Abstract
Recent work from a number of laboratories has provided new and important insights about how gene expression is altered by experience and how these molecular changes may provide a substrate for the long-term storage of new memories. Here we review a series of recent studies using aversive Pavlovian conditioning in rats as a well characterized model system in which experience-dependent alterations in gene expression can be manipulated and quantified within a specific neural circuit. We highlight some of the issues involved in using broad-spectrum inhibitors of mRNA and protein synthesis to study cellular changes underlying the formation and long-term stability of memory and discuss the idea that these changes occur over widespread, behaviorally-defined, networks of cells. We also discuss the idea that the maintenance of memory and its susceptibly to disruption after retrieval may relate to local protein synthesis in dendrites. Finally, a series of recent experiments from our laboratory studying the role of a specific signaling pathway (mTOR) which regulates translational processes and memory formation in the amygdala and hippocampus during fear conditioning are reviewed.
Introduction
Over the last 60 years or so the general idea that the cell biological substrate for long term memory storage critically involves the synthesis of new mRNA and protein has enjoyed increasingly widespread support. While the initial evidence for this position was often drawn from studies using large systemic doses of broad-spectrum inhibitors and a variety of species and behavioral paradigms, more recent experimental work has been able to go a bit deeper into describing the relationships between macromolecular synthesis and the consolidation of long-term memory.
Pavlovian fear conditioning (FC) in rodents has emerged as one of the more productive approaches to understanding the neurobiology of learning and memory. This robust form of learning is rapidly acquired, straightforward to measure, and relies on a fairly well described neural circuit that includes cortical and subcortical elements. Similar training procedures can be used in laboratory animals and human volunteers and current data suggest a large degree of similarity in neural mechanisms across species. A number of excellent recent reviews are available providing more detailed treatment of various aspects of the anatomy, physiology, and molecular biology of FC than space here permits (e.g., Blair, et al., 2001; Davis, 2000; Fanselow & Poulos, 2005; Goosens & Maren, 2002; Kim & Jung, 2006; LeDoux 2000; Maren 2000; Medina, et al., 2002; Pare, Quirk & LeDoux, 2004; Stork & Pape, 2002).
It is generally accepted that the amygdala plays a critical role in the formation of memory for aversive events although important details regarding the nature of its involvement are still a matter of some debate (McGaugh, 2004). Pioneering studies by LeDoux and colleagues showed that cells in the basolateral subdivision (BLA), including the lateral and basolateral amygdaloid nuclei (c.f., deOlmos et al., 1985), are necessary for normal learning in FC. BLA cells receive inputs capable of representing both auditory and contextual signals for shock (conditional stimuli, CS) as well as diencephalic projections that may help encode the shock unconditional stimulus (UCS). Electrophysiological studies have shown that unit responses in BLA are consistent with processing relevant presynaptic inputs and appropriate stimulation regiments result in long-term potentiation (LTP) at BLA synapses both in vitro and in vivo. Because of the large amount of systematic work being done on this region of the amygdala, a clear and detailed picture of the molecular and cellular events underlying FC-related synaptic plasticity in this structure is emerging (Blair, et al., 2001; Goosens & Maren, 2002; Maren & Quirk, 2004)
While the BLA is thought by many to be a site of critical synaptic plasticity in FC, the expression of fear-related conditional responses (CR) during subsequent behavioral performance based on this learning is thought to depend on intrinsic amygdaloid connections from BLA to the central nucleus (CeA) and from there to a widespread series of target structures more specifically involved in the generation of particular behavioral and physiological elements of the fear response. Expression of FC involves multiple simultaneous outputs and systems-level analyses have identified various unique contributions to components of this response. Early work showed that specific lesions of the CeA or destruction of major output pathways from the CeA to the brainstem results in general suppression of multiple CRs (Hitchcock & Davis, 1991; Helmstetter, 1992). Autonomic CRs appear to require CeA projections to the lateral hypothalamus (LH) but not projections to the periaqueductal gray or rostral medulla (e.g., LeDoux, et al 1988; Helmstetter & Tershner, 1994). Freezing behavior, on the other hand, is disrupted by manipulation of the PAG (e.g., Amorapanth et al., 1999; Kim, Rison & Fanselow, 1993) while changes in pain sensitivity in response to a CS for shock rely on a polysynaptic pathway from the CeA to subpopulations of cells in PAG and medulla (e.g. Bellgowan & Helmstetter, 1998; Helmstetter, et al. 1998).
BLA neurons receive multiple sensory inputs from cortical and subcortical sites. LeDoux’s early work was the first to show that direct input to the LA from the medial portion of the medial geniculate nucleus (MGm) was sufficient to support auditory FC. Subsequent studies have confirmed the importance of MGm and its projections to the amygdala. Conditioning to a contextual CS, on the other hand, appears to rely on a specific role of the hippocampus in processing contextual stimuli (Anagnostaras, et al. 2001; Rudy, & O’Reilly, 1999, 2001). Certain manipulations targeting the hippocampus show a time-limited and selective effect on learning about context without disrupting acquisition of tone-evoked CRs. While a role for the hippocampus in context processing seems clear at this point, the specific contribution of this structure to FC acquisition and the extent to which auditory CRs are really independent of the structure still needs to be worked out completely. Recent work by Rudy and others strongly supports the idea that hippocampal contributions to context FC reflect a more general role of this structure in forming mnemonic representations of complex multi-modal stimuli. In auditory FC when tones and shocks are presented in a distinctive context, potentially important learning-related changes in physiology simultaneously take place in hippocampal, neocortical, and thalamic inputs to the amygdala (see below).
Gene expression and fear conditioning
As the technology to quantify and anatomically localize alterations in gene expression in brain tissue became available it was applied to the study of memory formation generally, and to the neurobiology of FC specifically. Early work in this area focused largely on the expression of immediate early genes (IEGs) as potential markers for cellular activity and plasticity. Campeau and colleagues (1991) were the first to show that exposure to the training procedures used in FC was sufficient to produce an elevation in mRNA in the amygdala, in this case for c-fos. This basic training effect was later confirmed at the protein level and quantitative assays of FOS protein have been shown to correlate with behavioral performance (e.g., Radulovic, Kammermeier & Spiess, 1998). Importantly, synthesis of new protein can also be driven by the recall/retrieval/behavioral expression of FC as well as during the consolidation of new learning (Hall, Thomas & Everitt, 2001). Early studies also identified a specific role for zif268 / egr-1 in the lateral amygdala in the formation of new memory (Rosen et al., 1998; Malkani & Rosen, 2000). More recent work using DNA microarrays or related approaches with multiple probes assessed in parallel has shown, not surprisingly, that a rather large number of genes are likely to alter their expression following fear conditioning in the amygdala and hippocampus alone (e.g., Levenson, et al., 2004; Ressler, et al., 2002; Stork et al., 2001). Within this group one can find multiple functional classes including a variety of glutamate receptor subunits, IEGs, transcription factors and growth regulators as well as structural proteins.
The presence of new transcripts or proteins in a particular brain region (or at particular location within neurons) during the period after training, even if these molecules are only present in the cells of animals that learn compared to appropriate behavioral controls, obviously does not indicate the degree to which this change in gene expression is actually necessary for new memory to be formed. Similarly, evidence for training-related activity in various protein kinases and transcription factors thought to be contributing to synaptic modification does not mean that they are actually needed or specifically involved in memory formation per se. Direct manipulation of these gene products restricted to specific aspects of the training experience or time points during memory consolidation is necessary to draw firm conclusions in this regard. Early studies in rodents relied on very non-selective manipulations in which protein synthesis was globally suppressed and this suppression was not restricted to brain tissue causing a series of interpretive difficulties. These approaches did not permit detailed assessment of the role of a particular target protein or process or of the contribution of specific brain structures. Conversely, more recent technologies such as antisense oligodeoxynucletide treatment, RNA interference, transgenic animals, and directed expression of target genes allow for very selective manipulation of specific gene products, in some cases with good temporal and anatomical control. A large amount of work is currently underway in which specific gene products thought to be important for fear memory formation are targeted using these approaches. It appears that within a relatively simple behavioral paradigm such as FC there are multiple new proteins produced in multiple brain areas as a function of initial encoding as well as retrieval processes, and that the temporal profile for expression of each may also vary as a function of target protein, brain site, stimulus modality and specifics of the procedure being used (e.g., Hall, Thomas & Everitt, 2001; Gafford et al 2007; Parsons, et al., 2006a–d; Malkani & Rosen, 2000; Ressler et al., 2002, etc) In part because of this complexity, our approach in recent years has been to target rather broad classes of cellular process (e.g., transcription versus translation) and investigate their relationship to multiple brain areas and multiple phases of memory formation and storage.
Inhibition of mRNA and protein synthesis in the amygdala
Site specific local injections of protein synthesis inhibitors in the brain have a number of advantages over systemic administration but, of course, important limitations still exist. Early work on the direct application of protein synthesis inhibitors (e.g., cycloheximide) to the amygdala in rats showed that post-training injections could result in significant deficits in retention in a passive avoidance procedure (Berman, Kessner & Partlow, 1978). More recently, Bailey et al (1999) were the first to show that suppressing the synthesis of new mRNA in the lateral amygdala with the inhibitor actinomycin-D (ACT-D) around the time of training in FC resulted in dramatic deficits in long-term retention of fear evoked by both an auditory signal for shock and by the context in which training took place. Furthermore, this study included behavioral assessments indicating that amygdala injections of ACT-D had no significant effect on performance during the training session suggesting that motor impairments, short-term memory deficits, or a more general functional disruption of the amygdala was not responsible for the effect on long-term retention. Retraining animals one week after ACT-D injections resulted in normal acquisition and retention of fear memory indicating that cytotoxicity in the amygdala was not likely to be an issue. Finally, the Bailey study also included quantification of the degree to which local mRNA synthesis was suppressed by ACT-D injections which in turn appeared to be related to the degree of memory impairment.
Subsequent work has shown that preventing the translation of new protein from mRNA in the amygdala either during or immediately after the training session has the predicted effect in preventing memory formation (Schafe 2000; Parsons et al 2006a) and that these effects are likely due at least in part to the disruption of a series of specific signaling pathways in amygdala neurons including, but not limited to, protein kinase A, protein kinase C, ras/Erk/MAP kinase (Atkins, et al., 1998; Duvarci, Nader & LeDoux, 2005; Schafe, 2000b; Selcher, et al., 2002), CREB (Josselyn, et al., 2001; Han, et al., 2007; Ou & Gean, 2007), and mTOR (Parsons, et al 2006c). Taken together, the existing evidence indicates that exposure to CS-UCS pairings during FC triggers multiple intracellular signaling cascades within amygdala neurons that ultimately result in the availability of new proteins that contribute to the long term functional modification of synaptic efficacy (Ou & Gean, 2007; Rodrigues et al., 2004; Blair, et al., 2001)
Distributed plasticity
After the initial demonstration that suppressing mRNA synthesis in the BLA prevented the formation of fear memory, we were particularly interested to see if other sites within the basic circuit required for normal FC would also be sensitive to this manipulation and therefore suggest that the molecular changes supporting long term memory in FC were occurring at multiple sites rather than at a single location with a subpopulation of critical synapses. Immunohistochemical work mapping protein expression following FC (e.g., Beck & Fibiger, 1995; Milanovic, et al., 1998) as well as multi-cellular recording studies (e.g., Gabriel et al., 1991) supported the idea that learning was accompanied by molecular and physiological changes in many brain areas in addition to the amygdala. Metabolic mapping in rodents (Poremba et al., 1998; McIntosh & Gonzalez-Lima, 1993) and functional imaging in human subjects (Knight et al.,1999; 2004) also support the importance of network level interactions in the acquisition and performance of FC. However, since each of these techniques essentially reflect correlations between cellular activity and performance they do not address whether a particular change is actually required for the learning process or memory storage.
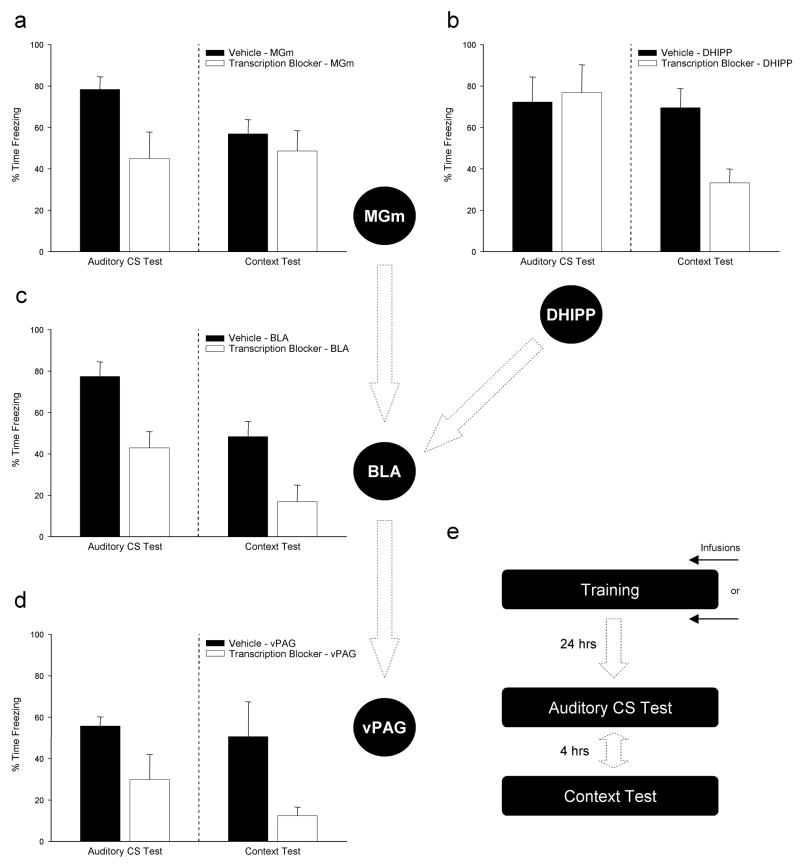
Figure 1 summarizes the results of a few experiments conducted at different times over the last several years, each using essentially the same methodology. In each case, adult male Long-Evans rats were prepared with chronic cannulae for local intracranial injections into the BLA and then trained and tested in an auditory FC procedure in which animals receive a series of four auditory stimuli paired with foot shock on Day 1 and then are returned to the lab on the following day and tested for reactions to the auditory CS and to the chamber (context) in which training occurs (see Bailey et al., 1999; Parsons, et al., 2006a, 2006b for details on the methods). Injections of the transcription inhibitors ACT-D (5 ng/μl) or DRB (20–200 ng/μl) were made either immediately before or immediately after the 15 min training session on Day 1. In no case did the injections produce any observable effect on gross motor activity, performance of the freezing response, short-term behavioral reactions to the shock, or gross morphology of Nissl stained tissue sections collected throughout the injection sites. Each of the basic findings summarized in Figure 1 comes from work originally done by David Bailey (Bailey, Sun, Kim & Helmstetter, 1997; Bailey & Helmstetter, 1998) which was subsequently replicated and extended by several other people working in the lab.
Figure 1.
Under typical training conditions fear conditioning requires a sequential circuit of brain areas involved first in processing the CS and ultimately in behavioral expression of the CR. Preventing the synthesis of new mRNA in any one of a number of brain areas either before, or immediately after, training will affect the long term retention of this memory. Performance in response to exposure to the auditory CS is selectively disrupted by mRNA inhibition in the auditory thalamus (a) while memory for a shock-associated context relies selectively on transcription in hippocampal neurons (b). In contrast, behavioral performance to both auditory and contextual stimuli are similarly affected by disrupting training-induced gene expression in the basolateral amygala (c) or periacquctal gray region of the midbrain (d). All experiments used similar manipulations (either ACT-D or DRB microinjections) and nearly identical training and testing procedures (e).
The data in Figure 1c are taken from Parsons, et al. (2006a) and essentially replicate the Bailey et al. (1999) study using DRB rather than ACT-D and immediate post-training, rather than pre-training, injections. Note that behavioral performance in response to both the auditory CS and training context are attenuated during retention testing at 24 hr indicating that 1) injections disrupted consolidation of fear memory during the post-training period and 2) new mRNA at training is required in the BLA for both discrete CS and context associations with the UCS. The results of this experiment are consistent with a rather large amount of converging evidence supporting the importance of BLA synapses in the formation of fear memory. Indeed, until very recently many people working in this area chose to focus on the BLA as “the site” for memory storage in fear conditioning (e.g., Fanselow & LeDoux, 1999; Maren, 2001).
A series of interesting and slightly more novel comparisons may be made between the results depicted in Figure 1a versus 1b. Prior influential work by LeDoux and colleagues showed that the BLA receives important sensory input through synaptic connections with cells projecting from the auditory thalamus (Clugnet, LeDoux, & Morrison, 1990; Clugnet & LeDoux, 1990). Unit responses recorded in auditory cortex and MGm during aversive learning support the idea that conditioning can have profound long term effects on stimulus evoked primary sensory processing (Bakin & Weinberger, 1990; Lennartz & Weinberger, 1992; Edeline & Weinberger, 1992). Somewhat comparable effects are seen in metabolic imaging studies (e.g., Poremba, Jones & Gonzalez-Lima, 1998). If synapses in the auditory thalamus were modified by experience in a manner similar to those in the BLA, then preventing the synthesis of new mRNA or protein in MGm might have an impact on the acquisition of auditory FC. We recently decided to test this idea directly and completed a series of studies examining the requirements for mRNA and protein synthesis in MGm during fear learning (see Parsons, et al. 2006b). Figure 1a summarizes the essential outcomes in that inhibition of mRNA and protein synthesis in MGm during training significantly attenuates the conditional fear response provoked by exposure to the auditory CS during retention testing. Critically, the animals’ memory for the context in which training took place was unaffected supporting a specific and selective role for macromolecular synthesis in MGm neurons in the formation long-term memory for tone-shock associations. It should be noted, however, that other recent studies on the role of MGm protein synthesis in fear conditioning have come to different conclusions about the nature and importance of plasticity in this structure (Maren et al., 2003; Apergis-Schoute et al., 2005).
A complementary set of outcomes can be seen in Figure 1b which shows a similar experiment in which the same drug was injected into the dorsal hippocampus immediately after the training session (Gafford et al., 2004). When the synthesis of new mRNA in the hippocampus is suppressed during the consolidation period after training, animals show normal acquisition of fear memory to the auditory CS. However, the same animals are significantly impaired with respect to their performance when returned to the context in which training was conducted. These data are consistent with the well established selective role of the hippocampus in context fear learning (e.g., Kim & Fanselow, 1992) and support more recent observations regarding the importance of hippocampal gene expression in rats’ ability to represent and recall distinctive environments (Barrientos, O’Reilly & Rudy, 2002; Guzowski, 2002; Huff, et al., 2006).
Taken together, our results with inhibitors applied to the MGm and dorsal hippocampus represent a “double dissociation” with respect to requirements for de novo protein synthesis in forming memory for two independent aspects of the same training experience based on the modality/physical characteristics of the particular CS. Learning the auditory CS–shock association appears to require synthesis-dependent modification of MGm synapses that do not contribute directly to context learning. Conversely, learning about the context in which fear conditioning takes place requires new protein in the dorsal hippocampus but not in the MGm. Each structure projects, directly or indirectly, to the BLA and the same manipulation applied to the BLA is equally (and simultaneously) effective against performance to each class of stimuli. While there are various theoretical perspectives that place different levels of emphasis on the importance of synaptic modifications in each of these three brain areas during fear learning, our results clearly indicate that macromolecular synthesis is required for long-term modification of synapses in each of these brain areas, and that accordingly each area may make an important contribution to long-term memory storage in FC.
One perspective that can be used to understand the pattern of results in Figure 1a–c is that learning and experience often modify the way stimuli are processed by sensory systems (Weinberger, 2007; Knight et al., 1999, 2004). During conditioning, localized long-term alterations may occur in cells that normally participate in processing the environmental signals for an aversive UCS and help to enhance processing of that CS on subsequent exposures. Many of the details regarding the relative importance of protein synthesis-dependent plasticity in the amygdala versus in brain structures that process inputs to the amygdala and project to it have yet to be worked out. It seems clear, however, that changes in gene expression outside of BLA neurons are likely to make important contributions to memory formation in this preparation and that understanding the details regarding the modification of amygdala synapses by learning will not be sufficient for a complete understanding of memory storage in FC.
Another perspective that could help to integrate the results shown in Figure 1 with the larger literature on systems-level changes in gene expression and metabolic processing as a consequence of conditioning centers on the idea that synaptic plasticity is a potentially ubiquitous process and that critical cellular alterations requiring new protein are not likely to be restricted to a single brain area such as the amygdala whose responsibility is to store “the fear conditioning engram”. Rather, one can view the essential neural circuit for FC as a collection of interconnected brain areas with a functional relevance that may be quite independent of learning or plasticity per se (Bellgowan & Helmstetter, 1996; Helmstetter, et al., 1998) but that undergoes modification at the circuit level as a normal consequence of experience. Using this logic one might expect cells and synapses throughout the circuit engaged by a particular behavioral function (e.g. responding to something fear-provoking) to modify their properties in a manner that requires new gene products induced by that experience. This account handles the observation that preventing protein synthesis in either the BLA, MGm, or dorsal hippocampus is effective in disrupting learning in a manner dependent on what (i.e. auditory CS versus context) is being learned about, but also leads to the prediction that other synapses at other points in the circuit might also show a requirement for new protein around the time animals are learning.
Working along these lines we asked if similar effects of mRNA and protein inhibition would be seen at sites thought to be receiving input from the amygdala cells already known to be critical in the formation of FC memories. Traditionally, output from the BLA has been thought to influence behavioral and physiological reactions related to FC through intrinsic connections to the central nucleus (CeA) which in turn projects in parallel to multiple brain areas. Efferent targets of the CeA include diencephalic and midbrain nuclei that are directly involved in the expression of autonomic and somatomotor components of a rat’s normal reaction to threat (Davis, 2000; McNaughton & Corr, 2004). The ventrolateral periaqueductal gray (vPAG), for example, receives substantial input from CeA. Lesions, reversible inactivation, or certain pharmacological manipulations applied here clearly compromise an animal’s ability to express freezing behavior or changes in pain sensitivity in response to a CS for foot shock (Bellgowan & Helmstetter, 1998; Helmstetter & Landiera-Fernandez, 1990; Kim et al., 1994). Importantly, lesions here do not result in a global performance deficit or general failure to learn or recall the training experience since other components of the CR, such as short latency autonomic reactions to the CS, are often spared (e.g. Helmstetter & Tershner, 1994). While there is considerable functional heterogeneity within the PAG and closely related regions of the midbrain, most current models view the vPAG primarily as an “output structure” or final common pathway for CR expression (but see McNally & Westbrook, 2006).
Figure 1d shows the results of treating animals with ACT-D prior to the training session through unilateral cannulae in the vPAG (5 ng/μl). Rats showed normal behavior during the training session (e.g. post-shock freezing) and during retraining/testing one week after injections. However, at the drug free retrieval test conducted 24 hr after CS-UCS pairings ACT-D animals showed a significant deficit in freezing in response to both the auditory cue and the context in which training was conducted. Note that the pattern of outcomes here closely resembles that seen for the BLA (Figure 1c). While a few details regarding this effect remain to be worked out, this outcome was rather surprising and suggests that training in FC not only results in widespread changes in the expression of multiple gene products but that these gene products expressed outside areas traditionally identified as “memory structures” may make critical contributions to the normal memory consolidation process. It is of particular interest to relate the effects seen in Figure 1d with those of a recent study showing that protein synthesis in CeA, also though to be an “output” structure, is important for normal FC acquisition (Wilensky et al., 2006). Taken together, this overall pattern of results supports the idea that critical plastic changes in neurons requiring the synthesis of new protein may be the “rule” rather than the “exception” during fear memory consolidation and that memory formation in FC may rely more on network level organization than on storage of information in “memory systems”.
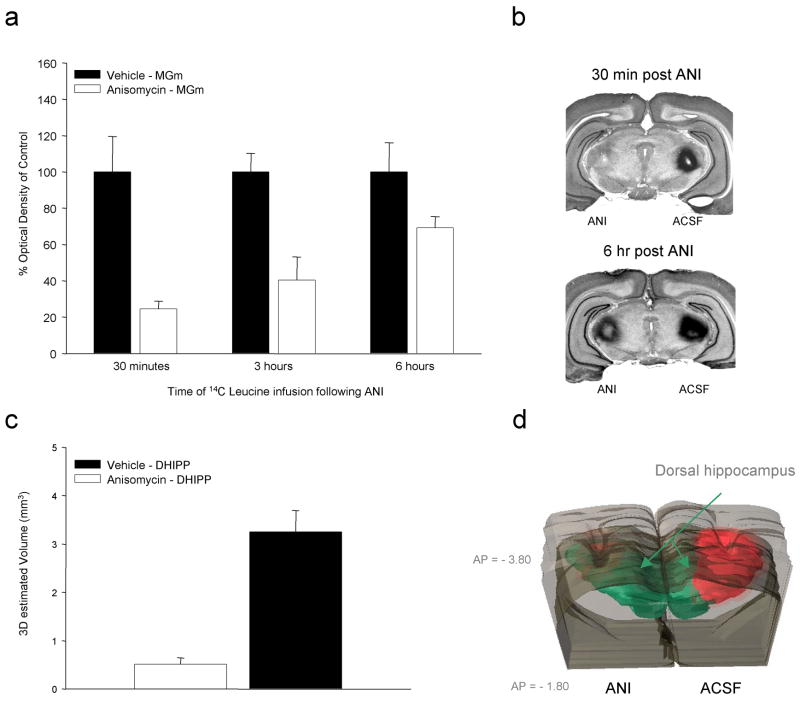
The data presented in Figure 1 represent a highly condensed summary of several series of experiments. In each case, the basic result seen here with mRNA synthesis inhibitors (ACT-D or DRB) has been replicated with anisomycin (ANI) supporting the need for both transcripts and protein during the period immediately following behavioral training and making it somewhat unlikely that these effects are due to some unique or non-specific effect of the particular compounds being used. For each injection target we were also able to show that the inhibitors do not produce notable acute effects on behavior following injections or any long term cytotoxicity or behavioral impairment at the doses tested. Finally, in many of these studies we have also tried to carefully quantify the effectiveness and document the anatomical specificity and time course of the effects of these manipulations. Figure 2 provides some examples from this work. Our basic approach has been to combine local injections of inhibitors at the dose and volume used for behavioral studies with radiolabeled leucine and uridine. Autoradiographic images are quantified with optical densitometry and overlaid on corresponding Nissl stained brain slices. The top two panels on Figure 2 show the effects of ANI injections in the medial geniculate on [14C]-Leucine incorporation. Rats with chronic cannulae received ANI (125 μg/μl) dissolved in artificial cerebrospinal fluid (ACSF) pressure injected into the MGm on one side and vehicle injection on the other. A larger volume (1.0μl/side) bilateral injection of [14C]-leucine followed at various time points after application of ANI. Rats were killed 60 min after the second injection and processed for autoradiography and 3-dimensional reconstruction of injection sites (see Parsons, et al., 2006a, 2006b & 2006c for details on method).
Figure 2.
Because of various issues related to the use of local microinjection of general synthesis inhibitors in behaving animals it is important to carefully quantify both the magnitude and duration of their effects on new message and protein. In (a) rats with MGm cannulae received 14C-Leu injections at various times after ANI applied to the same site. Leucine incorporation was most dramatically affected during the period immediately after ANI but was detectable out to approximately 6 hours after injection. Example Nissl stained tissue with autoradiographic overlays are shown in (b). The lower panels show the effects of ANI on leucine incorporation in the dorsal hippocampus after local injections. Serial reconstruction of autoradiographic data indicates that, at concentrations and volumes typically used in the literature, ANI produces a dramatic decrease in local protein synthesis. Injection of radiolabel into the control hemisphere also provides a useful indication of the relationship between injection volume and affected tissue.
Figure 2a shows that at 30min after ANI injection the side treated with inhibitor showed approximately 80% reduction in the incorporation of [14C]-leucine compared to ACSF injection in the same animals. Gradually decreasing but significant levels of protein synthesis inhibition were seen in this brain area out to 6 hours after ANI injection. These results indicate that using concentration and volume parameters typical for studies of this type, a single injection of ANI would be expected to impact both training-evoked and constitutive protein synthesis well into the post-training period. Figure 2b shows examples of the tissue sections averaged for the bar graph. Note that the ACSF injection (top, right side) corresponds to a dark region on the autoradiographic overlay signifying higher local concentration of 14C-leucine restricted largely to the lateral thalamus. At 30 min after ANI injection (top, left side) little evidence for new protein can be detected. By 6 hr after ANI, the site of the leucine injection is quite obvious but the intensity is still notably less than for the control injection (right side). Figure 2c and 2d summarize a similar analysis done in our lab by David Baruch and Brady Riedner applied to injections of ANI in the dorsal hippocampus. Again, bilateral injections were used with the side opposite ANI injection serving as a within-subject control. In this case, a series of two dimensional sections were collected at fixed anterior-posterior intervals throughout the region of the injection site and the total volume of tissue affected by the injection was estimated by serial reconstruction with threshold densitometry. Figure 2c shows that while a 1.0 μl injection of 14C-leucine applied to the dorsal hippocampus produced a localized region of incorporation of approximately 3 mm3 and largely restricted to hippocampus and immediately overlying cortex, ANI injections drastically reduced the effective region of incorporation essentially shutting down protein synthesis on the side of the injection. Our work on these issues indicates that at the doses at which transcription and translation inhibitors are typically used for local injections in behaving animals these treatments tend to result in a dramatic and long-lasting suppression of macromolecular synthesis which is largely restricted to the target structure.
Long term stability and “reconsolidation”
Soon after the requirement for new mRNA and protein in the amygdala during the consolidation of FC was established, Nader, Schafe & LeDoux (2000) reported a fascinating observation which has since received a great deal of experimental attention. They showed that long-term memory for auditory FC can be disrupted by infusion of ANI into the BLA immediately after a short CS presentation. The authors concluded that FC memory goes through a “reconsolidation” phase following retrieval and that the BLA needs to be able to synthesize new protein for the memory to remain intact. These data argue that consolidated memories are not necessarily consolidated in a formal sense, but instead suggests that the memory remains in a labile state, presumably capable of being changed and clearly capable of being disrupted. Although questions still remain regarding the nature of these post-retrieval performance disruptions (e.g. Lattal & Abel, 2004), this finding and dozens of similar results cast some doubt on the long-held notion that once a memory becomes consolidated it exists in a relatively fixed state (Nader, 2003).
Our initial efforts to replicate and extend the observations of Nader et al. (2000) involved testing the effects of transcription blockade in the BLA following retrieval operating with the assumption that inhibiting protein synthesis at the level of transcription would be functionally equivalent to the presumed effects of ANI on protein translation. In several early experiments we found that ACT-D or other similar inhibitors given over a large range of doses immediately after retrieval had no effect at all on subsequent tests of memory retention. Since this appeared inconsistent with Nader’s work we went on to directly compare the effects of ACT-D and ANI on both initial consolidation and reconsolidation of fear memory (Parsons et al., 2006a). Animals were trained with 3 unsignaled shocks and given intra-BLA infusions of ANI (125 μg/μl) or ACT-D (5 ng/μl) immediately after training. Rats treated with either ANI or ACT-D exhibited memory impairments when they were given a context test the next day, consistent with previous published results (Bailey et al., 1999; Schafe and LeDoux, 2000; Maren et al., 2003). This session allowed us to assess the effects of the post-training manipulations but it also functioned as a retrieval trial. Another round of infusions immediately followed and the animals were given an identical retention test 24 hours later. This final test showed that although ANI was effective in disrupting the stability of memory after retrieval, ACT-D had no effect. Subsequent work with another transcription blocker and higher doses did not yield positive findings, despite the fact that the same compounds consistently blocked initial memory formation and that intra-BLA infusion of ANI following retrieval consistently yielded positive results. A few other studies have directly tested the effects of transcription blockade on reconsolidation and observed positive effects (Sangha et al., 2003; Lee et al., 2004). While it is unclear exactly what is responsible for the discrepant findings, it is likely that some differences in procedure and dose played a major role (see Parsons et al., 2006a for a more complete discussion).
The pattern of findings from our lab suggests that while consolidation of FC depends on both gene expression and protein synthesis in the amygdala, reconsolidation is supported by proteins being translated from existing stores of mRNA in dendrites. A recent study (Mileusnic, Lancashire, & Rose, 2005) lends additional indirect support to the idea that reconsolidation depends primarily on dendritically synthesized protein. They showed that disabling intracellular transport in chick brains had no effect on the reconsolidation of passive avoidance memory, although the same treatment clearly disrupted initial learning. Much more work is needed to evaluate this rather interesting idea. Especially important will be studies that use techniques which selectively disrupt local protein synthesis or selective message transport/targeting in behaving animals.
Of course, it is also possible that ANI is effective in post-retrieval memory disruption (where ACT-D is not) because of effects that are independent of its role as a translation inhibitor. It is fairly well known that ANI suffers from the liability of multiple effects on neurons including a tendency to “superinduce” certain IEGs like c-fos and c-jun and activation of MAPK and other kinases (e.g., Edwards & Mahadevan, 1992; Hazzalin et al., 1998; Zink et al., 1995) as well as direct and indirect short-term effects on several important neurotransmitters (e.g. Canal, Chang, & Gold, 2007). While the observation that broad-spectrum translation inhibitors have serious shortcomings is certainly not new (e.g., Davis & Squire, 1984; Flexner & Goodman, 1975) it should be a source of some concern that over the last few years local injection of ANI has become the “standard manipulation” for blocking translation in the memory literature. Indeed, some authors have gone so far as to argue that because drugs like ANI and cycloheximide do more than just inhibit translation, experiments that rely on them do not provide adequate support for the more general requirement for de novo protein synthesis in memory formation (Canal, Chang & Gold, 2007; Routtenberg & Rekart, 2005). Unfortunately, simply dismissing the use of protein synthesis inhibitors as a method to evaluate the potential requirements for new proteins in memory formation puts one in a rather difficult position experimentally by rendering the hypothesis essentially untestable. Of course, one can still point to correlational data on gene expression, to interventional studies that target single gene products rather that all proteins being translated, or even to other classes of broad spectrum inhibitors with far fewer side effects (see above), but the best answers may come from developing “cleaner” approaches that selectively affect the translation of multiple gene products simultaneously.
Translational control through mTOR
Based on the idea that “reconsolidation” of fear memory may be independent of transcription and rely on translation of new protein from mRNA localized in dendrites (Parsons et al., 2006a) and based on the need for a better understanding of translational control that is independent of the problems seen with traditional translation inhibitors like ANI, we recently began to focus on the signaling pathway controlled by the mammalian target of rapamycin (mTOR) kinase. mTOR can regulate translation by controlling the phosphorylation of eIF4E-binding protein (4E-BP1) and p70s6 kinase (p70s6k) (see Raught et al., 2001) and helps to control the synthesis of locally translated proteins at active synapses (Cammalleri, et al., 2003; Gong et al., 2006; Tang, et al., 2002).
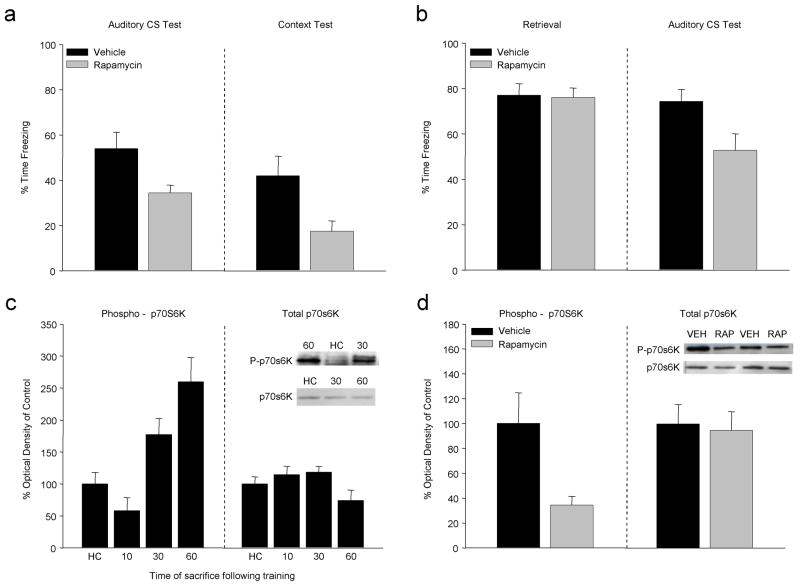
Figure 3 summarizes a portion of the results from a recent paper (Parsons, et al., 2006c) showing that rapamycin (RAP), which prevents phosphorylation of the mTOR kinase, significantly affects the formation and stability of FC memory when applied to the lateral amygdala. In Figure 3a groups of rats with amygdala cannulae were trained in our standard fear conditioning procedure (i.e. Figure 1e) after which they received a single immediate post-training injection of RAP (5μg/μl, .5μl per side). When tested for retention 24 hr later, RAP injected rats showed significantly less freezing in response to both the auditory CS and the training context compared to the vehicle injected group. While the magnitude of the behavioral disruption seen with RAP was similar to prior work with ANI or polymerase II inhibitors, quantification of 14C-leucine incorporation indicated that RAP reduced local protein synthesis by about 10% (Parsons, et al 2006c). Given that comparable injections of ANI can suppress synthesis in the BLA between 60% and 80% of controls, the significant behavioral outcomes with RAP may indicate that the subset of transcripts affected by this pathway are disproportionately critical for learning. Figure 3b shows that RAP, like ANI, can affect performance to an auditory CS when it is applied to the amygdala directly after “reactivation” of the memory by CS exposure on a single retrieval trial. When rats are trained with a series of CS-shock pairings and then killed at various time points afterward, there is a time dependent increase in the relative rate of phosphorylation of p70s6K isolated from amygdala tissue, but no change in total p70s6K protein levels (Fig 3c). The increased phospho-p70s6K expression seen 60 minutes following FC can be disrupted by a memory impairing dose of RAP applied to the amygdala immediately after training (Fig 3d).
Figure 3.
Manipulating translation related specifically to mTOR signaling overcomes some of the limitations of inhibitors such as ANI while still affecting large numbers of gene products thought to be important for synaptic plasticity. (a) Rapamycin, a specific inhibitor of mTOR, applied to the BLA immediately after training affects fear responses to both auditory and contextual CSs. (b) As in prior work with ANI, application of RAP to the amygdala immediately after retrieval results in long-term memory deficits on subsequent tests. These RAP effects are consistent with the temporal pattern of p70s6K phosphorylation observed during the period after training (c). RAP injections selectively inhibit p70s6k phosphorylation in a manner similar to its effects on learning (d). (Data redrawn from Parsons et al 2006c)
We investigated whether the mTOR pathway was activated in the hippocampus in response to encoding and recalling contextual FC (Gafford, Parsons, & Helmstetter, 2007). Rats were trained with a single foot shock applied either immediately, or with a 2 min delay, after being placed in an observation chamber (see Parsons, et al., 2006c for method). Immediate shock results in little or no learning while animals that received delayed shock learn normally. Sixty minutes after training all animals were deeply anesthetized and their brains were removed. An additional control group was sacrificed from the home cage. Western blot analysis conducted on tissue from the dorsal hippocampus (DH) showed a selective increase in phosphorylated p70s6K for animals that formed a fear memory compared to controls. No differences were seen in overall p70s6K protein levels in these same animals. To determine where within the DH the increase in phosphorylated p70s6K was localized, a subset of animals was processed for slice immunohistochemistry with the same phoso-p70s6K antibody. These images revealed an increase in immunolabeling in dendrites in the CA1 and granule cells in the dentate gyrus for animals in the delay shock condition. Figure 4 shows representative confocal images of phospho-p70s6K stained cells in the CA1 of animals that received immediate (Fig 4a) or delayed (Fig 4b) shock. Furthermore, representative immunofluorescence images also show clear differences in phospho-p70s6K staining in the dentate gyrus of animals in the immediate (Fig 4c) versus delay (Fig 4d) conditions. The cells labeled with phospho p70s6K were neurons as demonstrated by colocalization with an antibody for neuron-specific DNA binding protein NeuN (Fig 4e and 4f).
Figure 4.
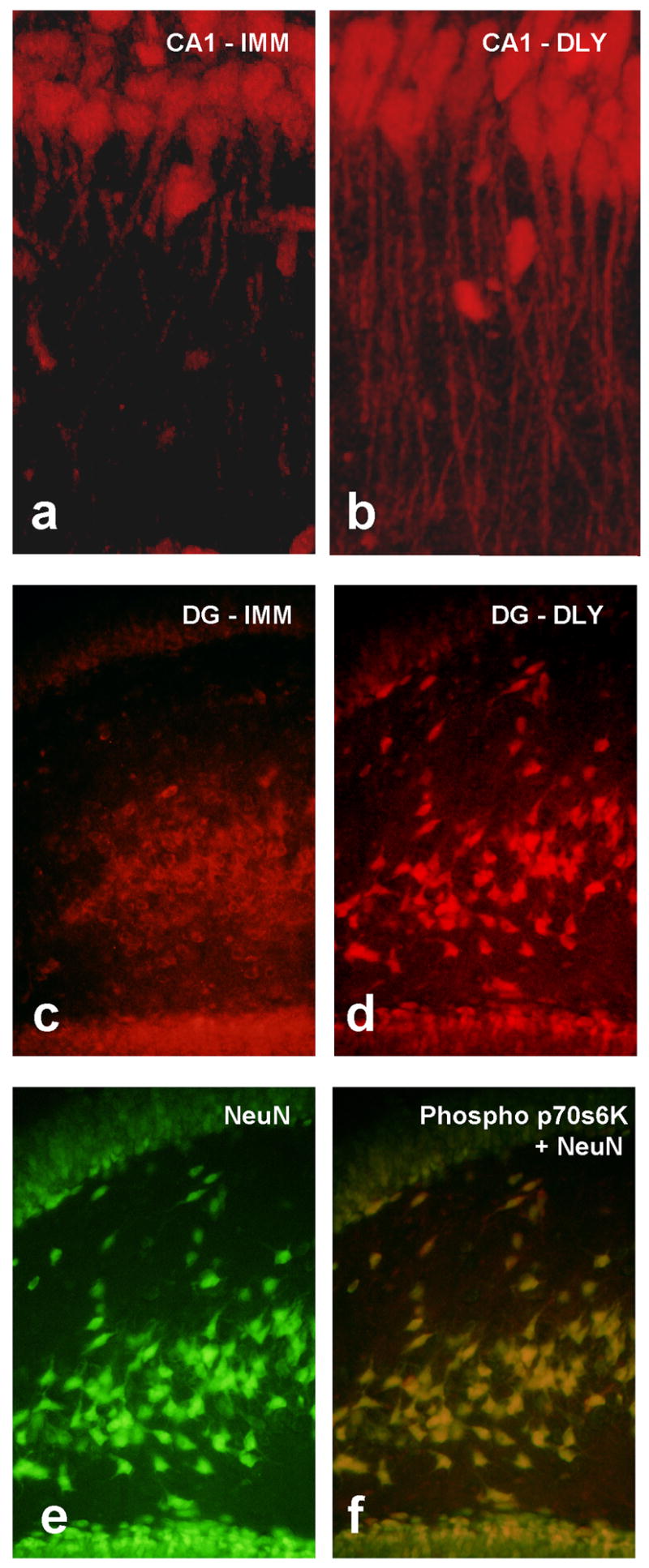
Immunofluorescence images of phosphorylated p70s6k in hippocampal tissue from rats given immediate (IMM) or delayed (DLY) shock after placement in a novel chamber. Confocal images show distinct patterns of staining in apical dendrites of CA1 pyramidal neurons in animals that learned (b) compared to those that did not (a) supporting the potential importance of mTOR-dependent translation in dendrites. Dentate gyrus showed a similar pattern in that more intense and more punctate staining was found in the DLY group (d). Double labeling with an antibody to the neuron-specific DNA binding protein NeuN (e) indicated that the p70s6k signal is seen primary in neurons. Quantitative protein assays on hippocampal tissue from these groups confirm these observations (see Gafford et al., 2007).
Since phosphorylation of p70s6K in the DH was selectively increased after learning we next tested whether the same was true after memory retrieval. In the second experiment one group of animals (A+/A) was trained with three shocks in the observation chamber and then re-exposed to this context 24 hours later. Three control groups were included in this experiment to verify that any difference was due to retrieval of the context fear memory rather than to some other component of the procedure. These included: 1) a group exposed to the chamber without shock and re-exposed to that same context on day 2 (A/A), 2) a group that was trained with shocks but exposed to a different, novel environment on day 2 (A+/B), and 3) an unstimulated home cage control group (HC). Brains were removed from animals 60 minutes after retrieval or at an equivalent time point for controls. Behavioral data confirmed that only rats exposed to the shock-associated apparatus showed fear memory. Western blots showed a significant increase in phosphorylated p70s6K after retrieval only for the group that was trained with shock and re-exposed to that same context the following day. No differences were found in expression of total p70s6K in the same samples.
These data demonstrate that mTOR-dependent translation is activated in DH neurons after recall of a contextual fear memory. Our final experiment was conducted to address whether mTOR-dependent gene products were important for maintaining the stability of memory after retrieval in the hippocampus (as is the case in the amygdala c.f. Parsons et al 2006c). RAP infused into the DH prior to retrieval of the memory, when p70s6K is normally active, disrupted reconsolidation as assessed by behavioral performance on the following day supporting the idea that mTOR dependent signaling in the DH is normally required during reconsolidation of contextual fear memory.
Taken together, the present findings show that mTOR dependent translation in the DH is involved in both consolidation and reconsolidation of contextual fear memory. Phosphorylated p70s6K was selectively increased in DH neurons during the period after learning when protein synthesis is normally required (Kandel, 2001). This observation fits well with prior work showing that activated p70s6K is located in dendrites after LTP in vitro (Cammalleri et al., 2003) and that mice with compromised mTOR signaling show learning deficits on tasks requiring the hippocampus (Banko et al., 2005).
It is particularly interesting that very similar translational events are initiated in response to memory retrieval at 24 hrs after training. Phosphorylation of p70s6K was greatest when measured after recall and was selective for the memory of the apparatus associated with shock. Since disruption of the mTOR pathway prior to retrieval resulted in disruption of memory for the environment in which shock occurred, it appears that both the formation and the long-term stability of cellular changes underlying this memory rely on mTOR.
Summary
Work in our lab conducted over the last few years clearly supports the general idea that the formation of new long-term memory in aversive Pavlovian conditioning depends upon activity-related changes in gene expression and the synthesis of new protein. Presumably, these new gene products contribute directly to the functional modification of synapses as is more directly supported by the work of other groups. In addition to the important role of the lateral amygdala in forming associations in FC, we find that very similar requirements for macromolecular synthesis in the period after learning are seen in multiple brain structures leading to the conclusion that synaptic plasticity and protein synthesis occurs throughout a network of brain areas important for responses to environmental threat. Although initial memory consolidation depends on gene expression and protein synthesis in the amygdala, our data suggests that reconsolidation is supported by proteins translated from existing stores of mRNA presumably in dendritic compartments. The mTOR pathway, which has been implicated in the regulation of dendritic protein synthesis, is critical for consolidation and reconsolidation in the amygdala and dorsal hippocampus.
Acknowledgments
This work was supported by NIMH grants MH060668, MH069558 (FJH) and MH071126 (RJP).
We would like to that David Bailey, Brady Riedner and David Baruch for important contributions to various aspects of the work summarized here. We would also like to that James R. Moyer, Jr. for his help with some of the slice histochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: A role for presynaptic plasticity in the fear system. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn Mem. 1999;6:491–499. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Pertratis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Sun W, Kim JJ, Helmstetter FJ. Inhibition of RNA synthesis in the amygdala and hippocampus selectively blocks acquisition of Pavlovian fear conditioning. Society for Neuroscience Abstracts.1997. [Google Scholar]
- Bailey DJ, Helmstetter FJ. Protein synthesis in the periaqueductal gray may be critical for the acquisition of fear conditioning. Society for Neuroscience Abstracts.1998. [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behavioral Neuroscience. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Beck C, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate-early gene c-fos with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgowan PSF, Helmstetter FJ. The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Res. 1998;791:83–89. doi: 10.1016/s0006-8993(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Bellgowan PSF, Helmstetter FJ. Neural systems for the expression of hypoalgesia during non-associative fear. Behavioral Neuroscience. 1996;110:727–736. doi: 10.1037//0735-7044.110.4.727. [DOI] [PubMed] [Google Scholar]
- Bellgowan PSF, Helmstetter FJ. The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Research. 1998;791:83–89. doi: 10.1016/s0006-8993(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear conditioning. Learning & Memory. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, Kind A, Simpson C, Francesoni W, Sana PP. Time-restricted role for dendridic activation of the mTOR-p70s6k pathway in the induction of late-phase long-term potentiation in the CA1. PNAS. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. Induction of the c-fos protooncogene in rat amygdala during unconditioned and conditioned fear. Brain Research. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci U S A. 2007;104:12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE, Morrison SF. Unit responses evoked in the amygdala and striatum by electrical stimulation of the medial geniculate body. J Neurosci. 1990;10:1055–1061. doi: 10.1523/JNEUROSCI.10-04-01055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear andanxiety. In: Aggleton JP, editor. The amygdala: A functional analysis. Oxford University Press; 2000. [Google Scholar]
- Davis HP, Squire LR. Psychological Bulletin. Vol. 96. 1984. Protein synthesis and memory: a review; pp. 518–59. [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Mahadevan LC. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms. EMBO Journal. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Ann Rev Psych. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Vogt BA, Kubota Y, Poremba A, Kang E. Training-Stage Related Neuronal Plasticity in Limbic Thalamus and Cingulate Cortex During Learning - A Possible Key to Mnemonic Retrieval. Behavioural Brain Research. 1991;46:175–185. doi: 10.1016/s0166-4328(05)80111-1. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Translational control by mTOR contributes to the formation and stability of fear memory in the hippocampus. Submitted to . Journal of Neuroscience 2007 [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Effects of post training hippocampal injections of midazolam on fear conditioning. Learning & Memory. 2005;12:573–578. doi: 10.1101/lm.51305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Parsons RP, Helmstetter FJ. Consolidation but not reconsolidation of contextual fear memory is sensitive to rapamycin and an inhibitor of mRNA synthesis in the dorsal hippocampus. Society for Neuroscience Abstracts; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: Evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Bio Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neuroscience. 2001;13:1453–1458. doi: 10.1046/j.0953-816x.2001.01531.x. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Hazzalin CA, Le Panse R, Cano E, Mahadevan LC. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol Cell Biol. 1998;18:1844–1854. doi: 10.1128/mcb.18.4.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Riedner BA, Baruch DE, Parsons RG. Trace fear conditioning requires hippocampal protein synthesis. Society for Neuroscience Abstracts.2004. [Google Scholar]
- Helmstetter FJ. Genetic Substrates of Memory: Amygdala. In: Byrne JH, editor. Learning & Memory. 2. New York: MacMillan; 2003. [Google Scholar]
- Helmstetter FJ, Tershner SA, Poore LH, Bellgowan PSF. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Research. 1998;779:104–118. doi: 10.1016/s0006-8993(97)01104-9. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Hypoalgesia in response to sensitization following acute noise stress. Behavioral Neuroscience. 1994;108:177–185. doi: 10.1037//0735-7044.108.1.177. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behavioral Neuroscience. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Tershner SA. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditional responses. Journal of Neuroscience. 1994;14:7099–7108. doi: 10.1523/JNEUROSCI.14-11-07099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiology and Behavior. 1992;51:1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is critical for the expression of conditional hypoalgesia. Behavioral Neuroscience. 1992;106:518–528. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Landeira-Fernandez J. Conditional hypoalgesia is attenuated by naltrexone applied to the periaqueductal gray. Brain Research. 1990;537:88–92. doi: 10.1016/0006-8993(90)90343-a. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci Bio Behav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of Amygdala, Hippocampus, and Periaqueductal Gray Lesions on Short-Term and Long-Term Contextual Fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. NeuroReport. 1999;10:3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency-specific receptive field plasticity in the medial geniculate body induced by pavlovian fear conditioning is expressed in the anesthetized brain. Behav Neurosci. 1992;106:484–497. doi: 10.1037//0735-7044.106.3.484. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Choi S, Lee SY, Cao YA, Ahn HJ, Worley KC, Pizzi M, Liou HC, Sweatt JD. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. J Neurosci. 2004;24:3933–3943. doi: 10.1523/JNEUROSCI.5646-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene I in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97:693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Ann Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Ann Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Large-scale functional connectivity in associative learning: Interrelations of the rat auditory, visual, and limbic systems. J Neurophysiol. 1998;80:3148–3162. doi: 10.1152/jn.1998.80.6.3148. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learning & Memory. 2006;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Bio Behav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the FOS protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Mileusnic R, Lancashire CL, Rose SP. Recalling an aversive experience by day-old chicks is not dependent on somatic protein synthesis. Learn Mem. 2005;12:615–619. doi: 10.1101/lm.38005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72:350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. European Journal of Neuroscience. 2006a;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Riedner BA, Gafford GM, Helmstetter FJ. The Formation of auditory fear memory requires the synthesis of protein and mRNA in the auditory thalamus. Neuroscience. 2006b;141:1163–1170. doi: 10.1016/j.neuroscience.2006.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. Journal of Neuroscience. 2006c;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Blockade of protein synthesis in the ventrolateral periaqueductal gray attenuates the formation of fear memory in rats. Society for Neuroscience Abstracts.2006d. [Google Scholar]
- Poremba A, Jones D, Gonzalez-Lima F. Classical conditioning modifies cytochrome oxidase activity in the auditory system. Eur J Neurosci. 1998;10:3035–3043. doi: 10.1046/j.1460-9568.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. Journal of Neuroscience. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Gingras AAC, Sonenberg N. The target of rapamycin (TOR) proteins. PNAS. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neuroscience. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J Neurosci. 2003;23:8034–8040. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Varga AW, Sweatt JD, Swank M. Protein kinase signal transduction cascades in mammalian associative conditioning. Neuroscientist. 2002;8:122–131. doi: 10.1177/107385840200800208. [DOI] [PubMed] [Google Scholar]
- Stork O, Pape HC. Fear memory and the amygdala: Insights from a molecular perspective. Cell & Tissue Res. 2002;310:271–277. doi: 10.1007/s00441-002-0656-2. [DOI] [PubMed] [Google Scholar]
- Stork O, Stork S, Pape HC, Obata K. Identification of genes expressed in the amygdala during the formation of fear memory. Learning & Memory. 2001;8:209–219. doi: 10.1101/lm.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. PNAS. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink R, Cahill M, Kracht M, Sachsenmaier C, Hipskind RA, Nordhein A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Bio. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]