Abstract
Drinking arsenic-contaminated water is associated with increased risk of neoplasias of the skin, lung, bladder and possibly other sites, as well as other diseases. Earlier, we showed that human lymphoblast lines from different normal unexposed donors showed variable sensitivities to the toxic effects of arsenite. In the present study, we used microarray analysis to compare the basal gene expression profiles between two arsenite-resistant (GM02707, GM00893) and two arsenite-sensitive lymphoblast lines (GM00546, GMO00607). A number of genes were differentially expressed in arsenite-sensitive and arsenite-resistant cells. Among these, γ-glutamyltranspeptidase 1 (GGT1) and NFB inhibitor-epsilon (NFKBIE) showed higher expression levels in arsenite-resistant cells. RT-PCR analysis with gene-specific primers confirmed these results. Reduction of GGT1 expression level in arsenite resistant lymphoblasts with GGT1-specific siRNA resulted in increased cell sensitivity to arsenite. In conclusion, we have demonstrated for the first time that expression levels of GGT1 and possibly NFKBIE might be useful as biomarkers of genetic susceptibility to arsenite. Expression microarrays can thus be exploited for identifying additional biomarkers of susceptibility to arsenite and to other toxicants.
Keywords: arsenite, human, lymphoblasts, microarray, NFKBIE, GGT1
INTRODUCTION
Epidemiological evidence demonstrates a strong association between arsenic exposure and human skin, lung, and bladder cancers (IARC, 1980; NRC, 2000). Arsenite is the most likely environmental carcinogenic species (Tinwell et al., 1991). Although arsenite alone does not cause skin tumors in animals, this laboratory previously demonstrated that arsenite in drinking water enhances ultraviolet irradiation-induced skin carcinoma in the mouse (Rossman, et al, 2001), and that this cocarcinogenic effect could be blocked by an organoselenium compound and Vitamin E (Uddin et al., 2005)
Not every individual exposed to arsenic in drinking water shows clinical signs of toxicity. For example, in Bangladesh and West Bengal, 24.47% and 15.02% (respectively) of people from arsenic polluted villages exhibited skin lesions, the most characteristic clinical symptom of arsenicosis (Chowdhury et al, 2000). Peripheral lymphocytes from different donors have shown large inter-individual variations in arsenite-induced sister chromatid exchanges (SCEs) (Grossen, 1983), chromosome aberrations (Wiencke and Yager, 1992) and aneuploidy (Vega et al, 1995). Using 14 human lymphoblast lines derived from clinically unaffected Caucasian female donors as a model to study genetic susceptibility to arsenite, this laboratory reported variability to the toxic effects of arsenite, as measured by growth inhibition (Li et al., 2004). No correlation was found between sensitivity to arsenite and initial arsenite accumulation rates.
Numerous epidemiological studies have demonstrated that individuals with reduced ability to completely methylate arsenic are at increased risk of developing cancers (Steinmaus et al., 2006 and references therein). Human lymphoblasts showed negligible rates of arsenic methylation (Styblo, personal communication), allowing us the opportunity to identify other pathways important to arsenite resistance. We used oligonucleotide microarray to determine basal RNA expression profiles in arsenite-resistant and arsenite-sensitive human lymphoblast lines. Although a number of studies have compared gene expression levels in control and arsenite-treated human cells (Yih, et al., 2002, Rea, et al., 2003, Andrews et al., 2003; Su, et al., 2006), and one study compared gene expression profiles in lymphocytes from arsenic-exposed individuals with and without skin lesions (Argos et al., 2006), gene expression profiles comparing cells from normal, unexposed humans with different sensitivities to arsenite have not been reported.
In this study, we compared gene expression profiles between two arsenite-resistant and two arsenite-sensitive lymphoblast cell lines. Gamma glutamyltransferase (GGT1) and NFkB inhibitor-epsilon (NFKBIE) were identified among the genes more highly expressed in both arsenite-resistant lymphoblast lines. Knockdown of GGT1 by siRNA increased the sensitivity to arsenite in both arsenite-resistant cell lines, demonstrating the importance of glutathione (GSH) regeneration in arsenite sensitivity.
MATERIALS AND METHODS
Cell lines and Culture Conditions
Normal human lymphoblastoid cell lines (Epstein-Barr virus transformed lymphocytes from clinically unaffected Caucasian females) were purchased from Coriell Institute for Medical Research (Camden, NJ). We have previously reported variability in sensitivity toward arsenite among 14 cell lines tested (Li et al., 2004). Two arsenite-resistant lines (GM00893 and GM02707) and two arsenite-sensitive lines (GM00607 and GM00546) were selected for these studies. Cells were maintained as suspension cultures in RPMI 1640 (Gibco BRL, Rockville, MD) containing 1% L-glutamine and 15% heat-inactivated fetal bovine serum (Omega Scientific, Inc., Tarzana, CA). All cell cultures were grown at 37°C in 5% CO2 atmosphere. Figure 1 shows the effects of growth in 0.2 µM arsenite for 10 days on these cell lines.
Figure 1. Effect of 0.2µM arsenite on the growth of human lymphoblast lines from four individuals.
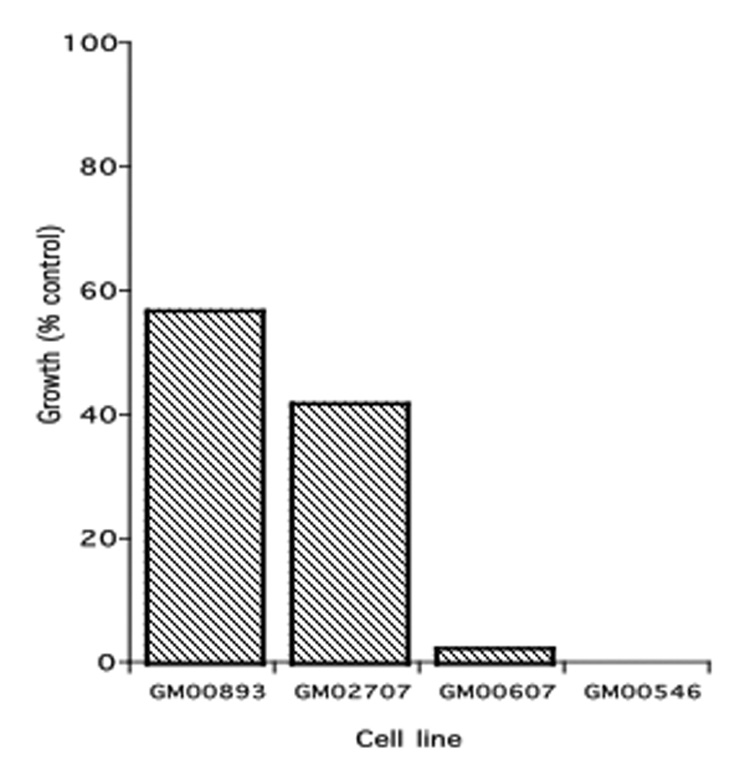
Cells were grown for 10 days in the presence and absence of arsenite. Results are expressed as % of the growth (final cell count minus initial innoculum) in the absence of arsenite.
Affymetrix microarray
The GeneChip Human Genome U133A (HG-U133A) Array (Affymetrix, Santa Clara, CA) was used for analysis of gene expression. In this array, more than 22,000 probe sets are employed to analyze the expression level of over 18,400 transcripts and variants, including more than 14,500 well-characterized human genes.
Total RNA was isolated from the cells by using RNeasy Mini Kit (QIAGEN Inc., USA) according to the manufacturer's protocol. Target labeling was performed using Affymetrix Two-Cycle cDNA Synthesis Kit according to the manufacturer’s recommendation. In brief, total RNA is first reverse transcribed using a T7-Oligo(dT) Promoter Primer in the first-strand cDNA synthesis reaction. Following RNase H-mediated second-strand cDNA synthesis, the double-stranded cDNA is purified and serves as a template in the subsequent in vitro transcription (IVT) reaction in the presence of T7 RNA Polymerase and unlabeled ribonucleotides for complementary RNA (cRNA) amplification. The unlabeled cRNA obtained from the first cycle of IVT amplification is then reverse transcribed in the first-strand cDNA synthesis step of the second cycle using random primers. Subsequently, the T7-Oligo(dT) Promoter Primer is used in the second-strand cDNA synthesis to generate double-stranded cDNA template containing T7 promoter sequences. The resulting double-stranded cDNA is then amplified and labeled using a biotinylated nucleotide analog/ribonucleotide mix in the second IVT reaction. The labeled cRNA is then cleaned up, fragmented, and hybridized to the GeneChip HG-U133A Array according to the instructions of the manufacturer.
GeneChips were scanned using a Gene Array Scanner (Hewlett Packard for Affymetrix). Analysis of microarray data was done using Affymetrix Microarray suite 5.0, GeneSpring version 4.1, GeneTraffic UNO (Stratagene, La Jolla, CA), and S-Plus 6.1 (Insightful, Seattle, WA). The data processing protocol began with the initial segment of the RMA (Robust Multiarray Analysis) (Bolstad et al., 2003) protocol as it is implemented in the GeneTraffic UNO program. The default output of the Affymetrix Microarray suit 5 was followed by the following data processing steps: (1) PM (Perfect Match) data was retained, but MM (Mismatch) data was eliminated, (2) Background noise was estimated and the signal re-estimated as the conditional mean of signal given noise (Bolstad et al., 2003), (3) Quantile normalization was performed on a set of six chips which included the four chips of this study. For purposes of comparison perhaps the simplest normalization is to rescale the data chip-by-chip so as to produce a common average for all chips. Quantile normalization consists not only of rescaling each chip to a common average, but in chip-by-chip transformations that produce a common distribution for all chips. This is achieved by averaging the quantiles of the individual chip distributions over all the chips. (Bolstad et al., 2003).
Differentially expressed genes were identified as follows: For each oligonucleotide each of the two sensitive cell lines’ normalized expression was divided by the same quantities of each of the two resistant cell lines, resulting in four ratios for each oligonucleotide. A gene was deemed as differentially expressed if for the nucleotide representing it if either all four ratios exceeded the two-fold criterion, or if all four ratios were less than one half. Identification and function of the genes was based on the Entrez Gene database (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene).
RT-PCR
For verification of the differential expression, RT-PCR analysis was done, using β-actin as housekeeping gene for normalization. Total RNA was isolated from cells with TRI Reagent according to manufacturer's protocol (Molecular Research Center, Inc., Cincinnati, OH). cDNA synthesis and PCR amplification were performed in a single tube using SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen Corp., Carlsbad, CA) following the manufacturer's recommendations.
The primers for NFKBIE were: 5’-CAGCACTCCATCTGGCTGTA for forward and 5’-CTAGGGCACCAGAAGAGCAC for reverse.
The primers for GGT1 were: 5'-CAGCACCATCAACCTCTACTTTG for forward and 5'-TATTGTGCTGCTCTGCTGCTCAC for reverse.
The primers for β-actin were: 5'-CAGATCATGTTTGAGACCTTCAACAC for forward and 5'-TCTGCGCAAGTTAGGTTTTGTCAAG for reverse.
Primers were purchased from Sigma Genosys (The Woodlands, TX). PCR parameters were: for cDNA synthesis, 55° C for 30 min; for denaturation, 94° C for 2 min; for PCR amplification, 94° C for 15 sec (denature), 56° C (GGT1) or 54°C (NFKBIE) for 30 sec (anneal), 68° C for 1 min (extend). PCR amplification was performed for 24 cycles (GGT1 and NFKBIE) and 18 cycles (β-actin). A linear relationship between input RNA and final product has been detected with RNA dilution series amplified at several different cycles numbers. cDNA was tested in 1% agarose gel electrophoresis followed by quantitation on a ChemiImager 4400 (Alpha Innotech. Corp.).
siRNA transfection
GGT1 siRNA (human) (Cat# sc-35473), control siRNA (Cat# sc-37007), and siRNA transfection kit (Cat# sc-45064) were purchased from Santa Cruz Biotechnology, Inc. (Santa-Cruz, CA). Transfection of cells with siRNA was performed according to manufacturer's recommendations. Briefly, prior to transfection suspension cells (2×105 cells per transfection per well) were collected by centrifugation at 400g for 5 min. The pellet was washed with transfection medium and resuspended with siRNA transfection mixture and transferred to 12 well tissue culture plate. After a 5-hr incubation, equal volumes of 2X growth medium containing various concentrations of sodium arsenite were added to each well. After 24 hr, cytotoxicity was assessed.
Short-term cytotoxicity measurements
A freshly prepared solution of sodium arsenite (Sigma, St. Louis, MO) was added to the cells to give final concentrations of 0.1–1.0 µM. We have previously shown that, because of delayed apoptosis, clonal survival assays are the most accurate method of assessing cytotoxicity induced by arsenite, followed by MTT or neutral red assays performed 72 hours after exposure (Komissarova et al., 2005). Because lymphoblasts grow in suspension, cytotoxicity cannot easily be measured by these methods. For convenience, and because we are interested in comparative rather than actual cytotoxicity, we have chosen to assess cytotoxicity here by counting cells unstained with trypan blue after a 24-h exposure to arsenite, knowing that this is a measure of necrosis and does not detect cells undergoing apoptosis (Komissarova et al, 2005).
RESULTS
Gene expression levels
To identify genes whose basal expression levels play a role in arsenite sensitivity, Affymetrix HG-U133A microarray was used to compare the gene expression profiles of arsenite-resistant (GM00893 and GM02707) and arsenite-sensitive (GM00607 and GM00546) human lymphoblast cell lines. We identified 19 genes whose expression levels were at least two-fold higher in both arsenite-resistant cells compared with both arsenite-sensitive lines (Table 1) and 21 genes whose expression levels were at least two-fold higher in both arsenite-sensitive lines compared with both arsenite-resistant lines (Table 2). Among those genes listed in Table 1, we chose to focus on GGT1, involved in glutathione metabolism, and NFKBIE, an inhibitor of NFkB.
Table 1.
Genes more highly expressed in resistant cell lines
| Gene Symbol | GenBank Accession Number(s) | Name, Other Symbols, and Functions |
|---|---|---|
| BHLHB2 | BG326045 | Basic helix-loop-helix protein; DEC1; Negative transcriptional regulator; anti-apoptotic; adaptation to hypoxia; role in differentiation |
| CAPG | NM_001747 | Capping protein (actin filiment), gelsolin-like; control of motility in non-muscle cells |
| CCL22 | NM_002990 | Small inducible cytokine subfamily A (Cys-Cys), member 22 Chemotactic; Role in trafficking of T lymphocytes |
| DDX17 | AW188131 | Dead box 17; RNA helicase; implicated in translesion initiation, splicing and ribosome assembly; Role in growth regulation and transcriptional co-activator or co-repressor |
| NM_030881 | ||
| U59321 | ||
| EMR1 | NM_001974 | Egf-like module containing, mucin-like, hormone receptor-like; Has 7 trans-membrane domains |
| GGT1 | NM_013421 | Gamma-glutamyltransferase 1; transfers glutamyl moiety from GSH to a variety of receptors; hydrolyzes gamma-glu-cys bond |
| NM_013430 | ||
| HLA-DMA | X76775 | Histocompatability complex, class II, DM-alpha, expressed in antigen-presenting cells |
| HLA-DQB1 | AI583173 | Histocompatability complex, class II, DR-beta 4, expressed in antigen-presenting cells |
| HLA-DRB4 | BC005312 | Histocompatability complex, class II, DQ-beta 1, expressed in antigen-presenting cells |
| IFITM2 | NM_006435 | Interferon induced transmembrane protein 2 (I-8D), function unknown |
| IL32 | NM_004221 | Natural killer cell transcript 4; Interleukin 32; induces TNF alpha |
| MYLIP | NM_013262 | Myosin regulatory light chain interacting protein; inhibits neurite outgrowth |
| NFKBIE | NM_004556 | IkB-ε; IKBE, cytoplasmic sequestration of and inhibitor of NFkB transcription factor |
| QPRT | NM_014298 | Quinolinate phosphoribosyltransferase; NAD biosynthesis |
| RAB31 | AF183421 | G protein, member of RAS oncogene family; GTPase-mediated signal transduction |
| BE789881 | ||
| NM_006868 | ||
| RGS1 | NM_002922 | Regulator of G-protein signalling 1; increases rate of conversion of GPT to GDP which reduces signaling |
| SOX9 | AI382146 | Sex-determining region Y box 9; SRY; regulates transcription |
| NM_000346 | ||
| SPIB | NM_003121 | Spi-B transcription factor (ETS family) |
| TSPYLS | AI096375 | TSPY-like 5 (function unknown) |
Table 2.
Genes more highly expressed in sensitive cell lines
| Gene Symbol | GenBank Accession Number(s) | Name, other symbols, and Functions |
|---|---|---|
| ARHGEF12 | NM_015313 | Rho guanine exchange factor 12; signaling |
| CREG1 | NM_003851 | Repressor of E1A-stimulated genes; may contribute to transcriptional control of growth and differentiation |
| EIF5A | NM_001970 | Translation initiation factor 5A; may regulate p53 |
| FEZ1 | NM_005103 | Fasciculation and elongation protein zeta 1; axon bundling and elongation |
| FLJ20245 | NM_017723 | unknown |
| IGF2R | BG031974 | Insulin-like growth factor 2 receptor; CD222; receptor for IGFII and M6P-containing proteins; involved in activation of latent TGF beta |
| IGSF4 | NM_014333 | Immunoglobulin superfamily, member 4; role in cell adhesion; tumor suppressor |
| MEIS2 | NM_020149 | Meis (mouse) homolog 2 |
| METTLTA | NM_014033 | Methyltransferase-like |
| MYH10-like | AI382123 | Similar to myosin heavy chain 10, non-muscle |
| AK026977 | ||
| NRN1 | NM_016588 | Neuritin; neurite growth |
| NUP 160 | D83781 | Nucleoporin; part of nuclear pore complex; mediates nucleoplasmic transport; promotes spindle assembly |
| PIK3R3 | BE622627 | Phosphoinosotide-3-kinase regulatory subunit (p55 gamma); signaling function |
| PLS3 | NM_005032 | Plastin 3 (T-isoform); actin-binding protein |
| PRKD3 | NM_005813 | Protein kinase D3; PKC-NU; protein phosphorylation; signaling function |
| PRKDC | U47077.5 | DNA-dependent protein kinase (with Ku); DNAPK; XRCC7; role in DNA repair and recombination |
| RANBP2 | D42063 | RAN-binding protein 2; localizes to nuclear pore complex; scaffold protein; role in sumoylation |
| RHOBTB3 | N21138 | Rho GPTase; KIAA0878; signaling function |
| NM_014792 | KIAA0125 | |
| ROCK2 | AL049383 | Rho-associated, coiled-coil containing protein kinase 2 |
| T | NM_003181 | Brachyury-like; TFT; transcription factor binding to palindromic T-site; role in differentiation |
| TUBB2A | NM_001069 | Tubulin beta; forms microtubules; modulates Notch signaling |
Validation of NFKBIE and GGT1 expression levels by RT-PCR
RT-PCR analysis supports the results of the oligonucleotide arrays. Expression levels of NFKBIE in both arsenite-resistant lymphoblast lines (GM00893 and GM02707) were approximately the same, and were about twice those of the two arsenite-sensitive lines (GM00607 and GM00546) (Fig. 2). The expression levels of GGT1 were also greater in the arsenite-resistant lines, and the differences between expression levels in the resistant and sensitive lines were larger (about 4 to 6-fold higher in resistant compared with sensitive lines) (Figure 3). Because of the greater differences in GGT1 expression levels, as well as the known importance of GSH metabolism for arsenic toxicology, we chose to determine the effects of GGT1 expression on arsenite sensitivity.
Figure 2. NFKBIE expression in arsenite-resistant and -sensitive lymphoblasts.
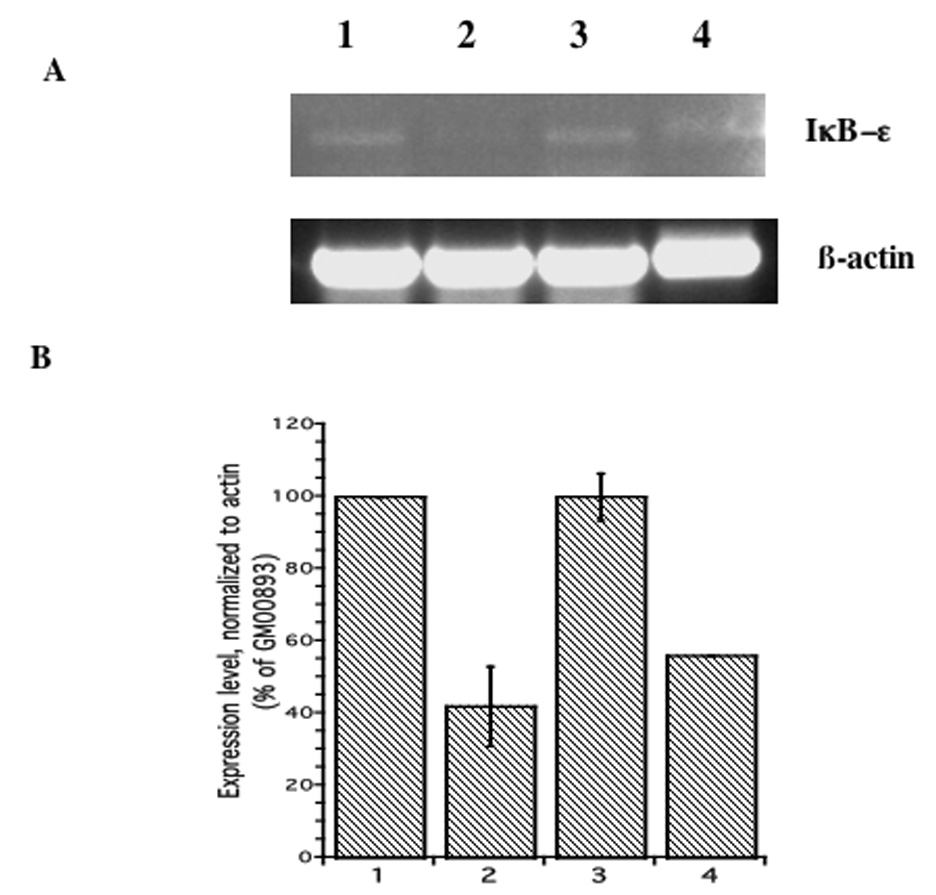
A. NFKBIE transcript was detected by RT-PCR using primers specific for NFKBIE, as described in Methods. Lane 1: GM00893 (resistant); Lane 2: GM00607 (sensitive); Lane 3: GM02707 (resistant); Lane 4: GM00546 (sensitive). B. Expression levels normalized to β-actin. Data represent the mean plus/minus standard error of the mean of three experiments.
Figure 3. GGT1 expression in arsenite-resistant and -sensitive lymphoblasts.
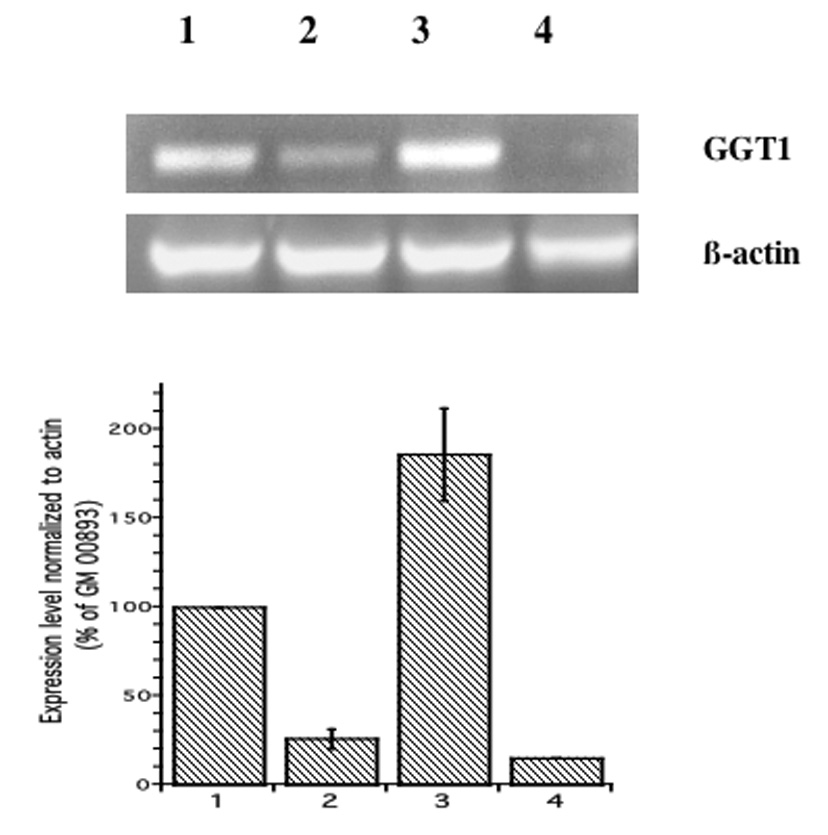
A. GGT1 transcript was detected by RT-PCR using primers specific for GGT1, as described in Methods. Lane 1: GM00893 (resistant); Lane 2: GM00607 (sensitive); Lane 3: GM02707 (resistant); Lane 4: GM00546 (sensitive). B. Expression levels normalized to β-actin. Data represent the mean plus/minus standard error of the mean of three experiments.
Analysis of GGT1 expression in arsenite-resistant lymphoblasts after GGT1 specific siRNA transfection
RNA interference using GGT1-specific siRNA was used to selectively knock down GGT1 function in the arsenite-resistant lymphoblasts, GM00893 and GM02707 (see Materials and Methods). RT-PCR analysis was performed to assess the knock down efficiency 24 hr after transfection. Transfection with GGT1-specific siRNA caused GGT1 transcripts to decrease to 38% and 31% of the levels in cells transfected with the control siRNA in GM02707 and GM00893, respectively (Figure 4).
Figure 4. Knock-down of GGT1 expression in arsenite-resistant lymphoblasts GM02707 and GM00893.
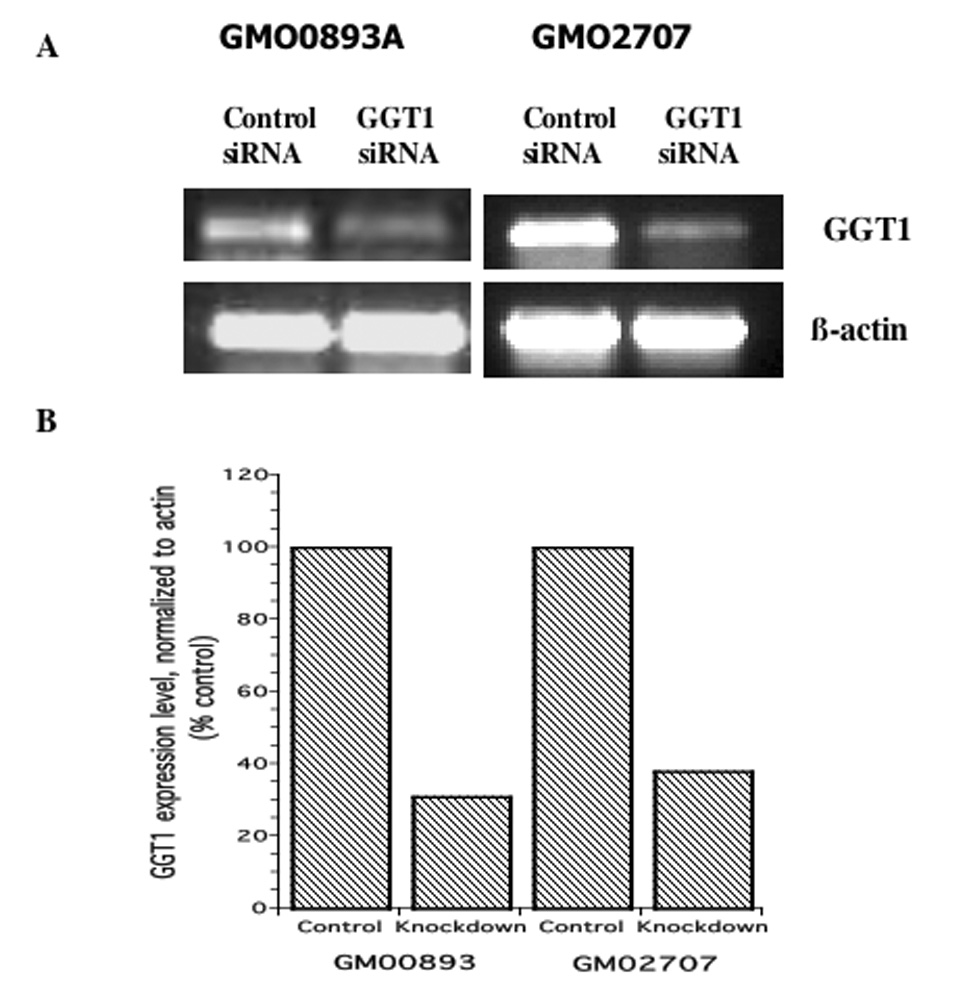
A. GGT1 transcript detected by RT-PCR; B. Expression levels normalized to β-actin.
Effect of reduced level of GGT1 expression on arsenite resistance
To determine if decreased expression of GGT1 affects arsenite resistance, GM02707 and GM00893 lymphoblasts were grown in the presence of different concentrations of arsenite (0.1–1µM) after transfection with GGT1-specific (or control) siRNA. After a 24-h exposure to arsenite, we observed a dose-dependent increase in cytotoxicity in the knocked-down cells, starting with the lowest dose (0.1 µM) (Figure 5). In contrast, lymphoblasts transfected with control siRNA and grown in 0.1µM arsenite showed significant increases in the number of living cells (147% for GM02707 and 219% for GM00893), reflecting cell growth. A decrease in viability occurred only after growth in 1µM arsenite.
Figure 5. Knock-down of GGT1 in arsenite-resistant lymphoblasts sensitizes cells to arsenite.
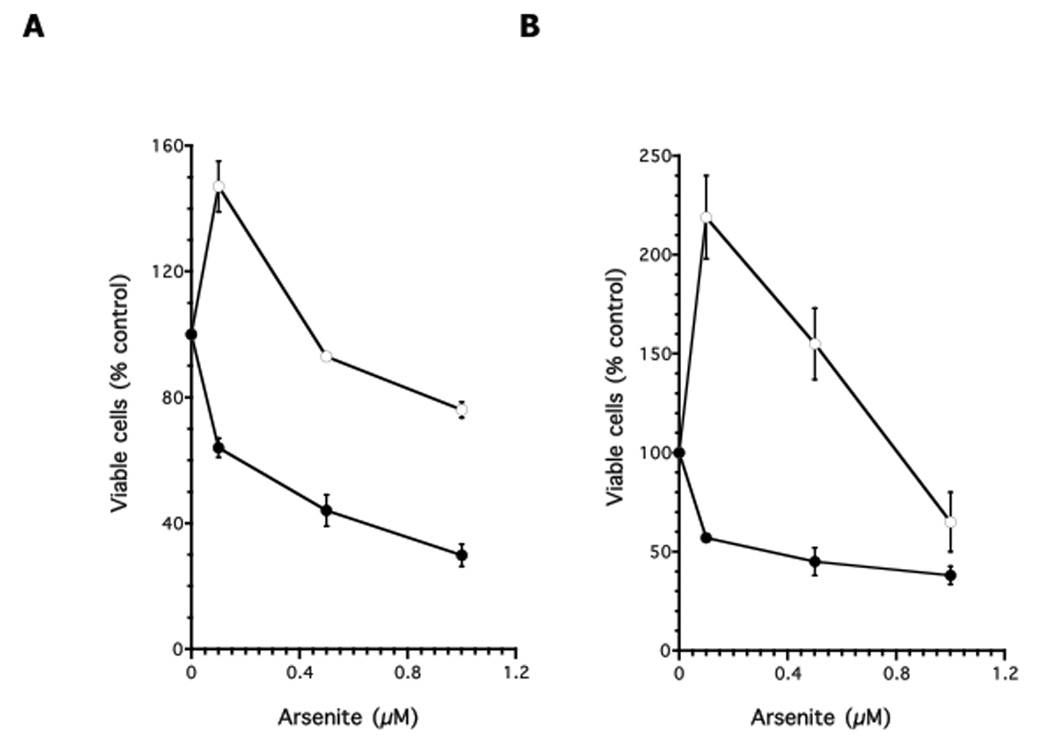
Open circles: lymphoblasts transfected with control siRNA; Closed circles: lymphoblasts transfected with GGT1 siRNA. A: GM02707; B: GM00893 Each assay was performed in duplicate. Data were expressed as the mean plus/minus standard error of the mean.
DISCUSSION
An analysis of basal gene expression levels in arsenite-resistant and arsenite-sensitive lymphoblasts identified 19 genes with greater expression levels (at least two-fold) in the two arsenite-resistant cell lines and 21 genes with grater expression levels in the two arsenite-sensitive cell lines (Table 1 and Table 2, respectively). The fact that a number of these genes were identified as differentially expressed with more than one chip sequence (DDX17, GGT1, RAB31, SOX9, MYH10-like, RHOBTB3) adds confidence to the accuracy of these calls. We selected two genes with higher expression levels in the resistant lymphoblasts for further study: GGT1, encoding γ-glutamyltransferase, important in glutathione metabolism and NFKBIE, encoding an inhibitor of the transcription factor NFκB. The differential expression of both genes was verified using RT-PCR analysis. Expression levels of NFKBIE were two fold higher in both arsenite resistant lymphoblasts, compared to both sensitive lines, and even greater differences were seen for expression of GGT1.
Interference by arsenite with signal transduction pathways involving transcription factor NFκB has been reported (Hu et al., 2002, Liao et al, 2004, Barchowsky et al., 1996). NFKBIE belongs to the family of NFκB inhibitory proteins (IκBs) that interact with NFκB in unstimulated cells. In response to stimuli, the IkBs are rapidly phosphorylated and degraded by ubiquitin-dependent proteolysis, resulting in the release of free NFκB dimers, which translocate to the nucleus to induce transcription of target genes (Karin and Delhase, 2000, Beinke and Ley, 2004). Alterations affecting the expression or function of the IkB family members have been observed in several cancers (Rayet and Gelinas, 1999). Recently, inactivating NFKBIE mutations were found in Hodgkin/Reed-Steinberg cells of classical Hodgkin lymphoma (Emmerich, et al. 2003). NFkB plays an important role in regulating the expression of anti-apoptotic proteins (c-IAP-1/2, AI, Bcl-2, Bcl-XL) and the cell-cycle regulator cyclin D1 (Karin and Lin, 2002, Karin, et al., 2002). Paradoxically, increasing NFKBIE expression might be expected to make activation of NFkB more difficult, thereby reducing expression of antiapoptotic proteins and rendering cells more sensitive to arsenite, not less. Whether the increased expression of NFKBIE observed in arsenite-resistant lymphoblasts is important for arsenite susceptibility requires further study.
To examine the role of GGT1 in arsenite resistance, we selectively down regulated its expression using siRNA in the arsenite-resistant lymphoblast lines GM02707 and GM00893 to 38% and 31% (respectively) of the level in control cells (Fig. 4). Down regulation of GGT1 resulted in increased sensitivity to arsenite in both cell lines (Fig. 5). It has been known for some time that glutathione (GSH) is important in arsenite detoxification. Arsenite toxicity increased markedly when cellular GSH was depleted with a GSH synthase inhibitor (Lee et al., 1989). At high arsenite concentrations, the mechanism underlying arsenic toxicity may be oxidative stress induced via enhanced generation of ROS-reactive oxygen species (Barchowski et al., 1996; Ding et al., 2000, Li, et al., 2002, Lee et al., 2005). GSH reacts with ROS and with toxic compounds to form GSH conjugates (reviewed in Anderson, 1998).
An important mechanism for protection of cells against toxicants is efflux of the toxicant from the cell. The efflux of arsenite from cells is mediated by glutathione transferase Pi (GST-Pi) and some members of the multi-drug-associated protein family (MRP). GST-Pi catalyzes the formation of the arsenic(III)-triglutathione complex As(GS)3, which can also form nonenzymatically (Wang and Lee, 1993; Delnomdedieu et al., 1994; Scott et al., 1993). As(GS)3 is extruded from cells by one or more MRP transporters (Kala et al., 2000; Vernhet et al., 2001; Leslie et al., 2004). As(GS)3 is also a substrate of yeast and Leishmania MRP homologs (Ghosh et al., 1999; Dey et al., 1996). During this process, GSH is exported from the cell. Most cells cannot take up extracellular GSH. It first must be hydrolyzed into its constituent amino acids, which can then be taken up by the cell (Meister et al, 1981).
GGT1 is a membrane-bound protein whose active site is on the outer surface of the cell membrane (reviewed in Hanigan, 1998). It can cleave the γ-glutamyl bond of GSH or GSH conjugates to produce cysteinylglycine (Cys-Gly) or Cys-Gly conjugates, respectively (McIntyre and Curthoys, 1979). These in turn may be cleaved by Cys-Gly hydrolase (McIntyre and Curthoys, 1982). The resulting amino acids can enter the cell to regenerate GSH. In this way, GGT activity protects cells from GSH depletion and oxidant-induced cytotoxicity (Rajpert-De Meyts, et al., 1992; Karp, 2001). Overexpression of GGT has been observed in human tumor cells resistant to chemotherapeutic drugs (reviewed in Hanigan, 1998).
Table 1 shows some other candidate genes whose expression is higher in arsenite-resistant cells and thus might be worth exploring as biomarkers of susceptibility. For example, DDX17 encodes a DEAD-box RNA helicase that is thought to be important in DNA repair. QPRT encodes quinolinate phosphoribosyltransferase and is a key enzyme in NAD synthesis. NAD is depleted during genotoxic stress when PARP becomes activated and catalyzes poly(ADP ribose) synthesis (Virag and Szabo, 2002).
In conclusion, we have demonstrated the utility of performing gene expression analysis using normal human lymphoblasts that vary in sensitivty to a toxicant as a method for identifying candidate biomarkers of sensitivity to that agent. In addition, we demonstrated for the first time that expression levels of GGT1 and possibly NFKBIE might be useful as biomarkers of genetic susceptibility to arsenite.
ACKNOWLEDGEMENTS
This work was supported by United States Public Health Service Grant P42 ES10344, and in part by the NYU/NIEHS Center ES000260 and the NYU Cancer Center CA016087.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson M. Glutathione: an overview of biosynthesis and modulation. Chemico-Biological Interactions. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Warren AJ, Barchowsky A, Temple KA, Klei L, Soucy NV, O’Hara KA, Hamilton JW. Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ. Health Perspect. 2003;111:825–838. doi: 10.1289/ehp.111-1241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Kibriya MG, Parvez F, Jasmine F, Rakibuz-Zaman M, Ahsan H. Gene expression profiles in peripheral lymphocytes by arsenic exposure and skin lesion status in a Bangladeshi population. Cancer Epidemiol. Biomarkers Prev. 2006;15:1367–1375. doi: 10.1158/1055-9965.EPI-06-0106. [DOI] [PubMed] [Google Scholar]
- Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radical Biol. Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-kB1 and NF-kB2 in immune cell biology. Biochem. J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Åstrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D. Groundwater arsenic contamination in Bangladesh and West Bengal, India (comment) Environ. Health Perspect. 2000;108:393–397. doi: 10.1289/ehp.00108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnomdediou M, Basti MM, Otvos JD, Thomas DJ. Reduction and binding of arsenate and dimethylarsenate by glutathione: a multinuclear magnetic resonance study. Chem.-Bio. Interact. 1994;90:139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Dey S, Ouellette M, Lightbody J, Papadopoulou B, Rosen BP. An ATP-dependant As(III)-glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc. Natl. Acad. Sci. USA. 1996;93:2192–2197. doi: 10.1073/pnas.93.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Shi X, Castranova V, Vallyathan V. Predisposing factors in occupational lung cancer: Inorganic minerals and chromium. J. Environ. Pathol. Toxicol. Oncol. 2000;19:129–138. [PubMed] [Google Scholar]
- Emmerich F, Theurich S, Hummel M, Haeffker A, Vry MS, Dohner K, Bommert K, Stein H, Dorken B. Inactivating I kappa B epsilon mutations in Hodgkin/Reed-Sternberg cells. J. Pathol. 2003;201:413–420. doi: 10.1002/path.1454. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Shen J, Rosen BP. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossen PE. Arsenic and SCE in human lymphocytes. Mutat. Res. 1983;119:415–419. doi: 10.1016/0165-7992(83)90194-x. [DOI] [PubMed] [Google Scholar]
- Hanigan MH. γ-Glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis. Chemico-Biological Interactions. 1998;111–112:333–342. doi: 10.1016/s0009-2797(97)00170-1. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NFkB DNA binding activity and related gene expression. Toxicol. Letters. 2002;133:33–45. doi: 10.1016/s0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- IARC (International agency for Research on Cancer) IARC monograph on the evaluation of carcinogenic risk of chemicals to human, v.23. Lyon, France: World Health Organization; Some metals and metallic compounds. 1980
- Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP/cMOAT transporter and As-glutathione complex formation are required for biliary excretion of As. J. Biol. Chem. 2000;275:33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Zhi-Wei L. NF-kB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Sem. Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Karp DR, Shimooku K, Lipsky PE. Expression of gamma-glutamyl transpeptidase protects ramos B cells from oxidation-induced cell death. J. Biol. Chem. 2001;276:3798–3804. doi: 10.1074/jbc.M008484200. [DOI] [PubMed] [Google Scholar]
- Komissarova EV, Saha SK, Rossman TG. Dead or dying: The importance of time in cytotoxicity assays using arsenite as an example. Toxicol. Appl. Pharmacol. 2005;202:99–107. doi: 10.1016/j.taap.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Lee PC, Ho IC, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol. Sci. 2005;85:541–550. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- Lee TC, Wei ML, Chang WJ, Ho IC, Lo JF, Jan KY, Huang H. Elevation of glutathione levels and glutathione S-transferase in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev. Biol. 1989;25:442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Haimeur A, Waalkes MP. As transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a triglutathione conjugate is required. J. Biol. Chem. 2004;279:32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- Li M, Cai Jiu-Feng, Chiu J-F. Arsenic induces oxidative stress and activates stress gene expression in cultured lung epithelial cells. J. Cell. Biochem. 2002;87:29–38. doi: 10.1002/jcb.10269. [DOI] [PubMed] [Google Scholar]
- Li P, Uddin AN, Liu Z, Mukhopadhyay R, Komissarova EV, Rosen BP, Rossman TG. Variability in sensitivity to arsenite does not correlate with arsenic accumulation rate in normal human lymphoblasts. Mol. and Cell. Biochem. 2004;255:79–85. doi: 10.1023/b:mcbi.0000007263.27349.ae. [DOI] [PubMed] [Google Scholar]
- Liao W-T, Chang K-L, Yu Ch-L, Chen G-Sh, Chang LW, Yu H-S. Arsenic induces human keratinocyte apoptosis by the FAS/FAS ligand pathway, which correlates with alterations in nuclear factor-kappa B and activator protein-1 activity. J. Invest. Dermatol. 2004;122:125–129. doi: 10.1046/j.0022-202X.2003.22109.x. [DOI] [PubMed] [Google Scholar]
- McIntyre TM, Curthoys NP. Renal catabolism of glutathione: Characterization of a particulate rat renal dipeptidase that catalyzes the hydrolysis of cysteinylglycine. J.Biol. Chem. 1982;257:11915–11921. [PubMed] [Google Scholar]
- McIntyre TM, Curthoys NP. Comparison of the hydrolytic and transfer activities of rat renal γ-glutamyltranspeptidase. J. Biol. Chem. 1979;254:6499–6504. [PubMed] [Google Scholar]
- Meister A, Tate SS, Greffith OW. γ-Glutamyltranspeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Arsenic in drinking water. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Rajpert-De Meyts E, Shi M, Chang M, Robinson TW, Groffen J, Heisterkamp N, Forman HJ. Transfection with γ-glutamyl transpeptidase enhances recovery from glutathione depletion using extracellular glutathione. Toxicol. Appl. Pharmacol. 1992;114:56–62. doi: 10.1016/0041-008x(92)90096-b. [DOI] [PubMed] [Google Scholar]
- Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH. Global alteration of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis. 2003;24:747–756. doi: 10.1093/carcin/bgg010. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Scott N, Hatidlid KM, MacKensie NE. Reaction of As(III) and As(V) species with glutathione. Chem. Res. Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Bates M, Yuan Y, Kalman D, Atallah R, Rey Omar A, Biggs M, Hopenhayn C, Moore L, Hoang B, Smith A. Arsenic Methylation and Bladder Cancer Risk in Case–Control Studies in Argentina and the United States. J. Occup. Environ. Med. 2006;48:478–488. doi: 10.1097/01.jom.0000200982.28276.70. [DOI] [PubMed] [Google Scholar]
- Su PF, Hu YJ, Ho IC, Cheng YM, Lee TC. Distinct gene expression profiles in immortalized human urothelial cells exposed to inorganic arsenite and its metabolites. Environ Health Perspect. 2006;114:394–403. doi: 10.1289/ehp.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinwell H, Stephens SC, Ashby J. Arsenite as the probable active species in the human carcinogenicity of arsenic: mouse micronucleus assay on Na and K arsenite, orpiment, and Fowler's solution. Environ. Health Perspect. 1991;95:205–210. doi: 10.1289/ehp.9195205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin AN, Burns FJ, Rossman TG. Vitamin E and organoselenium prevent the cocarcinogenic activity of arsenite with solar UV in mouse skin. Carcinogenesis. 2005;26:2179–2186. doi: 10.1093/carcin/bgi180. [DOI] [PubMed] [Google Scholar]
- Vega L, Gonsebatt ME, Ostrosky-Wegman P. Aneugenic effect of sodium arsenite on human lymphocytes in vitro: An individual susceptibility effect detected. Mutat. Res. 1995;334:365–373. doi: 10.1016/0165-1161(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Vega L, Styblo M, Patterson R, Cullen W, Wang C, Germolec D. Differential effects of trivalent and pentavalent arsernicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2001;172:225–232. doi: 10.1006/taap.2001.9152. [DOI] [PubMed] [Google Scholar]
- Vernhet L, Allain N, Payen L, Anger JP, Guillouzo A, Fardel O. Resistance of human multidrug resistance-associated protein 1-expressing lung tumor cells to the anticancer drug As trioxide. Biochem. Pharmacol. 2001;61:1387–1391. doi: 10.1016/s0006-2952(01)00606-2. [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo C. The Therapeutic Potential of Poly(ADP-Ribose) Polymerase Inhibitors. Pharmacol. Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Wang HF, Lee TC. Glutathione S-transferase pi facilitates the excretion from As-resistant Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 1993;192:1093–1099. doi: 10.1006/bbrc.1993.1529. [DOI] [PubMed] [Google Scholar]
- Wiencke JK, Yager JW. Specificity of arsenite in potentiating cytogenetic damage induced by the DNA crosslinking agent diepoxybutane. Environ. Mol. Mutagen. 1992;19:195–200. doi: 10.1002/em.2850190303. [DOI] [PubMed] [Google Scholar]
- Yih LH, Peck K, Lee TC. Changes in gene expression profiles of human fibroblasts in response to sodium arsenite treatment. Carcinogenesis. 2002;23:867–876. doi: 10.1093/carcin/23.5.867. [DOI] [PubMed] [Google Scholar]


