Abstract
CNS neurons use robust cytoprotective mechanisms to ensure survival and functioning under conditions of injury. These involve pathways induced by endogenous neuroprotective cytokines such as erythropoietin (EPO). Recently, in contrast to its well known deleterious roles, TNF has also been shown to exhibit neuroprotective properties. In the present study, we investigated the molecular mechanisms by which TNF receptor (TNFR)I mediates neuroprotection by comparing the gene expression profiles of lesioned cortex from WT and TNFRI KO mice after permanent middle cerebral artery occlusion. Several known neuroprotective molecules were identified as TNFRI targets, notably members of the Bcl-2 family, DNA repair machinery and cell cycle, developmental, and differentiation factors, neurotransmitters and growth factors, as well as their receptors, including EPO receptor (EPOR), VEGF, colony-stimulating factor receptor 1, insulin-like growth factor (IGF), and nerve growth factor (NGF). Further analysis showed that induction of EPOR and VEGF expression in primary cortical neurons after glucose deprivation (GD) largely depended on TNFRI and was further up-regulated by TNF. Also, EPO- and VEGF-induced neuroprotection against GD, oxygen-glucose deprivation, and NMDA excitotoxicity depended significantly on TNFRI presence. Finally, EPO prevented neuronal damage induced by kainic acid in WT but not TNFRI KO mice. Our results identify cross-talk between tissue protective cytokines, specifically that TNFRI is necessary for constitutive and GD-induced expression of EPOR and VEGF and for EPO-mediated neuroprotection.
Keywords: cDNA microarray, cytokine signaling, neuron death, transgenic mice, epilepsy
Cytokine-based therapeutic strategies that focus on endogenous brain proteins with direct neuroprotective properties are receiving increasing attention for the treatment of a broad spectrum of neurodegenerative diseases. Erythropoietin (EPO), which was originally described as a hematopoietic growth factor, has been shown to exert potent protective effects on CNS neurons against excitotoxic, metabolic, and ischemic cell death in vitro. In animals, it efficiently penetrates the blood–brain barrier and shows a large therapeutic window in models of experimental ischemia (1–3), traumatic injury (2, 4), NMDA- and kainate-induced excitotoxicity (5), and experimental autoimmune encephalomyelitis (2, 6). It is well tolerated and safe in humans, has been successful in a phase II trial for acute stroke (7), and is currently being assessed in early-phase trials for multiple sclerosis and schizophrenia (8, 9). Similarly, the cytokines VEGF and colony-stimulating factor (CSF) also show promise for neuroprotection. They are strongly neuroprotective in preclinical models of stroke (10, 11), spinal cord injury (12), diabetic neuropathy (13), and ALS (14, 15) and are now being assessed in humans for the management of diabetic neuropathy, ALS, and Parkinson's disease.
The proinflammatory cytokine TNF has been attributed both neurotoxic and neuroprotective effects in the CNS. The neurotoxic effects of TNF have been shown in various preclinical ischemia models, including those in which the administration of TNF exacerbated tissue injury in hypertensive rats (16), and inhibition of TNF offered cytoprotection (16, 17). In general, the deleterious effects of TNF have been associated with its inflammatory effects on glia and its procoagulative effects on the vascular system (18). However, TNF has been shown to exert direct protective effects on neurons through each of the two TNF receptors (19–21) and is necessary for ischemic preconditioning in animals and neuron cultures (19, 22). TNFRI signaling activates and sustains neuronal activity of the antiapoptotic transcription factor NF-κB (23), and TNFRII activates an Akt-dependent pathway (24), and the overall outcome of TNF–TNFR interactions is the maintenance of calcium homeostasis (19, 20), decrease of glutamate currents (25), and the induction of antiapoptotic mechanisms involving Bcl-2, Bcl-X, and FLIP (23, 26) and possibly growth factors.
The possibility that these versatile protective molecules use integrated signaling pathways in neurons has not been investigated. By applying a microarray approach to compare gene transcription in WT and TNFRI KO ischemic lesions, we identified here several neuroprotective molecules, including EPO receptor (EPOR) and VEGF, as TNFRI target genes. We further show that TNFRI is required for EPOR and VEGF expression and protective effects in primary cortical neurons after ischemic and excitotoxic injury.
Results
Microarray Analysis Reveals Mechanisms of TNFRI-Mediated Neuroprotection.
To determine whether TNFRI neuroprotective signaling, as has been defined in in vivo and in vitro models of ischemic injury (23), can interact with other known neuroprotective pathways, we performed gene expression profiling of lesions obtained from WT and TNFRI KO at 3 h (n = 2 and n = 2 respectively) and 6 h (n = 2 and n = 2) after permanent middle cerebral artery occlusion (pMCAO), as well as from corresponding regions from nonoccluded WT and TNFRI KO brain. We hybridized Atlas mouse cDNA array membranes, containing 588 or 1,185 known genes representing selected pathways, with radioactively labeled cDNA from WT and TNFRI KO ischemic lesions and corresponding nonoccluded brain regions. Genes that showed ≥2-fold difference in TNFRI KO compared with WT lesions were considered to be differentially regulated. Comparison between WT and TNFRI KO nonoccluded brain tissue revealed changes in the expression of 34 of 1,185 genes (2.9%) [supporting information (SI) Table S1]. At 3 h after occlusion, changes in the transcription of 51 of 588 genes were identified (8.7%). Notably, genes encoding for proapoptotic molecules of the Bcl-2 family (BAD, BID, BAG1, and PCD1) and DNA repair proteins (excision repair 1, ERCC-5, and translin) were mainly up-regulated, whereas signaling molecules and developmental factors (ephrin receptors B2 and B4) were down-regulated in the absence of TNFRI (Table S2). At 6 h after occlusion, transcriptional changes in 85 of 588 genes were identified (14.5%). Genes encoding proteins in three major functional categories, growth factor signaling [including EPOR, VEGF, EGF, FGFR1, CSF receptor (CSFR)1, IGF1, and NGF], differentiation (PAX6, BMP15, stromal cell-derived factor receptor 1, and TIMP3) and neurotransmission (serotonin receptors 2C and 1E and AMPA1) were down-regulated in the absence of TNFRI (Table S3). Only five genes in the abovementioned categories were constitutively differentially regulated in TNFRI KO nonoccluded brain tissue (BID, FRA-2, vABL, ubiquitin B, plasminogen activator inhibitor type I).
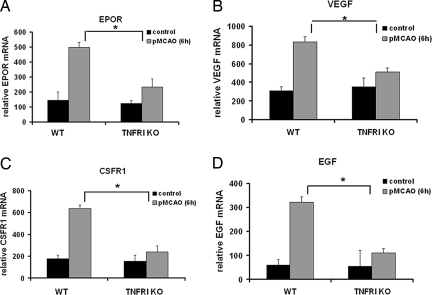
Interestingly, the genes differentially regulated by TNFRI included several molecules with well characterized neuroprotective function that have already progressed toward clinical trials for treatment of neurodegenerative conditions, specifically EPOR, VEGF, and CSFRI (7, 11, 27). The microarray results were validated by an independent analysis of the expression of four selected genes, EPOR, VEGF, CSFR-1, and EGF, by semiquantitative PCR in 6-h pMCAO lesions from WT (n = 3) and TNFRI KO (n = 3) mice. In agreement with the array results, these genes were significantly down-regulated in lesions from TNFRI KO mice (Fig. 1).
Fig. 1.
TNFRI is necessary for up-regulation of EPOR, VEGF, CSFR1, and EGF expression after pMCAO. The relative mRNA levels of EPOR (A), VEGF (B), CSFR1 (C), and EGF (D) 6 h after pMCAO were quantified by RT-PCR of cDNA samples derived from the occluded cortex of WT (n = 3) and TNFRI KO (n = 3) mice and from corresponding nonoccluded cortical areas as controls [WT (n = 2) and TNFRI KO (n = 3)]. Relative mRNA levels represent the mean densitometry values ± SEM (i.e., the gene of interest relative to actin of the same sample) from the independent samples. *, P < 0.05 for comparing the relative mRNA levels of each growth factor between WT and TNFRI KO 6 h after pMCAO.
EPO and VEGF Require Neuronal TNFRI to Mediate Neuroprotection in Vitro.
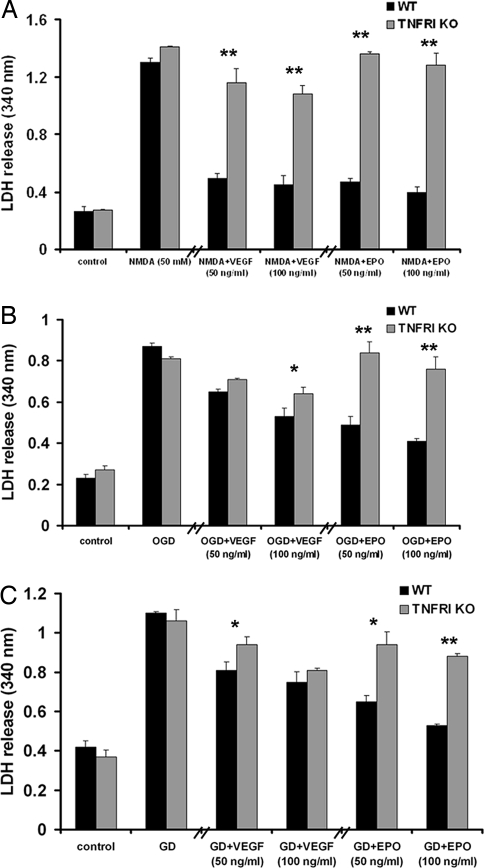
To investigate whether TNFRI is necessary for the neuroprotective functions of VEGF and EPO, we performed three models of neuron injury in vitro. First, we subjected primary cortical neurons from both strains to NMDA excitotoxicity. NMDA (50 μM) was applied to untreated and EPO- or VEGF-pretreated neurons for 24 h, and death was assessed by lactate dehydrogenase (LDH) release. NMDA-induced death was similar in WT and TNFRI KO neurons 24 h after addition of the neurotoxin. Both growth factors (at 50 and 100 ng/ml) significantly protected WT, but not TNFRI KO, neurons (Fig. 2A). Similarly, in a second model, oxygen-glucose deprivation (OGD), EPO significantly protected WT neurons but not TNFRI KO (Fig. 2B). In contrast, VEGF protected neurons from both strains against OGD, but protection was significantly stronger in WT compared with TNFRI KO neurons (Fig. 2B). In a third model, glucose deprivation (GD), EPO and VEGF significantly increased neuron survival of both WT and, to a lesser extent, TNFRI KO neurons (Fig. 2C). Taken together, these data show that TNFRI contributes significantly to EPO- and VEGF-induced neuroprotection in three diverse models of neuronal death.
Fig. 2.
EPO- and VEGF-mediated neuroprotection after ischemic, metabolic, and excitotoxic injury in vitro is TNFRI-dependent. LDH release was measured in the culture medium of WT and TNFRI KO neurons 24 h after NMDA exposure (50 μM) (A), OGD (B), and GD (C) with or without pretreatment with either EPO or VEGF at two different concentrations (50 and 100 ng/ml). The results shown represent the means ± SEM of triplicate samples from two independent experiments. *, P < 0.05; **, P < 0.001 for comparisons between WT and TNFRI KO neurons at each condition.
TNF/TNFRI Signaling Induces EPOR and VEGF Expression in Neurons.
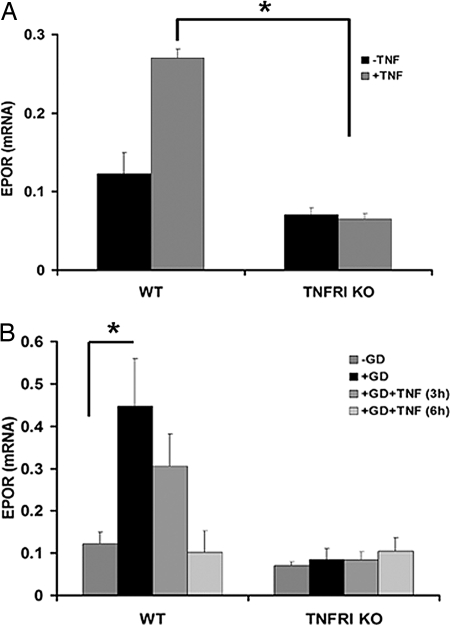
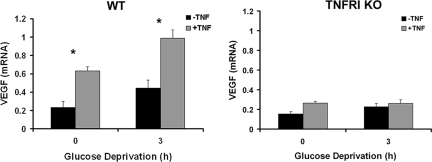
To further investigate the mechanism by which TNF/TNFRI signaling contributes to EPO- and VEGF-mediated neuroprotection, we assessed the expression of EPOR and VEGF in resting and GD-challenged WT and TNFRI KO neurons in the presence or absence of human (h)TNF, which selectively activates the murine TNFRI and not TNFRII (28). We chose the GD model, in which exogenous TNF is strongly neuroprotective, for this experiment. Treatment of WT neurons for 1 and 3 h with hTNF (10 ng/ml) had no effect on EPOR mRNA levels but significantly induced expression at 6 h, whereas it had no effect on TNFRI KO neurons at any time point studied (Fig. 3A and data not shown). EPOR protein expression was undetectable in primary cortical neurons of either strain (data not shown). GD induced the expression of EPOR (Fig. 3B) in WT, but not TNFRI KO, neurons, as measured by RT-PCR at 3 h after deprivation. Similarly, VEGF expression in WT, but not TNFRI KO, neurons was significantly induced 3 h after GD, and this induction was further enhanced by hTNF (10 ng/ml) (Fig. 4).
Fig. 3.
TNF- and GD-mediated induction of EPOR in neurons is TNFRI-dependent. The relative mRNA levels of EPOR in WT and TNFRI KO neurons were assessed by quantitative RT-PCR 6 h after hTNF treatment (A) and 3 h after GD with or without hTNF pretreatment (3 and 6 h) (B). The results shown represent the means ± SEM of triplicate samples from two independent experiments. *, P < 0.001 for comparing TNF-treated WT and TNFRI KO neurons (A); *, P < 0.02 for comparing WT neurons before and after GD (B).
Fig. 4.
hTNF- and GD-mediated induction of VEGF in neurons is TNFRI-dependent. The relative mRNA levels of VEGF were assessed by semiquantitative PCR in WT and TNFRI KO neurons 3 h after GD with or without hTNF treatment (6 h). *, P < 0.01 for comparisons of non-GD-treated and GD-treated WT neurons without or with hTNF treatment.
TNFRI and EPO in Kainate-Induced Neuronal Death.
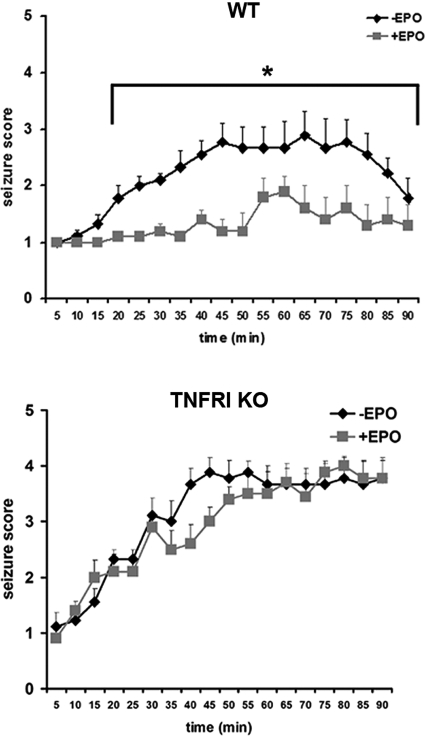
To verify the cooperation between the TNFRI- and EPO-mediated neuroprotective mechanisms in vivo, we performed kainate-induced neurotoxicity, an acute and selective model of neuronal death in which TNF and EPO have been shown independently to be neuroprotective (2, 20). We administered kainic acid (25 mg/kg) to WT (n = 21) and TNFRI KO (n = 24) mice and assessed seizure activity by using standard criteria every 5 min for 90 min. TNFRI KO mice exhibited more severe and sustained seizure activity and higher mortality rates compared with WT mice, which is consistent with the known neuroprotective effects of TNF (Fig. 5). The administration of recombinant human EPO (1,000 units/kg i.v.) 24 h before the injection of kainic acid significantly reduced seizure score in WT (n = 18), but not TNFRI KO (n = 20), mice (Fig. 5).
Fig. 5.
EPO-mediated neuroprotection against kainate-induced damage is TNFRI-dependent. Seizure responses of adult male mice to i.p. injection of kainic acid (25 mg/kg) [WT, n = 21 (A); TNFRI KO, n = 24 (B)] and after the i.v. administration of EPO (1,000 units/kg) [WT, n = 18 (A); TNFRI KO, n = 20 (B)]. *, P < 0.01 for comparison of seizure scores between untreated and EPO-pretreated WT mice at the same time point. It should be noted that five TNFRI KO mice reached a clinical score of 5 and were killed.
Discussion
CNS neurons can undergo cell death through multiple mechanisms, including apoptosis, necrosis, and autophagy, as well as others that are not yet fully characterized (29). During development and normal functioning of the adult CNS, and under diverse pathological conditions, neurons are exposed to potential death triggers. However, considering the vital importance of these cells to the organism, it is perhaps not surprising that they have developed elaborate mechanisms to protect from inappropriate neuronal losses. Although numerous effective neuroprotective molecules have been identified that have well characterized roles in individual pathways, it is impressive that many of these can protect neurons in a wide range of disease paradigms. For example, Bcl-2 mediates significant neuroprotection during ischemia (30, 31), spinal cord injury (32, 33), and excitotoxicity (34), even though neuron death is not restricted to only mitochondrial apoptosis. It is clear that a high level of integration between neuroprotective pathways must exist.
The cytokine EPO is a major neuroprotective factor that signals through the EPOR, which is expressed by most CNS cells including neurons (35). Recent evidence suggests that, in contrast to hematopoiesis, which is mediated by an EPOR homodimer, tissue protection employs a heteromeric receptor consisting of EPOR and the β common receptor used by GM-CSF, IL-3, and IL-5 (36). It is important to note that in the present study, only EPOR expression was monitored, and, therefore, a distinction between the homodimer and heteromer cannot be made. EPOR engagement by EPO induces the activation of NF-κB and Jak2 in cortical neurons and the induction of antiapoptotic molecules such as XIAP and c-IAP (37). EPO has given impressive results as a neuroprotective reagent in diverse preclinical models for neurodegeneration. EPO administered prophylactically has been shown to reduce the loss of CA1 neurons and improve learning ability after global ischemia (5), limit injury volume after pMCAO (1, 2) and blunt trauma (2), ameliorate the latency and severity of kainate-induced seizures (2), and delay the onset and reduce the clinical symptoms of experimental autoimmune encephalomyelitis (6). The direct neuroprotective effect of EPO has been demonstrated in cultures of cortical, hippocampal, and motor neurons subjected to NMDA-induced injury (5, 38), serum deprivation, and kainate-induced death (39). In this study, we show that EPOR expression and neuroprotection against in vitro ischemic-, metabolic-, and excitotoxic-mediated injury is TNFRI-dependent and that EPO cannot protect mice from kainate-induced damage in the absence of TNFRI. As far as we are aware, this gives the first evidence for crosstalk between two major neuroprotective pathways. Even though further details of this interaction are not yet available, TNFRI is a major inducer of NF-κB activity in neurons (23), whereas EPOR expression is known to be induced by NF-κB (40), suggesting that NF-κB may represent one link between the two pathways.
A second neuroprotective cytokine identified to be a TNFRI-dependent target in this study is VEGF. VEGF reduces ischemic infarction when directly administered to CNS tissue (10) and retinal neuron apoptosis in a model of ischemic reperfusion injury (41). Viral-based delivery of VEGF also has been shown to have beneficial results in a mouse model of ALS (14) and in a rat and nonhuman primate model of Parkinson's disease (42). Although VEGF is best known for its role as a growth factor for endothelial cells, its receptors are also expressed by neurons (13). VEGF prolongs the lifespan of mesencephalic neurons in culture (43) and rescues hippocampal and cortical neurons from serum deprivation (44), hypoxia (45), and glutamate-induced cell death (46). The mechanism by which TNFRI can regulate the expression of VEGF in pMCAO lesions and GD-treated neurons is not yet known. However, TNF, EPO, and VEGF have all also been implicated in ischemic tolerance (47), providing further support that their neuroprotective signaling pathways may be interrelated.
In addition to EPOR and VEGF, we detected changes in several other pathways that have been implicated in neuron death and survival in the absence of TNFRI. Genes encoding for proapoptotic members of the Bcl-2 family, BAD, BID, and PCD1, were all up-regulated by the absence of TNFRI at the 3 h time point, indicating that the mitochondrial pathway of apoptosis is triggered. By 6 h, growth factors represented the major functional category to be down-regulated in the absence of TNFRI. In addition to EPOR and VEGF, these included EGF, FGFR1, CSFR1, IGF1, and NGF, all of which have been implicated in neuroprotection (47). This finding suggests that TNF can be pivotal for orchestrating the trophic microenvironment of neuronal cells after injury. A second category of 6-h proteins included three neurotransmitters, serotonin 2C and 1F and AMPA1, and has been shown to be regulated in hippocampal neurons by TNF (48). Interestingly genes involved in cell cycle regulation and DNA excision repair system I were up-regulated in the absence of TNFRI. Cell cycle reactivation has been reported to be required for neuronal death both during development and in disease and trauma (49).
The results of this study reveal that neuronal TNFRI is required for the correct expression of genes involved in multiple pathways relevant for the protection of neurons after injury and ischemic tolerance, as well as for the physiological functioning of neurons. Finally, it is likely that the combined effect of several dysregulated neuroprotective pathways is responsible for the enhanced brain damage and increased mortality, as previously reported in TNFRI KO mice (20, 23). Given that TNF is a major proinflammatory cytokine expressed in the CNS in the acute phase of injury or infection and has the potential to induce tissue damage, it is likely that extensive crosstalk between protective pathways is necessary for neurons to ensure their survival and functioning.
Materials and Methods
MCAO Model.
Adult male WT and TNFRI KO (50) mice weighing at least 30 g were subjected to pMCAO, as described previously (1, 51, 52). During ischemia, physiological parameters remained in the normal range (body temperature, 37 ± 0.3°C; PaCO2, 40.9 ± 4.2 mm Hg; PaO2, 131.73 ± 3.97 mm Hg; 7.08 ± 0.08 pH). At 3 and 6 h after occlusion, mice were anesthetized and the occluded cortex was removed and frozen in liquid nitrogen until further processing. Surgical protocols were conducted in conformity with the institutional guidelines that comply with national and international laws and policies.
Kainic Acid-Induced Seizures.
WT and TNFRI KO mice were injected i.p. with kainic acid (A.G. Scientific) at 25 mg/kg of body weight. Kainic acid-induced seizures were scored every 5 min for 90 min (53) by using the following criteria: (i) arrest of motion; (ii) myoclonic jerks of head and neck; (iii) unilateral clonic activity; (iv) bilateral clonic activity; and (v) generalized clonic activity, loss of postural tone and continuous convulsions. Mice that reached a score of 5 were killed.
cDNA Microarray Analysis.
Total RNA was extracted from the occluded cortex of WT and TNFRI KO mice with TRIzol (Invitrogen) according to the instructions of the manufacturer. DNase-treated (Promega) RNA samples were reverse-transcribed to [α-33P]dATP-labeled cDNA. The resulting cDNA probes were hybridized to the Atlas mouse spotted nylon array membranes that contain 588 or 1,185 mouse cDNA fragments from selected known genes, according to the protocol of the manufacturer (Clontech). Membranes were exposed for 48 h on a Storm PhosphorImager Screen (Molecular Dynamics, Amersham), and hybridization patterns were quantified by using Image Quant 5.2. The signal for any given gene was calculated as the average of the signals from the two duplicate DNA spots and was normalized to the average expression of the entire array (global normalization) to allow comparisons of multiple cDNA array images. Changes in the mRNA profiles of WT and TNFRI KO lesions were considered significant if they were ≥2-fold.
RT-PCR.
RNA samples from WT and TNFRI KO occluded cortex and corresponding cortical regions from nonoccluded mice and from WT and TNFRI KO neurons before and after GD and TNF pretreatment were reverse-transcribed with M-MLV Reverse Transcriptase (Promega) and random hexamers (Roche Diagnostics). cDNAs were amplified by using the following set of primers: VEGF (forward, 5′-GCG GGC TGC CTC GCA GTC-3′; reverse, 5′-TCA CCG CCT TGG CTT GTC AC-3′), CSFR1 (forward, 5′-GAC CTG CTC CAC TTC TCC AG-3′; reverse, 5′-GGG TTC AGA CCA AGC GAG AAG-3′), EPOR (forward, 5′-GGA CAC CTA CTT GGT ATT GG-3′; reverse, 5′-GAC GTT GTA GGC TGG AGT CC-3′) and EGF (forward, 5′-AAA CAC TGC TGC AGA CAG GGG-3′; reverse, 5′-TCC TTT GTT CAA GCA CTG TAA-3′). Mouse β-actin was amplified as a loading control. Densitometric analysis was performed by using Image Quant 5.2 (Storm Scanner 600; Molecular Dynamics), and relative band intensities were determined by normalizing the densitometry value for the gene of interest to the respective actin value.
Quantitation of EPOR Expression Levels by Real-Time Quantitative PCR.
Reverse transcription (RT) was carried out at 37°C for 60 min in 50 μl of RT mixture containing 1 μg of total RNA, 400 units of reverse transcriptase (M-MLV, Invitrogen), 80 units of RNase inhibitor (RNaseOUT, Invitrogen), 0.6 mM each dNTPs (Amersham Biosciences), and 1 μg of random primers (Promega). Aliquots corresponding to 1/25 of the resulting complementary DNA (cDNA) were subjected to real-time quantitative PCR by using the TaqMan gene expression assays for mouse EPOR and for 18S rRNA as endogenous control (Applied Biosystems). All procedures, including data analysis, were performed on the ABI PRISM 7300 sequence detection system (Applied Biosystems) with the use of the software provided with the instrument. Quantification of EPOR mRNA was evaluated by using the comparative threshold cycle method, following Applied Biosystems guidelines. The results were expressed as EPOR mRNA arbitrary units, representing EPOR gene expression versus the calibrator sample. As a calibrator, we used a cDNA obtained from mouse kidney, which is known to express EPOR.
Western Blot.
Protein extracts from WT and TNFRI KO cortical neurons (100 μg) were resolved on 10–12.5% polyacrylamide gels under denaturing conditions and transferred onto nitrocellulose membranes. Blots were probed with an antibody against mouse EPOR (R&D; 1:100) and a secondary anti-mouse IgG (Jackson Immunoresearch Laboratories; 1:1,000). Antibody binding was detected by using the ECL Plus Detection system (Amersham Pharmacia).
Primary Cultures of Neocortical Neurons and Experimental Treatments.
Dissociated neocortical cell cultures were prepared from embryonic day 15 WT and TNFRI KO mice as described previously (23, 54). All experiments were performed on day 7 in vitro, in cultures containing <5% astrocytes, as determined by GFAP immunocytochemistry. GD and OGD of neuron cultures was performed as described previously (19, 55, 56). NMDA excitotoxic death was induced by exposure of neurons to 50 μM of NMDA for 24 h. Human recombinant TNF, EPO, and VEGF (R&D) were added to cultures 24 h before the onset and during the treatment. Neuronal injury in all cases was assessed by measurement of LDH released from damaged neurons into the culture medium, as described previously (57).
Statistics.
All statistical analyses were performed with Sigma Stat 2.0 for Windows (SPSS). All data are given as means ± SEM. To determine significant differences between seizure scores of WT and TNFRI KO mice before and after EPO administration, one-way ANOVA on ranks, followed by Dunn's test, was performed for pairwise comparisons at each time point because the group sizes were unequal. For comparisons of neuron viability, as assessed by LDH release, one-way ANOVA with Bonferroni correction was performed. For all RT-PCR analyses, a semiquantitative measurement of the band intensity was performed with Image Quant 5.2 (Storm Scanner 600; Molecular Dynamics) and expressed as pixel intensity per unit area. All densitometric values were normalized to their respective actin values. Relative mRNA levels were compared by using one-way ANOVA, followed by Tukey test for pairwise multiple comparisons. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Horst Bluethmann (Pharmaceutical Research Gene Technology, F. Hoffmann–La Roche Ltd., Basel, Switzerland) for TNFRI KO mice. This work was supported by the Sixth Framework Programme of the European Union, NeuroproMiSe, Grant LSHM-CT-2005-018637 (to L.P.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE8886).
This article contains supporting information online at www.pnas.org/cgi/content/full/0801447105/DCSupplemental.
References
- 1.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Brines ML, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junk AK, et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celik M, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakanaka M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnello D, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–134. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenreich H, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenreich H, et al. Erythropoietin: A candidate compound for neuroprotection in schizophrenia. Mol Psychiatry. 2004;9:42–54. doi: 10.1038/sj.mp.4001442. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenreich H, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–2588. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borlongan CV, Hess DC. New hope for stroke patients: Mobilization of endogenous stem cells. Can Med Assoc J. 2006;174:954–955. doi: 10.1503/cmaj.060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den BL, et al. Effects of vascular endothelial growth factor (VEGF) on motor neuron degeneration. Neurobiol Dis. 2004;17:21–28. doi: 10.1016/j.nbd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: Implications for neurological disorders. Semin Cell Dev Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- 14.Azzouz M, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 15.Storkebaum E, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 16.Barone FC, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 17.Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- 18.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 19.Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 20.Bruce AJ, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 21.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 22.Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Taoufik E, et al. FLIP(L) protects neurons against in vivo ischemia and in vitro glucose deprivation-induced cell death. J Neurosci. 2007;27:6633–6646. doi: 10.1523/JNEUROSCI.1091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontaine V, et al. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: Opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in. J Neurochem. 1998;70:1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- 26.Tamatani M, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 27.Lambrechts D, Storkebaum E, Carmeliet P. VEGF: Necessary to prevent motoneuron degeneration, sufficient to treat ALS? Trends Mol Med. 2004;10:275–282. doi: 10.1016/j.molmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Lewis M, et al. Cloning and expression of cDNAs for two distinct murine tumor. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicotera P, Leist M, Manzo L. Neuronal cell death: A demise with different shapes. Trends Pharmacol Sci. 1999;20:46–51. doi: 10.1016/s0165-6147(99)01304-8. [DOI] [PubMed] [Google Scholar]
- 30.Martinou JC, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 31.Shimazaki K, Urabe M, Monahan J, Ozawa K, Kawai N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: Prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7:1244–1249. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang HD, et al. Differential effects of Bcl-2 overexpression on hippocampal CA1 neurons and dentate granule cells following hypoxic ischemia in adult mice. J Neurosci Res. 1999;57:1–12. doi: 10.1002/(SICI)1097-4547(19990701)57:1<1::AID-JNR1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Wong LF, et al. Lentiviral-mediated delivery of Bcl-2 or GDNF protects against excitotoxicity in the rat hippocampus. Mol Ther. 2005;11:89–95. doi: 10.1016/j.ymthe.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Nagai A, et al. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 36.Brines M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 38.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Siren AL, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: Structural requirements and biological significance. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 41.Nishijima K, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambrechts D, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 43.Le Bras B, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 44.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor rescues HN33 neural cells from death induced by serum withdrawal. J Mol Neurosci. 2000;14:197–203. doi: 10.1385/JMN:14:3:197. [DOI] [PubMed] [Google Scholar]
- 45.Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA. Induction of vascular endothelial growth factor and hypoxia-inducible factor-1alpha by global ischemia in rat brain. Neuroscience. 2000;99:577–585. doi: 10.1016/s0306-4522(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 46.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 48.Bezzi P, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 49.Greene LA, Liu DX, Troy CM, Biswas SC. Cell cycle molecules define a pathway required for neuron death in development and disease. Biochim Biophys Acta. 2007;1772:392–401. doi: 10.1016/j.bbadis.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothe J, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 51.Welsh FA, Sakamoto T, McKee AE, Sims RE. Effect of lactacidosis on pyridine nucleotide stability during ischemia in mouse brain. J Neurochem. 1987;49:846–851. doi: 10.1111/j.1471-4159.1987.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 52.Valable S, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: Both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 53.Yang DD, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 54.Nicole O, et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: Involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci. 2001;21:3024–3033. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Culmsee C, et al. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci. 2003;23:8586–8595. doi: 10.1523/JNEUROSCI.23-24-08586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang WH, et al. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci USA. 2003;100:16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.