administered routinely for oleander poisoning and those who use a different regimen (the ideal dose is not known). To replicate the effect of activated charcoal noted in this study, it appears necessary for physicians to concomitantly administer atropine using the regimen described above
It is interesting that the most recent RCT did not improve mortality,(26) yet improvements in pharmacokinetics were observed from activated charcoal. The reasons for this are open to speculation, but may relate to absorption of a cardiotoxic dose prior to initiation of charcoal given that the median time to admission of these patients was 5.0-7.5h. Current recommendations are that activated charcoal should be administered within 1h of poisoning if it is to be effective, although this is based on data obtained primarily from pharmaceutical poisoning. (47) Alternatively, charcoal may have a low adsorptive capacity such that some cardenolides continued to be absorbed despite administration of charcoal. Another factor is the low baseline case fatality ratio from yellow oleander poisoning noted in the randomised controlled trial (4%), which may require a more pronounced effect on pharmacokinetics than we noted for statistical significance when using mortality as an endpoint. Further, some patients with the most severe toxicity were excluded from this study by being transferred to another hospital or administered the Fab antitoxin. While MDAC did not improve survival for the overall cohort of patients in the RCT at the final interim analysis, the increase in clearance noted here may suggest that there are individuals who could still benefit from its administration, in particular those who present to hospital early. This may be worthy of further investigation, given that activated charcoal is cheap and readily available.
Conclusion
This is the first study to assess the pharmacokinetics of Thevetia cardenolides in patients with acute oleander poisoning. In this sample of patients a favourable effect on cardenolide pharmacokinetics was noted in patients administered activated charcoal. Although potential trends in charcoal effect were evident, a difference in elimination was not noted between the two regimens of activated charcoal (SDAC and MDAC) in terms of AUC24, MRT24 or terminal half-life. The effect of SDAC or MDAC on cardenolide pharmacokinetics in patients with severe oleander poisoning has not fully been evaluated. Studies assessing the effect of early administration of activated charcoal may clarify the role of activated charcoal in the management of patients with acute yellow oleander poisoning.
Acknowledgements
We thank the consultant physicians, and medical and nursing staff of the study hospitals for their support and the study doctors for their invaluable work. Thanks also to Malcolm Rowland for helpful comments.
Sources of support: DMR acknowledges the support of the National Health and Medical Research Council (Australia). ME is a Wellcome Trust Career Development Fellow, funded by grant GR063560MA from the Wellcome Trust's Tropical Interest Group. The South Asian Clinical Toxicology Research Collaboration is funded by a Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant GR071669MA.
Footnotes
Potential conflicts of interest:
We declare that we have no conflicts of interest.
Reference List
- 1.Langford SD, Boor PJ. Oleander toxicity: an examination of the human and animal toxic exposures. Toxicol. 1996;109:1–13. doi: 10.1016/0300-483x(95)03296-r. [DOI] [PubMed] [Google Scholar]
- 2.Saravanapavananthan N, Ganeshamoorthy J. Yellow oleander poisoning - a study of 170 cases. Forensic Sci Int. 1988;36:247–50. doi: 10.1016/0379-0738(88)90150-8. [DOI] [PubMed] [Google Scholar]
- 3.Saraswat DK, Garg PK, Saraswat M. Rare poisoning with cerebra thevetia (yellow oleander). Review of 13 cases of suicide attempt. J Assoc Physicians India. 1992;40:628–9. [PubMed] [Google Scholar]
- 4.Bose TK, Basu RK, Biswas B, et al. Cardiovascular effects of yellow oleander ingestion. J Indian Med Assoc. 1999;97(10):407–10. [PubMed] [Google Scholar]
- 5.Eddleston M, Ariaratnam CA, Meyer WP, et al. Epidemic of self-poisoning with seeds of the yellow oleander tree (Thevetia peruviana) in northern Sri Lanka. Trop Med Int Health. 1999;4(4):266–73. doi: 10.1046/j.1365-3156.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DM, Eddleston M. Yellow oleander poisoning. In: Nayyar V, editor. Critical Care Update 2004. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2005. pp. 189–200. [Google Scholar]
- 7.Radford DJ, Gillies AD, Hinds JA, et al. Naturally occurring cardiac glycosides. Med J Aust. 1986;144:540–4. doi: 10.5694/j.1326-5377.1986.tb112283.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao EV. Cardiac glycosides of Thevetia peruviana and Cerbera odollam. The Indian J Pharm. 1973;35(4):107–13. [Google Scholar]
- 9.Kyerematen G, Hagos M, Weeratunga G, et al. The cardiac glycosides of Thevetia ovata A.DC. and Thevetia nereifolia Juss. ex Stend. Acta Pharm Suec. 1985;22(1):37–44. [PubMed] [Google Scholar]
- 10.Middleton WS, Chen KK. Clinical results from oral administration of thevetin, a cardiac glycoside. Am Heart J. 1936;11:75–88. [Google Scholar]
- 11.Bhatia ML, Manchanda SC, Roy SB. Haemodynamic studies of peruvoside in human congestive failure. Br Med J. 1970;3:740–3. doi: 10.1136/bmj.3.5725.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora RB, Sharma JN, Bhatia ML. Pharmacologic evaluation of peruvoside, a new cardiac glycoside from Thevetia neriifolia with a note on its clinical trials in patients with congestive heart failure. Indian J Exp Biol. 1967;5:31–6. [PubMed] [Google Scholar]
- 13.Arnold HL, Middleton S, Chen KK. The action of thevetin, a cardiac glucoside, and its clinical application. Am J Med Sci. 1935;189:193–206. [Google Scholar]
- 14.Schwind K. Bericht über die klinische prüfung von peruvosid. [Report on the clinical testing of peruvoside]. Medizinische Welt. 1969;3:134–42. [PubMed] [Google Scholar]
- 15.Frülich JC, Falkner FC, Watson JT, et al. Metabolism of peruvoside in man. Eur J of Clin Pharmacol. 1972;5(2):65–71. [Google Scholar]
- 16.Kramer P, Willms B, Horenkamp J, et al. Blutspiegelkinetik und renale clearance von H3-peruvosid. [Blood level kinetics and renal clearance of H3 peruvosid]. Klin Wochenschr. 1969;47(21):1157–66. doi: 10.1007/BF01483745. [DOI] [PubMed] [Google Scholar]
- 17.Garbe A, Nowak H. Zur pharmakokinetik des peruvosid. II. Vergleichende untersuchungen mit H3-ouabain und H3-digitoxin am meerschweinchen. [On the pharmacokinetics of peruvoside. II. Comparative studies with H3-ouabain and H3-digitoxin in guinea pigs]. Arzneimittelforschung. 1968;18(12):1597–601. [PubMed] [Google Scholar]
- 18.Forth W, Fubukawa E, Rummel W. Intestinale resorption von herzglykosiden in vitro und in vivo# Intestinal absorption of cardiac glycosides in vitro and in vivo Naunyn Schmiedebergs Arch Pharmak exp Path 1969. 262 1 53 72 [PubMed] [Google Scholar]
- 19.Lalonde RL, Deshpande R, Hamilton PP, et al. Acceleration of digoxin clearance by activated charcoal. Clin Pharmacol Ther. 1985;37:367–71. doi: 10.1038/clpt.1985.55. [DOI] [PubMed] [Google Scholar]
- 20.Reissell P, Manninen V. Effect of administration of activated charcoal and fibre on absorption, excretion and steady state blood levels of digoxin and digitoxin. Evidence for intestinal secretion of glycosides. Acta Med Scand Suppl. 1982;668:88–90. doi: 10.1111/j.0954-6820.1982.tb08527.x. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell JH, Caldwell PB, Murphy JW, et al. Intestinal secretion of digoxin in the rat. Augmentation by feeding activated charcoal. Naunyn Schmiedebergs Arch Pharmacol. 1980;312(3):271–5. doi: 10.1007/BF00499157. [DOI] [PubMed] [Google Scholar]
- 22.Park GD, Goldberg MJ, Spector R, et al. The effects of activated charcoal on digoxin and digitoxin clearance. Drug Intell Clin Pharm. 1985;19(12):937–41. doi: 10.1177/106002808501901216. [DOI] [PubMed] [Google Scholar]
- 23.Ibanez C, Carcas AJ, Frias J, et al. Activated charcoal increases digoxin elimination in patients. Int J Cardiol. 1995;48:27–30. doi: 10.1016/0167-5273(94)02212-2. [DOI] [PubMed] [Google Scholar]
- 24.Eddleston M, Rajapakse S, Rajakanthan, et al. Anti-digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet. 2000;355:967–72. doi: 10.1016/s0140-6736(00)90014-x. [DOI] [PubMed] [Google Scholar]
- 25.de Silva HA, Fonseka MMD, Pathmeswaran A, et al. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: a single-blind, randomised, placebo-controlled trial. Lancet. 2003;361:1935–8. doi: 10.1016/s0140-6736(03)13581-7. [DOI] [PubMed] [Google Scholar]
- 26.Eddleston M, Juszczak E, Buckley NA, et al. Randomised controlled trial of routine single or multiple dose superactivated charcoal for self-poisoning in a region with high mortality. Clin Toxicol. 2005;43:442–3. [Google Scholar]
- 27.Juurlink DN, Sivilotti MLA. Multidose activated charcoal for yellow oleander poisoning. Lancet. 2003;362:581. doi: 10.1016/S0140-6736(03)14136-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheung K, Hinds JA, Duffy P. Detection of poisoning by plant-origin cardiac glycosides with the Abbott TDx analyser. Clin Chem. 1989;35:295–7. [PubMed] [Google Scholar]
- 29.Hoffman RS. Non-pharmacological cardioactive steroids. J Toxicol Clin Toxicol. 2002;40(3):285–6. [Google Scholar]
- 30.Uber-Bucek E, Hamon M, Pham Huy C, et al. Determination of thevetin B in serum by fluorescence polarization immunoassay. J Pharm Biomed Anal. 1992;10(6):413–9. doi: 10.1016/0731-7085(92)80059-v. [DOI] [PubMed] [Google Scholar]
- 31.Rowland M, Tozer TN. Clinical Pharmacokinetics - Concepts and Applications. 2nd ed Philadelphia: Lea & Febiger; 1989. [Google Scholar]
- 32.Nakashima E, Benet LZ. General treatment of mean residence time, clearance, and volume parameters in linear mammillary models with elimination from any compartment. J Pharmacokinet Biopharm. 1988;16(5):475–92. doi: 10.1007/BF01062381. [DOI] [PubMed] [Google Scholar]
- 33.Lindenbaum J, Mellow MH, Blackstone MO, et al. Variation in biological activity of digoxin from four preparations. N Engl J Med. 1971;285(24):1344–7. doi: 10.1056/NEJM197112092852403. [DOI] [PubMed] [Google Scholar]
- 34.Jounela AJ, Pentikäinen PJ, Sothmann A. Effect of particle size on the bioavailability of digoxin. Eur J Clin Pharmacol. 1975;8(5):365–70. doi: 10.1007/BF00562664. [DOI] [PubMed] [Google Scholar]
- 35.Modell W, Kwit NT, Dayrit C, et al. Thevetin, a cardiac glycoside with an unusual speed of action in man. J Pharmacol Exp Ther. 1948;94:44–52. [PubMed] [Google Scholar]
- 36.Kohli JD, Vohra MM. Pharmacological studies on peruvoside - a new cardiac glycoside from Thevetia peruviana Juss. Arch Int Pharmacodyn. 1960;126(3-4):412–25. [PubMed] [Google Scholar]
- 37.Green R, Sitar DS, Tenenbein M. Effect of anticholinergic drugs on the efficacy of activated charcoal. J Toxicol Clin Toxicol. 2004;42(3):267–72. doi: 10.1081/clt-120037426. [DOI] [PubMed] [Google Scholar]
- 38.Bennett PN, Davies DS, Hawksworth GM. In vivo absorption studies with paraquat and diquat in the dog. Br J Pharmacol. 1976;58:284P. [PMC free article] [PubMed] [Google Scholar]
- 39.Rashid MU, Bateman DN. Effect of atropine on gastric emptying, paracetamol absorption, salivary flow and heart rate in young and fit elderly volunteers. Br J Clin Pharmacol. 1990;30:25–34. doi: 10.1111/j.1365-2125.1990.tb03739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivera-Calimlim Problems of therapeutic drug monitoring of chlorpromazine. Ther Drug Monitor. 1982;4:41–9. doi: 10.1097/00007691-198204000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Consolo S, Morselli PL, Zaccala M, et al. Delayed absorption of phenylbutazone caused by desmethyl imipramine in humans. Eur J Clin Pharmacol. 1970;10(2):239–42. doi: 10.1016/0014-2999(70)90278-5. [DOI] [PubMed] [Google Scholar]
- 42.Nimmo J, Heading RC, Tothill P, et al. Pharmacological modification of gastric emptying: effects of propantheline and metoclopramide on paracetamol absorption. Br Med J. 1973;1(5853):587–9. doi: 10.1136/bmj.1.5853.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan JP, Nathan G, Rivera-Calimlim L, et al. Imipramine caused interference with levodopa absorption from the gastrointestinal tract in rats. J Pharmacol Exp Ther. 1975;192(2):451–7. [PubMed] [Google Scholar]
- 44.Chopra RN, Mukerjee B. The pharmacological action of ‘Thevetin’ - a glucoside occurring in Thevetia neriifolia (Yellow Oleander) Indian J Med Res. 1933;20:903–12. [Google Scholar]
- 45.Anonymous Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1999;37(6):731–51. doi: 10.1081/clt-100102451. [DOI] [PubMed] [Google Scholar]
- 46.Chen KK, Chen AL. The action of crystalline thevetin, a cardiac glucoside of Thevetia neriifolia. J Pharm Exp Ther. 1934;51:23–34. [Google Scholar]
- 47.Chyka PA, Seger D, Krenzelok EP, et al. American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: single-dose activated charcoal. Clin Toxicol. 2005;43(2):61–87. doi: 10.1081/clt-200051867. [DOI] [PubMed] [Google Scholar]
- 48.Lohmöller G, Lydtin H. Mechanocardiographische untersuchungen über die wirkung von peruvosid auf das suffiziente menschliche herz. [Mechano cardiographic studies on the effect of peruvoside on the normal human heart]. Arzneimittel-Forschung (Drug Res) 1971;21(10):1567–71. [PubMed] [Google Scholar]
- 49.Chemical Abstracts Service . SciFinder Scholar. Columbus, Ohio: 2006. [Google Scholar]
- 50.Marks BH, Dutta S, Gauthier J, et al. Distribution in plasma, uptake by the heart and excretion of ouabain-H3 in human subjects. J Pharmacol Exp Ther. 1964;145:351–6. [PubMed] [Google Scholar]
- 51.Micromedex® Healthcare Series. Greenwood Village, Colorado: Micromedex Inc.; 2006. [Google Scholar]
- 52.The Merck index : an encyclopedia of chemicals, drugs and biologicals. 12th ed. Whitehouse Station, N.J.: Merck & Co.; 1996. [Google Scholar]
- 53.Caldwell JH, Bush CA, Greenberger NJ. Interruption of the enterohepatic circulation of digitoxin by cholestyramine: II. Effect on metabolic disposition of tritium-labelled digitoxin and cardiac systolic intervals in man. J Clin Invest. 1971;50(12):2638–44. doi: 10.1172/JCI106764. [DOI] [PMC free article] [PubMed] [Google Scholar]