Abstract
Persistent infection with the high-risk subset of genitotropic human papillomavirus (HPV) genotypes is a necessary cause of cervical cancer. Given the global burden of cervical cancer, a low-cost, broadly protective vaccine is needed. RG-1 is a cross-neutralizing and protective monoclonal antibody that recognizes residues 17–36 of HPV16 minor capsid protein L2. Because this epitope is highly conserved in divergent HPV types, we determined whether vaccination with HPV16 L2 17–36 peptide is broadly protective. The peptide was administered to BALB/c mice three times at monthly intervals, either alone or in the context of a synthetic lipopeptide vaccine candidate (P25-P2C-HPV) produced by linkage of the HPV peptide with a broadly recognized T helper epitope (P25) and the Toll-like receptor-2 (TLR2) ligand dipalmitoyl-S-glyceryl cysteine (P2C). In contrast to vaccination with the L2 17–36 peptide or P25-P2C alone, a potent L2-specific antibody response was generated to the P25-P2C-HPV lipopeptide when delivered either s.c. or intranasally. Sera from mice vaccinated with the P25-P2C-HPV lipopeptide neutralized not only HPV16 pseudovirions but also other evolutionarily divergent oncogenic genital (HPV18, HPV45) and cutaneous (HPV5, BPV1) types. The L2-specific antibody response depended on MHC class II, CD40, and MyD88 signaling. Additionally, vaccination with the P25-P2C-HPV lipopeptide protected mice from homologous challenge with HPV16 pseudovirions at cutaneous and genital sites and heterologous challenge with HPV45 pseudovirions. If provided in the appropriate context, therefore, HPV16 L2 17–36 might be used in a totally synthetic cross-protective HPV vaccine.
Genitotropic human papillomavirus (HPV) infections are considered the most common sexually transmitted infection in the United States (1). The major manifestations of anogenital HPV include genital warts (condyloma acuminatum) and anogenital intraepithelial neoplasia. If left untreated, a small fraction of persistent high-risk HPV infections progresses to cancer. The presence of HPV DNA has been reported in 99.7% of cervical carcinomas worldwide, indicating that HPV infection is a necessary cause of this cancer and that this disease can be prevented by prophylactic HPV vaccination (2).
Approximately 35 of the >100 subtypes of HPV are specific for the anogenital epithelium and have varying potentials for malignant transformation (3). Of the 15 oncogenic genital HPV types, HPV16 is the most common, followed by HPV18 and HPV45 (contributing ≈50%, 20%, and 10%, respectively, of cervical cancer cases worldwide). Public health efforts have successfully reduced the incidence and mortality of cervical cancer with the implementation of cervical cytology screening programs. Women who do not undergo regular screening account for most of the patients with invasive cancers (4), and cervical cancer remains the second most common cause of cancer death in women worldwide and the most prevalent cancer in women of sub-Saharan Africa, Central America, south-central Asia, and Melanesia (5). Approximately 471,000 cases of invasive cervical carcinoma are diagnosed annually (5). The disease burden resulting from the plethora of oncogenic HPV types suggests that a broadly protective vaccine would be of enormous advantage. To realize the full potential of HPV prevention globally, the vaccine must be safe and effective, stable at ambient temperature to facilitate delivery in remote locations, inexpensive to manufacture, and preferably available in a single-dose formulation. Needleless administration also might simplify and reduce the cost of vaccination.
The HPV genome is surrounded by a 60-nm, nonenveloped icosahedral capsid (6) that contains the two genetically unrelated major (L1) and minor (L2) capsid proteins. Recombinant L1 self-assembles into virus-like particles (VLPs) that are morphologically and immunologically similar to native virions (7). L1 VLP-based vaccines are highly protective against infection corresponding to the papillomavirus type used to derive the immunogen, but are ineffective against all but the most closely related HPV types (8, 9). Licensed HPV vaccines are multivalent and protect against only a subset of anogenital HPV infection. Cervarix contains L1 VLPs derived from HPV16 and HPV18, whereas Gardasil also contains HPV6 and HPV11 L1 VLPs for prevention of benign genital warts. The expense and need for refrigeration of these L1 VLP vaccines, however, renders them impractical for use in low-resource and remote areas where they are most needed. Furthermore, because these vaccines are ineffective against a significant fraction of oncogenic HPV types, costly cytologic screening of vaccinees is still necessary.
Immunization with minor capsid protein L2 peptides in animal models protects them from experimental papillomavirus infection at both mucosal and cutaneous sites (10, 11). Protection is mediated by neutralizing antibodies, and the work of several laboratories has identified cross-neutralizing epitopes (10–18). We have previously reported that monoclonal antibody RG-1, which is specific for residues 17–36 of L2 from HPV16, neutralizes both HPV16 and HPV18 and protects naïve mice from challenge with HPV16 (12). Although attempts have been made to create an L2 peptide-based vaccine (19), L2 is less immunogenic than L1 VLP, suggesting that novel vaccine strategies are necessary if such a vaccine is to be effective.
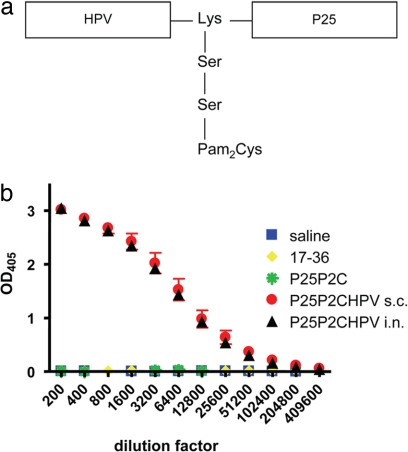
The assembly of totally synthetic and self-adjuvanting vaccine candidates that contain the target epitope together with the TLR2 ligand Pam2Cys (P2C) and a promiscuous T helper epitope (P25) in a simple branched configuration has emerged as a promising vaccine strategy even for poorly immunogenic self-epitopes (20). Herein, we examine the potential of this approach when applied to the L2 17–36 epitope as a candidate vaccine for the prevention of infection by several clinically significant HPV types (P25P2C HPV; Fig. 1a).
Fig. 1.
All components of the P25-P2C-HPV vaccine are required to generate a potent L2-specific antibody response. (a) Schematic representation of the three components of the lipopeptide construct P25-P2C-HPV. (b) Mice vaccinated with P25-P2C-HPV were bled 2 weeks after the third immunization (week 10). The titer for HPV16 L2-specific antibody was determined by HPV16 L2 17–36 peptide-based ELISA. HPV, HPV16 minor capsid protein L2 amino acids 17–36; P25, Th epitope derived from the fusion protein of the morbillivirus canine distemper virus; Lys, lysine; Ser, serine; Pam2Cys, lipid component of macrophage-activating lipopeptide 2; s.c., subcutaneous; i.n., intranasal; OD405, optical density at a wavelength of 405 nm.
Results
P25-P2C-HPV Generates Potent Immune Responses.
Vaccination with HPV16 L2 17–36 peptide in Freund's adjuvant failed to induce neutralizing antibody or protect mice against experimental viral challenge (data not shown). Given these results and the impracticability of this adjuvant for clinical use, we explored a self-adjuvanting approach to generate a potent humoral response to the HPV16 L2 17–36 peptide (20). To measure immune responses generated by vaccination with P25-P2C-HPV, sera from immunized BALB/c mice were tested by using HPV16 L2 17–36 peptide (Fig. 1b), HPV16 L2 11–200 polypeptide (data not shown), whole L2 protein (data not shown), and HPV16 L1/L2 VLP (data not shown) in ELISAs. Similar results were obtained in all cases. Preparations of P25-P2C-HPV vaccine administered either s.c. or intranasally (i.n.) (without adjuvant) generated potent antibody responses. Multiple ANOVA demonstrates that the antibody elicited by vaccination with P25-P2C-HPV vaccines was significantly different from the responses produced by control immunizations (P < 0.0001). Within group analysis of HPV16 L2 17–36, ELISAs demonstrate that these differences are significant to titers of 51,200. Analysis with Bonferoni pairwise comparisons established that HPV16 L2 17–36 peptide alone or P25-P2C alone generated antibody responses that were similar in magnitude to those achieved by the saline controls (P > 0.05).
L2 Antibody Responses to P25-P2C-HPV Depend on MyD88, Class II MHC, and CD40.
To further understand the mechanism by which P25-P2C-HPV stimulates the production of L2-specific antibodies, mice deficient for the TLR signaling mediator MyD88 were immunized with the P25-P2C-HPV lipopeptide construct. These mice failed to generate detectable antibody (Fig. 2), which is consistent with the role of MyD88 in the downstream signaling initiated by TLR-Pam2Cys ligand interactions (21). Similarly, both class II MHC and CD40-deficient mice also failed to generate L2-specific antibodies after vaccination with P25-P2C-HPV (Fig. 2), demonstrating a necessary role for T cell help in the mechanism of immunogenicity. It can be seen (Fig. 2) that C57BL/6 mice do not recognize P25 as well as do BALB/c mice, and, consistent with this observation, lower anti-L2-specific antibody is elicited in C57BL/6 animals.
Fig. 2.
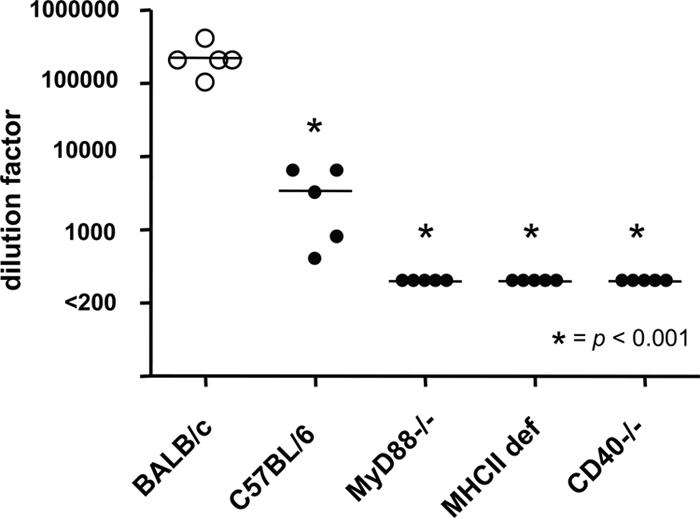
Class II MHC and MyD88 signaling are critical for an L2-specific antibody response to P25-P2C-HPV. BALB/c or C57BL/6 wild-type mice as well as MyD88−/−, class II MHC-deficient (def) or CD40−/− mice were vaccinated with P25-P2C-HPV and were subsequently bled 2 weeks after the third immunization (week 10). The titer of HPV16 L2-specific antibody was determined by HPV16 L2 17–36 peptide ELISA. L2-specific antibody was not detected in sera diluted 200 and derived from MyD88−/−, class II MHC def, or CD40−/− mice that were vaccinated with P25-P2C-HPV.
I.n. Vaccination with P25-P2C-HPV.
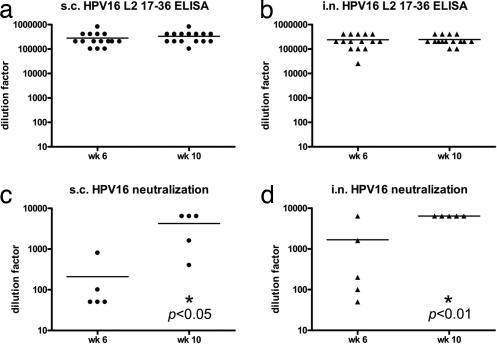
To investigate alternative, including needle-free, routes of immunization with the P25-P2C-HPV vaccine, BALB/c mice were immunized with the lipopeptide construct either s.c. or i.n. Antibody titers of sera obtained from these mice were measured 2 weeks after the second and third immunizations (Fig. 3 a and b). Simple t tests comparing the i.n. and s.c. routes of administration demonstrate the generation of equivalent L2-specific serum antibody titers in these groups (P > 0.05) at both time points. Further analysis of anti-L2 antibody titers show that both s.c. and i.n. P25-P2C-HPV-vaccinated BALB/c mice generated similar titers after two (week 6) or three (week 10) immunizations (P > 0.05) (Fig. 3 a and b).
Fig. 3.
Vaccination with P25-P2C-HPV via s.c. or intranasal routes induces high titers of L2-specific HPV16-neutralizing serum antibodies. (a–d) BALB/c mice vaccinated with P25-P2C-HPV via the s.c. (a and c) or i.n. (b and d) routes were bled 2 weeks after the second immunization (week 6) or 2 weeks after the third immunization (week 10). The titer for the HPV16 L2-specific antibody was determined by HPV16 L2 17–36 peptide-based ELISA (a and b). In vitro HPV16 neutralization titers also were determined (c and d). s.c., subcutaneous; i.n., intranasal. Statistical significance is indicated by an asterisk and P value.
Sera from P25-P2C-HPV-Vaccinated Mice Neutralize HPV16 Pseudovirions.
To test the ability of antibodies generated by immunization with the P25-P2C-HPV vaccine to neutralize homologous HPV virions, the antisera from P25-P2C-HPV mice were titrated in an HPV16 pseudovirion infectivity assay. Vaccination with P25-P2C-HPV induced equivalently high titers of HPV16-neutralizing antibody regardless of the route of administration (P > 0.05) (Fig. 3). However, in contrast to the L2 ELISA data reported above, analysis of HPV16 neutralization titers across two different time points shows that mice inoculated with P25-P2C-HPV generated significantly different immune responses after the second (week 6) and third (week 10) immunizations in both groups (P < 0.05 for s.c; P < 0.01 for i.n.) (Fig. 3 c and d). These findings suggest that the third P25-P2C-HPV immunization confers an increased neutralizing serum antibody titer for both s.c.- and i.n.-vaccinated mice.
Sera from P25-P2C-HPV-Vaccinated Mice Cross-Neutralize Multiple Heterologous HPV Pseudovirions.
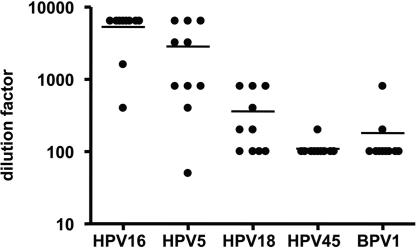
Because L2 17–36 has been previously shown to be a highly conserved epitope across multiple HPV subtypes (12), we tested the ability of mice antiserum generated by three immunizations with P25-P2C-HPV vaccine to neutralize heterologous HPV5, HPV18, HPV45, and BPV1 pseudovirions. These additional types represent different HPV species and papillomavirus genera and papillomaviruses with distinct tropisms defined by epithelial type (cutaneous vs. mucosal types) and host species as compared with HPV16. Using serial dilutions of antiserum, mean neutralization titers of 5320, 2845, 360, 110, and 180 were obtained for HPV16, HPV5, HPV18, HPV45, and BPV1 pseudovirions, respectively (Fig. 4), suggesting the potential for broad cross-neutralization.
Fig. 4.
Antibodies elicited by vaccination with P25-P2C-HPV cross-neutralizes multiple heterologous HPV pseudovirions. The ability of antiserum generated by immunization of mice with P25P2CHPV vaccine to neutralize heterologous HPV5, HPV18, HPV45, and BPV1 pseudovirions was tested. Using serial dilutions of antiserum, mean titers of 5320, 2845, 360, 110, and 180 were generated when reacted with HPV16, HPV5, HPV18, HPV45, and BPV1, respectively.
P25-P2C-HPV Vaccination Protects Mice Against Cutaneous Challenge with HPV16 and HPV45 Pseudovirions.
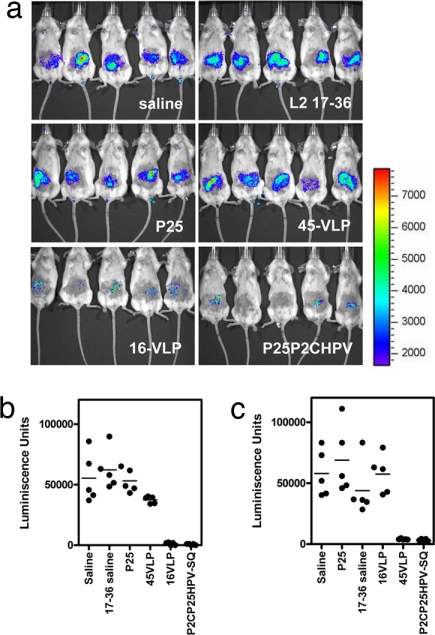
Because native HPV does not produce visible lesions in nonhuman hosts, we used an HPV pseudovirion construct carrying a luciferase reporter gene for challenge of vaccinated and control mice (12). Cutaneous infection of naive mice is detected 3 days after challenge as a bioluminescent signal after injection of the challenged mice with luciferin. Background bioluminescence is determined by using challenge with a noninfectious pseudovirus lacking L2 (data not shown). One-way ANOVA demonstrates that protection from HPV16 infection with P25-P2C-HPV and control immunizations were significantly different (P < 0.001) (Fig. 5 a and b). HPV16 L1 VLP vaccination protected mice from cutaneous challenge with HPV16 pseudovirions, whereas HPV45 L1 VLP vaccination did not. Vaccination with the 17–36 peptide alone failed to protect the mice, consistent with its failure to induce L2-specific antibody. However, P25-P2C-HPV vaccination protected mice as effectively as HPV16 L1 VLP vaccination from HPV16 pseudovirus challenge.
Fig. 5.
P25-P2C-HPV vaccination protects mice from cutaneous challenge with HPV16 and HPV45. (a–c) BALB/c mice were vaccinated s.c. three times with saline, HPV16 L2 17–36 peptide, P25 peptide, HPV45 L1 VLP, HPV16 L1 VLP, or P25-P2C-HPV and challenged on their belly with HPV16 (a and b) or HPV45 (c) pseudovirions carrying a luciferase reporter 2 weeks after the third immunization (week 10). To detect pseudoinfection, the mice were injected with luciferin 3 days after viral challenge and imaged for bioluminescence by using the IVIS 200 imaging system [see a for HPV16 challenge and supporting information (SI) Fig. S1 for HPV45 challenge]. The bioluminescence was quantified in relative light units by using Living Image 2.20 software for HPV16 challenge (b) or HPV45 challenge (c). rlu, relative light units.
HPV45 is phylogenically divergent from HPV16 (species 7 and 9, respectively, according to a recent papillomavirus classification scheme) (22), but sera of mice vaccinated with P25-P2C-HPV (which contains HPV16 L2 17–36) neutralized HPV45 pseudovirions with a mean titer of 110. To address the potential for cross-protection against a divergent HPV type and to evaluate the in vivo significance of the titer value, we challenged P25-P2C-HPV-vaccinated mice with HPV45 pseudovirions and measured the levels of infection 72 h after challenge (Fig. 5c). ANOVA demonstrates that the neutralization responses to vaccination with P25-P2C-HPV vaccines and controls were significantly different for HPV45 challenge (P < 0.001). Post hoc Bonferoni pairwise comparisons demonstrate that luminescence measured in cutaneously challenged mice vaccinated with homologous HPV45 L1 VLP and P25-P2C-HPV vaccine is significantly similar (P > 0.05). Similarly, luminescence in mice immunized with heterologous VLPs and L2 17–36 was statistically equivalent to saline controls. In summary, with the use of an animal model, saline, L2 17–36 peptide, and heterologous VLP did not protect against challenge with luciferase-expressing HPV pseudovirions, whereas homologous VLP and P25-P2C-HPV effectively prevented cutaneous HPV infection.
P25-P2C-HPV Vaccination Protects Mice Against Vaginal Challenge with HPV16.
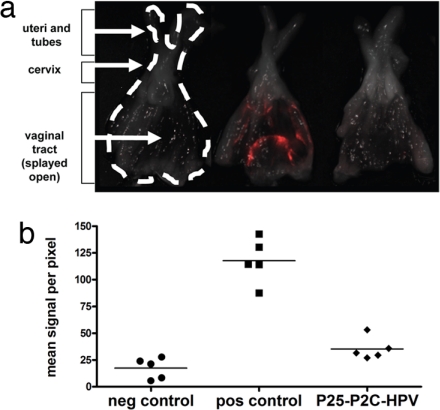
Because the primary site of HPV16-related pathology is in the genital tract, we also tested the ability of P25-P2C-HPV vaccination to protect against vaginal challenge with HPV16 pseudovirions carrying the red fluorescent protein (RFP) reporter (Fig. 6) (23). Baseline negative control genital tracts from unchallenged mice emitted mean signals of 17.4 ± 8.39 fluorescence units. In the mice challenged with RFP-expressing HPV16 pseudovirions, unvaccinated mice (positive controls) emitted a signal of 118 ± 9.24 units, whereas vaccinated mice emitted 35.4 ± 4.70 units (Fig. 6). P25-P2C-HPV vaccination demonstrated significant protection from vaginal challenge (P < 0.001, ANOVA), which was consistently observed in three independent experiments. In additional experiments, a similar level of protection was observed in mice vaccinated intranasally with P25-P2C-HPV (data not shown). Thus, vaccination with P25-P2C-HPV protects against HPV pseudovirions carrying two different reporters (luciferase and RFP) (see Figs. 5 and 6, respectively) at two different biological sites (cutaneous and vaginal) (see Figs. 5 and 6, respectively) demonstrated that the protective effect is independent of the reporter and anatomic site of infection.
Fig. 6.
P25-P2C-HPV vaccination protects mice from vaginal challenge with HPV16. BALB/c mice were vaccinated s.c. three times with P25-P2C-HPV or not (controls) and challenged in their genital tracts with HPV16 pseudovirions carrying an RFP reporter gene 2 weeks after the third immunization (except for negative control). To detect pseudoinfection, the mice were killed 3 days after viral challenge, and their genital tracts were dissected and isolated. (a) The lumen of each genital tract was imaged for red fluorescence by using a Maestro instrument. (b) The red fluorescence was quantified by using Image J software.
Discussion
The licensing of the HPV vaccines based on L1 VLPs represents a major public health milestone and demonstrates the potential to eradicate HPV-associated cancers by prophylactic vaccination. Nevertheless, significant hurdles remain for global implementation of HPV vaccination. Notably, ≈85% of cervical cancer deaths worldwide occur in developing countries (24). Most of these countries lack effective cytologic screening and intervention programs, scenarios in which HPV vaccines have the potential for the greatest impact and are needed most. In addition to safety and effectiveness, vaccine properties that might facilitate global implementation include low-cost, broad protection against all oncogenic HPV types, needle-free delivery, single dose, and stability at ambient temperature. The currently licensed HPV vaccines are costly, target only a subset of the oncogenic HPV genotypes, and require refrigeration and three doses delivered by needles.
A simplified manufacturing process (and additional market competition) might reduce the cost of an HPV vaccine. Direct chemical synthesis of vaccines has potential advantages over current biological systems for the preparation of antigen, in aspects relating to scale-up of manufacture, purification, reproducibility, stability, and regulatory requirements (25). Here, we have examined the potential of a totally synthetic and self-adjuvanting preventive HPV vaccine.
L1 VLP vaccines produce type-restricted immunity, although partial protection against the genotypes most closely related to those genotypes utilized to generate the L1 VLP vaccine has been demonstrated (8, 9). Thus, one of the complexities associated with the manufacture of the currently available licensed HPV vaccines is the need to include L1 VLPs from multiple HPV genotypes to protect against the >15 known oncogenic HPV types (26). In contrast, we have previously shown that vaccination with HPV16 L2 17–36 induces broadly neutralizing antibodies in rabbits (12). In this study, we demonstrate that vaccination with this epitope protects mice from challenge with HPV pseudovirions that are evolutionarily divergent; in this case, HPV16 and HPV45 from species 9 and 7, respectively (22). The lipopeptide vaccine candidate described here combines minimal sequences of the HPV16 L2 17–36-neutralizing epitope with a Th epitope and a TLR2 ligand. The lipopeptide acts as a self-adjuvanting immunogen, facilitating the generation of an antibody response by triggering dendritic cell maturation via the TLR2–MyD88 pathway and enhancing the class II MHC epitope presentation to and activation of CD4 T cells (21). Activation of the antibody response depends on CD40 signaling, which is consistent with the requirement for class II MHC signaling and a CD4 T cell-dependent humoral response.
P25-P2C-HPV elicits similar ELISA titers at weeks 6 and 10 after priming immunization, representing time points 2 weeks after first and second boost doses, respectively, but the accompanying neutralization titers were significantly different. This finding suggests that an L2-based ELISA is an imperfect surrogate for in vitro neutralization assays (17). This finding also has implications for potential vaccination strategies in humans and may indicate that prime-double boost may be superior to prime-single boost regimens.
Because viral capsid proteins are not expressed in the basal epithelial cells that harbor HPV infections, neither L1- nor L2-based vaccines have demonstrated therapeutic benefit (17, 27). Nevertheless, the lipopeptide approach described here has considerable potential as a preventive HPV vaccine. Furthermore, because lipopeptides also effectively induce cytotoxic T cell responses, similar constructs, including class I MHC epitopes of early HPV proteins, may provide therapeutic benefit. Lipopeptide-induced therapeutic efficacy in a tumor model has been demonstrated (19, 20).
HPV L1 VLP vaccines are injected i.m., and efforts at needle-free i.n. immunization have resulted in variable immune responses and require larger doses (28). Furthermore, licensed HPV L1 VLP vaccines require an adjuvant (either alum or MPL). In contrast, s.c. and i.n. administration of the self-adjuvanting P25-P2C-HPV vaccine each generate similarly potent and consistent serum-neutralizing antibody responses in mice, suggesting that this approach should be tested in patients. Thus, this lipopeptide approach has advantages of direct chemical synthesis, stability, and potential to be delivered without needles and generate cross-protection that would facilitate delivery in resource-limited settings.
Materials and Methods
Synthesis and Assembly of Lipidated, Epitope-Based Vaccine.
The assembly, purification, and characterization of synthetic lipopeptides has been described in detail previously (29). All peptide constructs were synthesized by using standard Fmoc chemistry. In brief, the vaccine consisted of the P25 Th epitope synthesized contiguously and N terminally to the broadly neutralizing epitope of HPV16 minor capsid protein L2 residues 17–36 (QLYKTCKQAGTCPPDIIPKV). The Th epitope (P25) has the sequence KLIPNASLIENCTKAEL and is derived from the fusion protein of the morbillivirus canine distemper virus (30). P25 and the HPV16 L2 17–36 epitope were separated in sequence by a single lysine residue. The lipid moiety Pam2Cys, corresponding to the lipid component of macrophage-activating lipopeptide 2 isolated from mycoplasma (31), was attached to the ε-amino group of the intervening lysine through two serine residues. A diagrammatic representation of the structure can be found in Fig. 1a and elsewhere (20).
Immunization of Mice.
All animal experimental work was done in accordance with Johns Hopkins Medical Institutions Animal Care and Use Committee guidelines. BALB/c and C57BL/6 (National Cancer Institute) wild-type or MyD88 (D. Golenbock, University of Massachusetts Medical Center, Amherst, MA), CD40, or class II MHC-deficient mice (The Jackson Laboratories) on a B6/129S background were used. Mice ages 4–6 weeks were immunized with P25-P2C-HPV vaccine or various controls. All vaccine and control peptides were dissolved in PBS. Mice received two booster immunizations at 4 and 8 weeks after primary immunization in the same fashion and dose.
The s.c. Inoculation.
Each mouse was administered with 20 nmol of vaccine in a total volume of 100 μl at the base of the tail.
Intranasal Inoculation.
Anesthesia induction was accomplished within 3–5 min by using a chamber filled with 2.5% isoflurane (Baxter). While anesthetized, each mouse was administered 20 nmol of lipopeptide in a total volume of 50 μl of vaccine via controlled micropipetting into the nares.
Collection and Quantification of HPV-Specific Antibody Responses.
Blood was collected from mice via the tail artery at 6 and 10 weeks after priming immunization. Presence of serum antibodies against HPV16 was assessed by ELISAs. Maxisorpflat-bottom 96-well plates (Nunc) were coated with 100 ng per well of HPV16 L2 17–36 (Sigma–Aldrich/Genosys), HPV16 L2 11–200, whole L2 protein, or HPV16 particles (see below) as described previously (12). The antibody titers were reported as the reciprocals of the highest dilution showing positive reactivity in each assay. Sera were designated ELISA-positive at a given dilution if the absolute optical density was greater than or equal to four SDs above the mean optical density of control wells in which preparations containing preimmune mouse serum were used as the primary antibody.
Production and Purification of HPV Pseudovirions.
In Vitro Neutralization of HPV Pseudovirions.
The in vitro neutralization of pseudovirions has been described in full previously (32–34). Sera from individual mice were collected and serially diluted 2-fold by using a 1:50 dilution as the initial concentration tested. Diluted sera were incubated with pseudovirions [that contain pYSEAP and express secreted alkaline phosphatase (SEAP)] at 4°C for 1 h. The pseudovirus solution was then used to infect 293TT cells. Supernatants were analyzed for SEAP activity after 54 h by using 2 M diethanolamine in water with 1 mM MgCl2 and 0.5 mM ZnCl2 adjusted to pH 9.8. Neutralization titers were reported as the reciprocals of the highest dilution showing 50% reduction in SEAP activity in each assay.
Cutaneous HPV Challenge (12).
A patch of skin on the ventral torso of anesthetized BALB/c mice was shaved with an electric razor, taking care not to traumatize the epithelium. Challenge was performed by application of 3 × 109 pYLUC-expressing pseudovirion particles (100 ng) in 10 μl of 0.6% carboxymethylcellulose (CMC; Sigma–Aldrich) to the freshly shaved epithelial patches. Three days later, mice were reanesthetized and injected with luciferin (100 μl at 7 mg/ml), and their image was acquired for 10 min with an IVIS 200 bioluminescent imaging system (Xenogen). Equal areas encompassing the site of virus inoculation were analyzed by using Living Image 2.20 software (Xenogen), and the background was determined by challenge with noninfectious HPV pseudovirions lacking L2.
Vaginal HPV Challenge (23).
Female BALB/c mice aged 6–8 weeks were pretreated 4 days before infection by s.c. injection of 3 mg of Depo-Provera (Pfizer). The mice were anesthetized by isoflurane inhalation as described above. To mimic the microtrauma of coitus, a standard plastic cytobrush (Fisher Scientific) was gently rotated 10 times within the vaginal vault. An aliquot of 4.5 × 107 HPV16 pseudovirion particles containing L1 and L2 capsid proteins and the encapsidated RFP reporter construct and suspended in 10 μl of 0.6% CMC (Sigma–Aldrich) was instilled in the vagina by using a 10-μl siliconized pipette tip. Mice were killed at 72 h after challenge, and their genital tracts (uterine horns, cervix, and vaginal tract) were dissected, isolated, and splayed opened to reveal the mucosal epithelium. Specimens were stored in PBS on ice for no more than 6 h before imaging. A Maestro (Cri) imaging device with a green excitation filter and a 580-nm long-pass emission filter was used to obtain images from 550–900 nm in 10-nm-wavelength increments. By using the spectral signature of RFP in infected tissues as signal and the background autofluorescence in uninfected tissues as noise, a spectral unmixing algorithm was applied to the composite images to determine the intensity and location of infection. The open-source software Image J was used to calculate the mean signal per pixel in a region of interest in the grayscale representation of unmixed signal.
Supplementary Material
Acknowledgments.
This work was supported by a Howard Hughes Medical Institutions Medical Student Research Training Fellowship (to H.H.A.), National Cancer Institute grants (to R.B.S.R.), and SPORE in Cervical Cancer Grants P50 CA098252 and R01 CA118790 and intramural National Institutes of Health funding (to J.N.R. and J.T.S.). This work was also supported by funds from the National Health and Medical Research Council of Australia and VacTX Pty. Ltd. (W.Z. and D.C.J.).
Footnotes
Conflict of interest statement: R.B.S.R. is a paid consultant of Knobbe, Martens, Olson, and Bear LLC. Under a licensing agreement among PaxVax Inc. and Acambis, the National Cancer Institute, and Johns Hopkins University, R.G. and R.B.S.R. are entitled to a share of royalty received on the sales of products described in this article. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800868105/DCSupplemental.
References
- 1.CDC Report to Congress. Prevention of Genital Human Papillomavirus Infection. Atlanta, GA: Centers for Disease Control; 2004. [Google Scholar]
- 2.Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman MS, Cavanagh D. Cervical cancer: Screening and prevention of invasive disease. Cancer Control. 1995;2:503–509. doi: 10.1177/107327489500200603. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 6.Baker TS, et al. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper DM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 9.Paavonen J, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 10.Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J Virol. 2002;76:9798–9805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roden RB, et al. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 12.Gambhira R, et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007;81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawana Y, et al. Human papillomavirus type 16 minor capsid protein l2 N-terminal region containing a common neutralization epitope binds to the cell surface and enters the cytoplasm. J Virol. 2001;75:2331–2336. doi: 10.1128/JVI.75.5.2331-2336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181:572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- 15.Fleury MJ, et al. Identification of type-specific and cross-reactive neutralizing conformational epitopes on the major capsid protein of human papillomavirus type 31. Arch Virol. 2006;151:1511–1523. doi: 10.1007/s00705-006-0734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawana K, et al. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine. 2001;19:1496–1502. doi: 10.1016/s0264-410x(00)00367-4. [DOI] [PubMed] [Google Scholar]
- 17.Gambhira R, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007;81:11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastrana DV, et al. Cross-neutralization of cutaneous and mucosal papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Kawana K, et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine. 2003;21:4256–4260. doi: 10.1016/s0264-410x(03)00454-7. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DC, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci USA. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 22.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JN, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Can Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 25.Jackson DC, Purcell AW, Fitzmaurice CJ, Zeng W, Hart DN. The central role played by peptides in the immune response and the design of peptide-based vaccines against infectious diseases and cancer. Curr Drug Targets. 2002;3:175–196. doi: 10.2174/1389450024605436. [DOI] [PubMed] [Google Scholar]
- 26.Roden R, Monie A, Wu TC. The impact of preventive HPV vaccination. Discov Med. 2006;6:175–181. [PubMed] [Google Scholar]
- 27.Hildesheim A, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: A randomized trial. J Am Med Assoc. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 28.Nardelli-Haefliger D, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23:3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol. 2002;169:4905–4912. doi: 10.4049/jimmunol.169.9.4905. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Walker J, Jackson DC. Identification of canine helper T-cell epitopes from the fusion protein of canine distemper virus. Immunology. 2001;104:58–66. doi: 10.1046/j.0019-2805.2001.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastrana DV, Vass WC, Lowy DR, Schiller JT. NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology. 2001;279:361–369. doi: 10.1006/viro.2000.0702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.