Abstract
Damage to peripheral nerves often cannot be repaired by the juxtaposition of the severed nerve ends. Surgeons have typically used autologous nerve grafts, which have several drawbacks including the need for multiple surgical procedures and loss of function at the donor site. As an alternative, the use of nerve guidance channels to bridge the gap between severed nerve ends is being explored. In this paper, the electrically conductive polymer—oxidized polypyrrole (PP)—has been evaluated for use as a substrate to enhance nerve cell interactions in culture as a first step toward potentially using such polymers to stimulate in vivo nerve regeneration. Image analysis demonstrates that PC-12 cells and primary chicken sciatic nerve explants attached and extended neurites equally well on both PP films and tissue culture polystyrene in the absence of electrical stimulation. In contrast, PC-12 cells interacted poorly with indium tin oxide (ITO), poly(l-lactic acid) (PLA), and poly(lactic acid-co-glycolic acid) surfaces. However, PC-12 cells cultured on PP films and subjected to an electrical stimulus through the film showed a significant increase in neurite lengths compared with ones that were not subjected to electrical stimulation through the film and tissue culture polystyrene controls. The median neurite length for PC-12 cells grown on PP and subjected to an electrical stimulus was 18.14 μm (n = 5643) compared with 9.5 μm (n = 4440) for controls. Furthermore, animal implantation studies reveal that PP invokes little adverse tissue response compared with poly(lactic acid-co-glycolic acid).
Keywords: conductive polymer, oxidized polypyrrole, nerve regeneration, PC-12 cells, electrical stimulation, neuronal stimulation
The current clinical approach to repair a peripheral nerve over a gap involves the utilization of autologous nerve grafts (1). However, this procedure has several disadvantages, including loss of function at the donor nerve graft site and mismatch of damaged nerve and graft dimensions. As an alternative to nerve autografts, natural and synthetic tubular guidance channels have been the subject of intensive research (2). Guidance channels help direct axons sprouting off the regenerating nerve end, provide a conduit for diffusion of neurotrophic and neurotropic factors secreted by the damaged nerve stump, and minimize infiltrating fibrous tissue. Materials that have been investigated for nerve repair range from autologous veins (3) and muscles (4), to prosthetic tubes derived from collagen, laminin, fibronectin (5–7), silicone (8), and various other biodegradable and nonbiodegradable polymers (2). However, the engineering of an ideal nerve guidance channel that provides an attractive clinical alternative to nerve autografts remains a challenge.
Past work has demonstrated that electrical charges play an important role in stimulating either the proliferation or differentiation of various cell types. For example, it has been shown that neurite outgrowth is enhanced on electrets such as poled polyvinylidene fluoride (9, 10) and poled polytetrafluoroethylene (11). Electrets are broadly defined as materials possessing quasi-permanent surface charge because of trapped monopolar charge carriers. In piezoelectric materials such as poled polyvinylidene fluoride, transient surface charges are generated as a result of minute mechanical deformations of the material, whereas poled polytetrafluoroethylene displays a static surface charge. Enhanced neurite outgrowth has been attributed to the presence of these surface charges. Furthermore, extensive research both in vitro and in vivo has shown that electromagnetic fields play an important role in neurite extension and regeneration of transected nerve ends (12–14).
It occurred to us that a polymer scaffold or guidance channel derived from an electrically conducting polymer could prove potentially useful in not only providing neuronal guidance but in localizing electromagnetic stimulation as well. The electrically conducting polymer, oxidized polypyrrole (PP), was chosen for this study because of its inherent electrical conductive properties, ease of preparation, flexibility of altering surface characteristics, and its in vitro compatibility with mammalian cells (15, 16). In its oxidized form, polypyrrole is a polycation with delocalized positive charges along its highly conjugated backbone and is an electronic conductor (17). Charge neutrality is achieved by the incorporation of negatively charged ions termed “dopants.” PP also exhibits reversible electrochemistry allowing for the control of surface-charge density by varying the oxidation state of the polymer. For example, the application of a reduction potential to PP converts it from a conductive form to its neutral (insulative) form with the release of dopant anions or the incorporation of cations to maintain charge neutrality. Potential biomedical applications have been developed based on the electrochemical properties of PP. These include the use of PP as a matrix for controlled delivery of dopamine (18) and as a biosensor for detection of glucose (19) or proteins (20).
Surface characteristics such as charge density and wettability play a key role in protein adsorption and cell–substrate interactions. Recently, it has been shown that both cell–surface interactions and cellular functions (e.g., DNA synthesis) on PP thin films can be controlled by either changing the oxidation state of the polymer (15) or by changing the wettability (hydrophobicity) of the polymer film using appropriate dopants (16). Surface characteristics are critical because the interaction of endogenous proteins with a biomaterial at the implantation site will have a significant impact on the adhesion, differentiation, and proliferation of surrounding cells. Flexibility at the level of controlling protein adsorption provides a means to engineer materials that yield predictable and desirable cell–surface interactions. Therefore, PP is an interesting candidate for tissue engineering applications, by virtue of its inherent electrical conductivity, the ease with which one can control crucial surface properties such as wettability and charge density, and its compatibility with mammalian cells.
In this study, the utility of the electrically conductive polymer, PP, as a substrate to enhance nerve cell differentiation in culture was evaluated with the hope of ultimately using electrically conducting polymers to stimulate in vivo nerve regeneration. We show that PP is a suitable material for in vitro nerve cell culture and that application of an electric stimulus through PP enhances neurite outgrowth. We also show that PP does not elicit an adverse tissue response when implanted in rats.
MATERIALS AND METHODS
Preparation of Polypyrrole Films.
PP was synthesized electrochemically (21). Indium tin oxide (ITO)-conductive borosilicate glass (Delta Technologies, Still Water, MN) was used as the electrically conductive surface for PP film deposition. ITO glass slides (75 × 25 mm or 50 × 25 mm, 40Ω/square) were cleaned before use by sonication in hexane, methanol, and methylene chloride for 5 min each. A three-electrode setup consisting of an ITO glass working electrode, platinum gauze counter electrode, and a Ag/AgCl reference was used for the electrochemical synthesis of PP. PP film was deposited at a constant potential of 0.7 V versus Ag/AgCl reference from an aqueous solution of 0.1 M pyrrole (Aldrich) containing 0.1 M sodium salt of poly(styrenesulfonate) (PSS) (Aldrich). A Pine Instruments AFRDE4 bipotentiostat (Grove City, PA) was used as the source of constant voltage. Films of two different thickness were synthesized: 0.1–0.15 μm (thin film) and 1.8–2.0 μm (thick film). The film thickness was controlled by the passage of charge (21), which was determined by integrating current over time. Thin films were optically transparent and were used for in vitro analyses, and thick films were used for in vivo implantation studies. Polypyrrole, with PSS as the dopant, will be referred to here simply as PP.
Due to the fragile nature of freestanding PP thick films, they were laminated with PLGA films, a polymer that has been approved by the Food and Drug Administration for certain applications, to yield processible and suturable disks for in vivo studies. In brief, the PP thick film was gently peeled off the ITO glass and then floated in distilled deionized water and subsequently transferred onto a clean glass slide. The PP film was then wetted with methylene chloride and layered with PLGA film. PLGA 85:15 (Birmingham Polymers, Birmingham, AL) films of 40 ± 2-μm thickness were solvent cast from a methylene chloride solution (100 mg of purified PLGA in 1 ml of methylene chloride) at room temperature overnight. For implantation, laminated PP films were cut into 5-mm-diameter disks and sterilized by exposure to UV light for 30 min.
Cell Culture.
Rat PC-12 cells (American Type Tissue Collection) (22) were used for in vitro studies. PC-12 cells respond reversibly to nerve growth factor (NGF) by differentiation into the neuronal phenotype (extension of neurites). In addition, sciatic nerve explants were isolated from 16-day chick embryos as previously described (23).
PC-12 cells were cultured in 85% high-glucose DMEM, 10% heat-inactivated horse serum, and 5% fetal bovine serum. Cells were maintained in a humid, 5% CO2 incubator and passaged using 0.25% trypsin at 1:2 dilution every other day. PC-12 cells were “primed” by the addition of 25 ng/ml NGF (Boehringer Mannheim) 24 h prior to seeding cells for an experiment. During experiments, cells were maintained in medium supplemented with 25 ng/ml NGF. Cells were seeded into wells formed by the attachment of sterile Plexiglas wells (1 cm × 1.5 cm, inner dimension) to PP films using autoclaved vacuum grease. In all experiments, 1 ml of medium containing 2–2.5 × 104 cells was used per well (1.33–1.67 × 104 cells/cm2). Chick sciatic nerve explants were maintained in DMEM supplemented with 10% fetal bovine serum, glucose to a total of 6 mg/ml, 1 mM sodium pyruvate, and 25 ng/ml NGF.
In Vitro Electrical Stimulation.
Primed PC-12 cells were plated into cell wells assembled onto PP thin films (see above) at densities of 1.33 × 104 cells/cm2 and then incubated for 24 h to permit attachment and spreading. After this initial 24-h period, the PC-12 cells were subjected to a steady potential of 100 mV for 2 h. For electrical stimulation, the PP film served as the anode and a gold (Au) wire placed at the opposite end (along the length) of the well served as the cathode. A silver (Ag) wire served as a quasi-reference electrode. A bipotentiostat (Pine Instruments) was used as the source of constant voltage. Cells were maintained in a CO2 incubator for the duration of electrical stimulation. After electrical stimulation (S), the cells were incubated for an additional 24 h (a total of 48 h from the start of the experiment). Images of cells were obtained both before (24 h) and after exposure to the constant potential (48 h). PC-12 cells plated on PP thin films that were not subjected to any electrical stimulation (NS) served as controls. Cells grown on tissue culture polystyrene (TCPS) and not subjected to any electrical stimulus served as additional controls. A seeding density of 1.33 × 104 cells/cm2 was maintained for all experiments. The neurite lengths of cells under stimulation were compared with controls to estimate the extent of differentiation.
To assess any effects of possible convective ion transport or oxidation/reduction products on neurite outgrowth, cells grown on PP were subjected to a constant potential (100 mV) that was applied through the medium and not the polymer film (“solution control”). To accomplish this, two gold wires were affixed to opposite ends of the cell well and served as anode and cathode. A silver wire on a third side of the well was used as a quasi-reference.
In addition to the application of a constant potential, electrical leads were directly attached to opposite ends of the PP film and constant current of 10 μAmp was passed through the polymer film. For comparison, the constant potential scenario of 100 mV corresponds to a current of about 100 μAmp, given that the resistance of the PP film is about 1 kΩ. The use of this magnitude of current is justified based on previous electrical stimulation studies in rats using currents of 0.6 μAmp (13), 10–30 μAmp (24), and 400 μAmp (25).
Neurite Length Measurements.
Thin PP films permitted the use of light microscopy and quantitative image analysis tools to study cell–material interactions in detail. Cells were viewed using an inverted phase contrast microscope (Diaphot-TMD; Nikon) and a 20× objective. Images from the microscope were acquired using a CCD video camera (HVC-20; Hitachi, Japan) and were subsequently digitized using nih image software and a Scion image capture board (LG-3; Scion, Frederick, MD). The lengths of the individual neurites for each cell were measured using the nih image software. Length was defined as the straight-line distance from the tip of the neurite to the junction between the cell body and neurite base. In the rare case of branched neurites, the length of the longest branch was measured from the tip of the neurite to the cell body, then each branch was measured from the tip of the neurite to the neurite branch point.
Neurite Data Analysis and Statistical Evaluation.
For a single experiment, each condition (S, NS, solution control, or TCPS) was run in duplicate cell wells. For each cell well, approximately 10–20 images were acquired randomly by scanning the wells from left to right and top to bottom. Experiments were repeated on at least two separate days. The total number of neurites (N) for each condition is reported.
The measured neurite lengths were not normally distributed, and, therefore, standard statistical parameters (e.g., population mean and standard deviation) were not computed. Instead, the actual distributions of neurite lengths are presented for each condition along with the population median. In addition, the Kruskal–Wallis H test (26) was used to assess statistical differences between the neurite populations. The Kruskal–Wallis H test is a nonparametric statistical test analogous to ANOVA.
Scanning Electron Microscopy.
Samples were fixed with 1% gluteraldehyde for 10 min, then exposed to increasing concentrations of ethanol (50%, 60%, 80%, 90%) for 2 min each, and finally allowed to dry overnight. Images of cells were obtained using an environmental scanning electron microscope (ESEM) (Electro Company, Boston, MA) equipped with a Trecor detector set at an accelerating voltage of 15 kV under a vacuum of 4.9 torr and 6% humidity.
In Vivo Tissue Response.
To evaluate both short-term and long-term tissue response, PLGA-laminated PP disks were implanted in adult male Lewis rats in subcutaneous and intramuscular locations. The PP disks along with the surrounding tissue were harvested at 1, 2, and 14 weeks postimplantation for histological evaluation. The tissues were fixed in formalin, embedded in paraffin, sectioned (≈4.5-μm thickness), and stained using hemotoxylin and eosin.
RESULTS
In Vitro Nerve Cell Cultures.
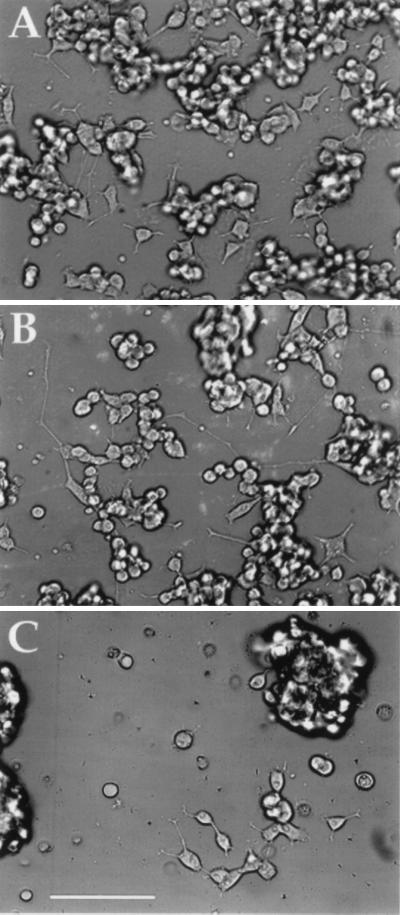
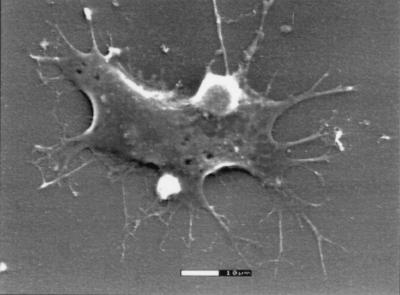
Phase-contrast optical micrographs of PC-12 cells cultured for 48 h in the presence of NGF on TCPS, PP film (without electrical stimulation), and poly(l-lactic acid) (PLA) film are shown in Fig. 1. For these experiments, cells were seeded at 25,000 cells per well. It is apparent from the images that PC-12 cells attach and differentiate equally well on TCPS (A) and PP (B) films and more poorly on PLA films (C). PC-12 cells also attach poorly to films of PLGA and ITO surfaces (data not shown). Scanning electron microscopy was used to verify that PC-12 cells adhered to and extended healthy neurites on PP thick films (Fig. 2). In addition to the PC-12 cell line, PP also supports the growth of chick sciatic nerve explants for up to at least 1 week in culture (data not shown). These explants contained Schwann cells, fibroblasts, and other neuronal support cells in addition to neurons. Thus, PP is capable of sustaining primary nerve cells and the necessary support cells that are critical for regeneration.
Figure 1.
Differentiation of PC-12 cells on polypyrrole compared with controls. Cells were grown on tissue culture polystyrene (TCPS) (A), oxidized polypyrrole (PP) (B), and poly(l-lactic acid) (PLA) (C) for 48 h in the presence of NGF. Bar = 100 μm. [Reproduced with permission from Materials Research Society (Copyright 1996).]
Figure 2.
Scanning electron microscopy of a PC-12 cell on polypyrrole. PC-12 cells were cultured in NGF-supplemented medium for 48 h on thick disks of PP, then processed for scanning electron microscopy. Bar = 10 μm.
In Vitro Electrical Stimulation.
To elucidate the effect of electrical stimulation through PP films on neuronal differentiation, experiments were performed in which PC-12 cells grown on PP were subjected to a potential of 100 mV for 2 h through the film (S). Controls consisted of: (i) PC-12 cells grown on PP but not subjected to any stimulation (NS), (ii) cells grown on PP and subjected to a potential through the solution only (solution control), and (iii) cells grown on TCPS. Cells were cultured for 24 h on PP films prior to electrical stimulation and then cultured for an additional 24 h (48 h total). Controls not requiring an electrical stimulus were cultured uninterrupted for a total of 48 h. For these experiments, cells were seeded at a density of 20,000 cells per well (1.33 × 104 cells/cm2).
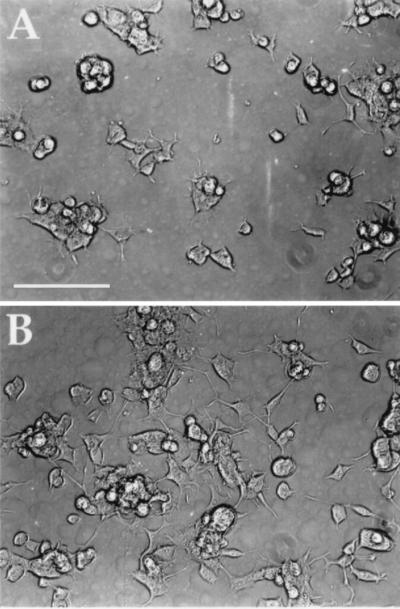
The application of an external electrical stimulus through the PP substrate significantly enhanced differentiation of PC-12 cells (Fig. 3). Qualitatively, cells exposed to a potential (S, Fig. 3B) extended longer neurites compared with cells grown on PP but not exposed to any electrical stimulus (NS, Fig. 3A). In addition, cell spreading for the stimulated cell population was more pronounced than for any of the control cases. Similar effects were observed for cells exposed to a constant current of 10 μAmp, in which case electrode leads were directly attached to the PP at opposite ends of the cell well (data not shown). There were no signs of cytotoxic effects in any of the experiments, and no measurable differences in pH or temperature were detected. Estimates of the electrical resistance of PP thin films and DMEM (culture medium) were obtained using a multimeter (electrode spacing was ≈2 cm) and were 1 kΩ and ≈180 kΩ, respectively.
Figure 3.
PC-12 cell differentiation on polypyrrole without (A) and with (B) application of an electric potential. PC-12 cells were grown on PP for 24 h in the presence of NGF, then exposed to electrical stimulation (100 mV) across the polymer film, S (B). Images were acquired 24 h after stimulation. Cells grown for 48 h but not subjected to electrical stimulation, NS, are shown for comparison (A). Bar = 100 μm. [Reproduced with permission from Materials Research Society (Copyright 1996).]
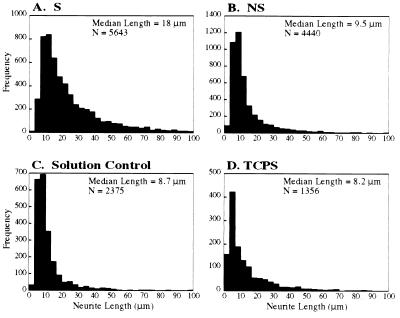
Image analysis was used to quantify the effect of electrical stimulation on PC-12 differentiation. The distributions of the neurite lengths for the different conditions (S, NS, solution control, TCPS) are shown in Fig. 4, where frequency is the number of neurites of a given measured length and N is the total number of neurites measured for each condition over multiple experiments. These data show that the neurite distribution in the stimulated cell population is shifted to the right (to longer neurites) compared with controls. This becomes more obvious if the peak frequencies are normalized by the total number of neurites measured (N) (e.g., the normalized peak frequency for the stimulated case is the number of peak neurites/total neurites, or 820/5,643). The normalized peak frequencies are 0.14, 0.27, 0.29, and 0.31 for the S, NS control, solution control, and TCPS cases, respectively. This indicates that the distribution of the neurite lengths in the “S” case is “flattened” and shifted to the right compared with the negative controls. The fact that the median neurite length (18.14 μm, n = 5643) in the “S” case is about twice that of the negative controls [9.5 μm for NS (n = 4440), 8.66 μm for solution control (n = 2375), and 8.23 μm for TCPS (n = 1356)] confirms that the neurite distribution in the “S” case reveals a significant increase in the neurite lengths. Finally, a Kruskal–Wallis H test verified that the distribution of neurite lengths for the “S” case is significantly different from the distributions for the negative controls (P < 0.0001).
Figure 4.
Neurite length histograms. Shown are histograms of neurite lengths for cells on PP with (S) (A) and without (NS) (B) potential applied through PP, on PP with potential applied through the solution (C), and on tissue culture polystyrene (TCPS) (D). Images are after 48-h total incubation time.
In addition, constant current of 10 μAmp applied directly through the PP film resulted in a median neurite length of 15.3 μm (n = 273), which is greater than the median neurite length for PP without an electrical stimulus (9.5 μm, n = 4440).
In Vivo Tissue Response to Oxidized Polypyrrole.
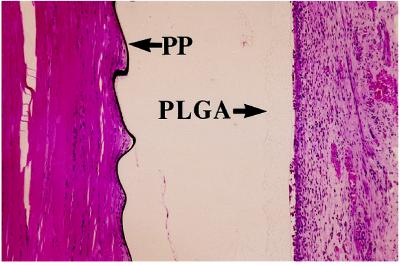
PLGA-laminated PP disks were implanted in adult male Lewis rats in subcutaneous and intramuscular sites to determine the in vivo tissue response to PP. It was observed that the inflammation associated with the PP film was less severe than that induced by the PLGA film (Fig. 5). The histological section is an intramuscular implant harvested 2 weeks after implantation. The black material adjacent to the muscle at the left side of the figure is PP, and the translucent film adjacent to the tissue at the right side of the image is the PLGA film (original magnification, 100×). More inflammatory cells are associated with the Food and Drug Administration-approved PLGA film compared with PP, suggesting that PP is a relatively inert and biocompatible material. Furthermore, at 14 weeks there was minimal evidence of a chronic inflammatory response as a result of PP implantation (data not shown).
Figure 5.
Histology of tissue response to polypyrrole. Shown is a histological tissue section stained with hemotoxylin and eosin of a PP/PLGA disk that had been implanted in rat muscular tissue for 2 weeks. The PP is seen as a black film (Left), and the PLGA is almost transparent (Right). The PP and PLGA films separated during histological processing, explaining the gap in the center of the image. (×100.)
DISCUSSION
This study demonstrates that the electrically conducting polymer, oxidized polypyrrole, is a suitable material for in vitro nerve cell culture and for in vivo implantation. The favorable interaction of PC-12 cells with PP compared with PLA and PLGA may be the result of increased adsorption of positively charged matrix proteins onto PP’s highly negatively charged surface (16, 17). The high surface negative charge is due to the incorporation of the polyanionic dopant poly(styrenesulfonate). PP also supports the growth and/or differentiation of primary chick sciatic nerve explants containing Schwann cells, fibroblasts, and other ganglion-derived cells in addition to neurons. Thus, PP is capable of sustaining primary nerve cells and the support cells that are critical for regeneration.
Application of an electrical stimulus to PC-12 cells cultured on PP significantly enhanced PC-12 neurite outgrowth and spreading. The median neurite length for PC-12 cells grown on PP and subjected to an electrical stimulus through the film (18.14 μm, n = 5643) was nearly doubled compared with cells grown on PP without the application of a constant potential (9.5 μm, n = 4440). This represents a 90% increase in neurite length, which is significantly greater than the 20–40% increase in neurite lengths observed for primary nerve cultures on piezoelectric meterials (10). Cells grown either on TCPS or on PP in which charge was passed through the solution and not through the polymer film possessed neurite length distributions indistinguishable from the “NS” controls (Fig. 4). This suggests that the observed effects result primarily from electronic conduction through the PP and not from ionic conduction through solution.
The precise mechanism for the observed effect in this study is unclear and is the subject of a current investigation. Several theories have been suggested to explain the effect of electric stimulation on nerve regeneration, including (i) electrophoretic redistribution of cell membrane growth factor and adhesion receptors or cytoskeletal proteins such as actin (27, 32), all of which are involved in growth cone migration, (ii) favorable membrane or extracellular matrix protein conformational changes (10), (iii) direct depolarization or hyperpolarization of nerves (14), (iv) enhancement of protein synthesis (12), and (v) field-induced gradients of ions and molecules in the culture medium or tissue fluid. Patel and Poo (27) used an extracellularly applied electric field to show that neurites preferentially orient toward the cathode. In their studies, they found a cathodal redistribution of growth-controlling surface glycoproteins, suggesting that a field-induced electrophoretic redistribution of charged membrane proteins gives rise to growth cones that migrate preferentially toward the cathode. Results from this study also argue against the formation of field-induced gradient of ions and molecules in the culture medium or tissue fluid. Similarly, studies in Xenopus have shown that cell surface receptors for neurotransmitters undergo a cathodal reorientation in an electric field (28), although other studies also in Xenopus demonstrate that neurites can turn either toward the cathode or anode depending on the identity of the substratum (29). In contrast, Davenport and McCaig (31) found that dendrites turn cathodally while axons do not respond, whereas Cork et al. (30) found that PC-12 cell neurite turn toward the anode. Thus, the true effect of electrical stimulation on neurite outgrowth is still the subject of debate.
In the present study, electrical charges pass through the underlying PP film on which cells are attached via an adsorbed protein monolayer. Because the resistance of PP is significantly lower than that for the electrolyte cell medium (1 kΩ and ≈180 kΩ, respectively), charge will pass preferentially through the PP film until charge transfer reactions are absolutely necessary for current to reach the gold counter electrode at the far end of the cell well. This suggests that enhanced neurite extension results primarily from the passage of electronic current through the material, and not from ionic flow of current through the electrolyte medium. Also, enhanced effects on neurite outgrowth were observed when constant current was passed directly through the PP film and in which electrochemical reactions were absent [median neurite length = 15.3 μm (n = 273) compared with 9.5 μm for controls (n = 4440)]. This indicates that the contribution of ionic current on neurite outgrowth can be excluded.
Furthermore, the effects of convective ion transport and oxidation/reduction products can be considered negligible based on two additional results. First, the “solution control,” in which cells grown on PP were exposed to 100 mV that was passed exclusively through the medium and not the polymer film, did not resemble the “S” case and more closely compared with the “NS” control. This suggests that products from oxidation/reduction reactions, under these experimental conditions, did not either adversely or positively affect neurite outgrowth. Second, the inability to detect a significant pH change during these short-term experiments also indicates that products from the electrochemical reactions did not likely contribute to the observed positive effect of electrical stimulation. These data are consistent with the conclusion by Patel and Poo (27) that field-induced gradients of ions or molecules in the cell medium do not play a role in enhanced neurite outgrowth.
In our study, upon electrical stimulation no directional bias of neurite outgrowth toward either electrode was observed. It was observed that neurite extension was enhanced uniformly in all directions for cells exposed to either a constant potential or a constant current applied through the PP film. This observation argues against an electrophoretic redistribution of cell matrix proteins or of cell membrane proteins, which would likely give rise to an overall directional bias. Thus, these data support the hypothesis that an electrical stimulus enhances overall differentiation rather than influencing the direction of neurite extension. Others have shown increased protein synthesis in neurons with electrical stimulation (12), which may help account for the observations presented here if proteins essential to neurite outgrowth were up-regulated. Furthermore, Kojima et al. (33) have shown that protein production in tumor cells plated on platinum and indium tin oxide surfaces is stimulated by the application of a constant potential. However, the mechanism by which protein synthesis is activated is not known. Studies are currently underway to understand the changes in electrophysiology of the cells during and after electrical stimulation.
The use of PP in vivo has also been evaluated in the present investigation. Both short-term and long-term tissue compatibility studies in the rat reveal that PP promotes little negative tissue or inflammatory response. In its present form, PP is nondegradable, and relatively thin films of PP, backed with PLGA for support, were used for in vivo implantation. Even after 14 weeks, the PP films were found to be intact, although the PLGA backing had completely degraded. Studies are currently in progress to determine the effect of an electrical stimulus applied through PP conduits on actual sciatic nerve regeneration in rats.
There are two key advantages to considering electrically conducting polymers for use in tissue engineering applications. First, unlike exogenous electromagnetic fields, electrical stimulation through such polymers would be predominantly focused to the area around the polymer, allowing for spatial control of stimulation. In the use of exogenous electromagnetic fields for neuronal stimulation, the inability to localize the stimulation has been cited as a disadvantage (34). Although piezoelectric materials such as polyvinylidene difluoride can aid in localized stimulation, the external control over stimulation is lacking. Other electret materials such as polytetrafluoroethylene display a static surface charge, but the stability and distribution of charges are highly dependent on processing conditions. Although electrets may hold promise for nerve regeneration applications (11), the possibility that electrical stimulation can be externally controlled and precisely regulated with electrically conducting polymers such as PP may prove advantageous. Second, the flexibility of synthesizing electrically conducting polymers, such as PP, with varying surface properties offers many advantages. PP is easy and inexpensive to synthesize, and different dopant ions can be incorporated into the PP chain during synthesis to provide varied surface characteristics. For instance, we found that PC-12 cells do not adhere to PP films having the chloride ion as a dopant, lending the possibility of patterning a surface with adhesive and nonadhesive areas. Finally, matrix analogs and growth factors could be either physically incorporated and subsequently released or tethered to the PP film (16). This offers the possibility of enhancing neuronal regeneration using multiple forms of stimuli. Furthermore, it may be possible that electrically conducting polymers can be used as an interactive scaffold material for stimulation of other tissue types such as bone.
Acknowledgments
We thank N. Chen, I. George, R. Bellamkonda, P. Basser, D. Odde, T.-H. Kim, R. Solomon, I. Joris, M. Frangelo, and S. Charnick for their help. This work was supported by a National Science Foundation Grant (BES-9525913) to R.L. and a National Institutes of Health postdoctoral fellowship to C.E.S.
ABBREVIATIONS
- PP
polypyrrole
- PLA
poly(l-lactic acid)
- PLGA
poly(lactic acid-co-glycolic acid)
- TCPS
tissue culture polystyrene
- ITO
indium tin oxide
- NGF
nerve growth factor
References
- 1.Millesi H. In: Operative Nerve Repair and Reconstruction. Gelberman R H, editor. Philadelphia: Lippincott; 1991. pp. 525–544. [Google Scholar]
- 2.Valentini R F. In: The Biomedical Engineering Handbook. Bronzino J D, editor. Boca Raton, FL: CRC; 1995. pp. 1985–1996. [Google Scholar]
- 3.Chiu D T, Janecka I, Krizek T J, Wolff M, Lovelace R E. Surgery. 1982;91:226–233. [PubMed] [Google Scholar]
- 4.Fawcett J W, Keynes R J. J Neurosurg. 1986;65:354–363. doi: 10.3171/jns.1986.65.3.0354. [DOI] [PubMed] [Google Scholar]
- 5.Archibald S J, Krarup C, Shefner J, Li S-T, Madison R A. J Comp Neurol. 1991;306:685–696. doi: 10.1002/cne.903060410. [DOI] [PubMed] [Google Scholar]
- 6.DaSilva C F, Madison R. Soc Neurosci. 1986;12:190.11. [Google Scholar]
- 7.Tong X-J, Hirai K-I, Shimada H, Mizutani Y, Izumi T, Toda N, Yu P. Brain Res. 1994;663:155–162. doi: 10.1016/0006-8993(94)90473-1. [DOI] [PubMed] [Google Scholar]
- 8.Fields R D, Ellisman M H. Exp Neurol. 1986;92:48–74. doi: 10.1016/0014-4886(86)90124-x. [DOI] [PubMed] [Google Scholar]
- 9.Aebischer P, Valentini R F, Dario P, Domenici C, Galletti P M. Brain Res. 1987;436:165–168. doi: 10.1016/0006-8993(87)91570-8. [DOI] [PubMed] [Google Scholar]
- 10.Valentini R F, Vargo T G, Gardella J A, Aebischer P. Biomaterials. 1992;13:183–190. doi: 10.1016/0142-9612(92)90069-z. [DOI] [PubMed] [Google Scholar]
- 11.Valentini R F, Sabatini A M, Dario P, Aebischer P. Brain Res. 1989;480:300–304. doi: 10.1016/0006-8993(89)90196-0. [DOI] [PubMed] [Google Scholar]
- 12.Sisken B F, Kanje M, Lundborg G, Herbst E, Kurtz W. Brain Res. 1989;485:309–316. doi: 10.1016/0006-8993(89)90575-1. [DOI] [PubMed] [Google Scholar]
- 13.Kerns J M, Fakhouri A J, Weinrib H P, Freeman J A. Neurosci. 1991;40:93–107. doi: 10.1016/0306-4522(91)90177-p. [DOI] [PubMed] [Google Scholar]
- 14.Basser P J. IEEE Trans Biomed Eng. 1994;41:601–606. doi: 10.1109/10.293248. [DOI] [PubMed] [Google Scholar]
- 15.Wong J Y, Langer R, Ingber D E. Proc Natl Acad Sci USA. 1994;91:3201–3204. doi: 10.1073/pnas.91.8.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shastri, V. R. (1995) Ph.D. Dissertation (Rensselaer Polytechnic Institute, Troy, NY).
- 17.Prezyna L A, Qiu Y-J, Reynolds J R, Wnek G E. Macromolecules. 1991;24:5283–5287. [Google Scholar]
- 18.Miller L L, Zhou Q-X. Macromolecules. 1987;20:1594–1597. [Google Scholar]
- 19.Couves L D. Synth Metals. 1989;28:C761–C768. [Google Scholar]
- 20.Sadik O A, Wallace G G. Anal Chim Acta. 1993;279:209–212. [Google Scholar]
- 21.Diaz A F, Castillo J C, Logan J A, Lee W Y. J Electroanal Chem. 1981;129:115–132. [Google Scholar]
- 22.Greene L A, Tischler A S. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y-W, Mezei C. Can J Biochem. 1971;49:320–327. doi: 10.1139/o71-047. [DOI] [PubMed] [Google Scholar]
- 24.Mraovitch S, Costantino I, Ruggiero D A, Reis J. Brain Res. 1985;341:283–296. doi: 10.1016/0006-8993(85)91067-4. [DOI] [PubMed] [Google Scholar]
- 25.Kow L-M, Pfaff D W. Brain Res. 1985;347:1–10. doi: 10.1016/0006-8993(85)90883-2. [DOI] [PubMed] [Google Scholar]
- 26.Randles R H, Wolfe D A. Introduction to the Theory of Nonparametric Statistics. New York: Wiley; 1979. pp. 395–396. [Google Scholar]
- 27.Patel N B, Poo M-M. J Neurosci. 1982;2:483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erskine L, McCaig C D. Dev Biol. 1995;171:330–339. doi: 10.1006/dbio.1995.1285. [DOI] [PubMed] [Google Scholar]
- 29.Stewart R, Erskine L, McCaig C D. Dev Biol. 1995;171:340–351. doi: 10.1006/dbio.1995.1286. [DOI] [PubMed] [Google Scholar]
- 30.Cork R J, McGinnis M E, Tsai J, Robinson K R. J Neurobiol. 1994;25:1509–1516. doi: 10.1002/neu.480251204. [DOI] [PubMed] [Google Scholar]
- 31.Davenport R W, McCaig C D. J Neurobiol. 1993;24:89–100. doi: 10.1002/neu.480240108. [DOI] [PubMed] [Google Scholar]
- 32.Luther P W, Peng H B, Lin J J-C. Nature (London) 1983;303:61–64. doi: 10.1038/303061a0. [DOI] [PubMed] [Google Scholar]
- 33.Kojima J, Shinohara H, Ikariyama Y, Aizawa M, Nagaike K, Morioka S. Biotechnol Bioeng. 1992;39:27–32. doi: 10.1002/bit.260390106. [DOI] [PubMed] [Google Scholar]
- 34.Evans B A, Litchy W J, Daube J R. Muscle Nerve. 1988;11:1074–1078. doi: 10.1002/mus.880111011. [DOI] [PubMed] [Google Scholar]