Abstract
The non-Mendelian inheritance of organelle genes is a phenomenon common to almost all eukaryotes, and in the isogamous alga Chlamydomonas reinhardtii, chloroplast (cp) genes are transmitted from the mating type positive (mt+) parent. In this study, the preferential disappearance of the fluorescent cp nucleoids of the mating type negative (mt−) parent was observed in living young zygotes. To study the change in cpDNA molecules during the preferential disappearance, the cpDNA of mt+ or mt− origin was labeled separately with bacterial aadA gene sequences. Then, a single zygote with or without cp nucleoids was isolated under direct observation by using optical tweezers and investigated by nested PCR analysis of the aadA sequences. This demonstrated that cpDNA molecules are digested completely during the preferential disappearance of mt− cp nucleoids within 10 min, whereas mt+ cpDNA and mitochondrial DNA are protected from the digestion. These results indicate that the non-Mendelian transmission pattern of organelle genes is determined immediately after zygote formation.
In nearly all eukaryotes, chloroplast DNA (cpDNA) and mtDNA are inherited from only one parent (1–3). The non-Mendelian inheritance of organelle DNA is a phenomenon common to diverse taxa of plants and animals, but its actual molecular mechanism remains a mystery.
In the unicellular alga Chlamydomonas reinhardtii, the chloroplast cpDNA is transmitted only from the mating type positive (mt+) parent (4), whereas mitochondrial genes are believed to be inherited from the mating type negative (mt−) parent (5). The mt+ and mt− gametes are the same size (isogamous) and contribute the same copy numbers of cp and mtDNA molecules to the zygote.
There are approximately 80 copies of the 196-kb cpDNA molecule per cell (6), and they are organized into 5–8 cpDNA–protein complexes (cp nucleoids). The nucleoids can be visualized under a fluorescent microscope by using DNA-specific fluorochrome 4′,6-diamidino-2-phenylindole (7). This technique permits direct observation of the number, distribution, and behavior of cp nucleoids in gametes and zygotes of C. reinhardtii. In 1982, Kuroiwa et al. (8) discovered that the cp nucleoids derived from the mt− parent disappear preferentially within 60 min after zygote formation, whereas the cp nucleoids of the mt+ parent remained unaffected.
The preferential disappearance of fluorescent mt− cp nucleoids occurs well before the DNA digestion is detected by biochemical techniques 6 hr after zygote formation (4). Therefore, the changes in cpDNA molecules that cause the preferential disappearance of fluorescent mt− cp nucleoids remain controversial. Several possibilities have been proposed. One is that the dispersal of cpDNA molecules, presumably caused by the digestion or relaxation of the proteins that form the cp nucleoids, might lead to the disappearance of fluorescent cp nucleoids (9, 10). Another is that the rapid digestion of cpDNA molecules might lead to the disappearance of cpDNA nucleoids (1, 11, 12). To understand the molecular mechanism that causes the preferential disappearance of fluorescent mt− cp nucleoids, more precise cytological observation and further molecular analysis of the phenomenon are indispensable. In this study, the process of preferential disappearance is visualized in a living zygote, and the changes in cpDNA molecules during preferential digestion are investigated further by using optical tweezers.
The use of optical tweezers is a novel technique for manipulating living cells or organelles in suspension without physically touching or damaging them (13–15). Using this technique, we isolated a single zygote under direct observation, according to the presence or absence of fluorescent mt− cp nucleoids. The mt+ and mt− cpDNA molecules were labeled separately by using bacterial aadA (aminoglycoside adenyl transferase) sequences (16, 17). Single zygotes obtained in this manner were subjected to highly sensitive nested-PCR analysis. Consequently, it was found that the rapid digestion of mt− cpDNA molecules causes the disappearance of fluorescent mt− cp nucleoids, whereas mt+ cpDNA and mtDNA molecules are protected from the digestion.
Materials and Methods
Cell Strain and Culture.
The wild-type strain, mt+ and mt−, was derived from strain 137c of C. reinhardtii. Cells were grown separately on agar plates [1.2% agar in Snell’s medium (18, 19)] at 22°C under 12 h of light followed by 12 h of darkness to synchronize cell division, which occurs in the middle of the dark period. The light intensity was approximately 6,600 lux at the surface of the flat culture container.
The chloroplast genome of L03c was transformed with plasmid pUCCEBaadA 53(NN), which is derived from the plasmid constructed by Goldschmidt-Clermont (16) and contains an aadA cassette driven by the atpA promoter and the aadA coding region and the rbcL 3′ region downstream from the coding region. The bacterial aadA gene encodes aminoglycoside adenyl transferase and is expressed in chloroplasts conferring resistance to spectinomycin and streptomycin. Chloroplast transformation was carried out with a particle gun as described in a previous report (17). The aadA cassette was inserted into the noncoding region downstream from the rbcL gene in the wild-type chloroplast genome by homologous recombination. This mutant strain was maintained on Snell’s medium supplemented with 100 μg/ml spectinomycin (Sigma). The mating reaction was induced by incubating the vegetative cells in N-free medium (Tsubo mating buffer: 0.6 mM MgSO4/1.2 mM Hepes⋅NaOH, pH 6.8) according to the previously described method (19). For UV irradiation, each gamete culture (mt+ and mt−) was exposed to UV (2,270 erg s−1⋅cm−1; 1 erg = 0.1 μJ) for 5 min immediately before mixing.
SYBR Green I Staining.
To stain the DNA in live gametes and zygotes, SYBR Green I nucleic acid stain (Molecular Probes) was added to the sample to give a final dilution of 1:1,000 (20). In all the experiments, the mixture was kept under the same conditions as the original culture and either illuminated or placed in the dark. The cells were observed under blue (B) excitation with a fluorescence microscope, and the squashed, motionless, live cells were photographed.
Optical Tweezers.
The optical tweezers used were similar to those described by Ashkin and colleagues (13–15). The main components of the optical tweezers device are a laser and a microscope system. The laser was a diode-pumped neodymium-YAG (yttrium/aluminum/garnet) laser ADLAS DPY 421 (Adlas, Lubeck, Germany) that emits continuous infrared (IR) light at 1,064 nm with a maximum power of 2,000 mW. The IR laser beam is deflected into a Zeiss photomicroscope (Axiovert 135; Zeiss) equipped with a PALM laser interface system (PALM, Brnrid, Germany; ref. 21) and focused with a Neofluar ×100 objective into the optical field. The laser power in the 1- to 2-μm focal spot was varied from 10 to 2,000 mW. In this study, optical manipulations were conducted at a power of 1,000 mW. The optics of the optical tweezers were improved by adding filters to allow observation of the fluorescent images of cells or organelles during the manipulation.
Immobilization of Cells.
To manipulate C. reinhardtii cells with the optical tweezers, the cells were immobilized by deflagellation or formaldehyde fixation, because optical tweezers cannot trap the swimming gametes or zygotes. The flagella were removed by following the method described by Witman et al. (22). For some experiments, the cells were fixed with formaldehyde. Formaldehyde solution in methanol was added to the cell suspension to give a final concentration of 4%. The suspension was incubated for 10 min at room temperature. Then the cells were harvested by centrifugation at 3,000 rpm for 1 min and resuspended in cold Tsubo mating buffer.
Nested-PCR Analysis.
The nested-PCR method was used to detect rbcL (ribulose 1,5-biphosphate carboxylase oxygenase large subunit: cpDNA), cox I (cytochrome c oxidase subunit I: mtDNA), and aadA (bacterial gene introduced into cpDNA of L03c strain) genes in single cells of C. reinhardtii. Nested-PCR primer sets specific for the rbcL, cox I, and aadA regions were generated. The primer sequences were as follows: (i) rbcL_F1: 5′-CATGGACTACAGTATGGACTGACGG-3′, rbcL_R1: 5′-GTACAAGCTTCAAGAGCTACACGGT-3′; (ii) rbcL_F2: 5′-TGCTTACGTTAAAACATTCGTAGGT-3′, rbcL_R2: 5′-ATACGTGAATACCGCCTGAAGCAAC-3′; (iii) cox I_F1: 5′-GCCTTCTTTGGCGGTTTGCTAGGTA-3′, cox I_R1: 5′-TACCATAGCACGACCCTCGTGGTAA-3′; (iv) cox I_F2: 5′-TTGCTACCAATCATGATCGGTGCCC-3′, cox I_R2: 5′-GGCACCCATAGCGCAAATCATACCA-3′; (v) aadA_F1: 5′-CTCTAGCTAACTTAGTATAC-3′, aadA_R1: 5′-GAAGTATCGACTCAACTATC-3′; (vi) aadA_F2: 5′-CTATCAGAGGTAGTTGGCGTCATCG-3′, aadA_R2: 5′-GCACTACATTTCGCTCATCGCCAGC-3′.
The expected sizes of the fragment amplified with primer pairs 1–6 were 1.09, 0.73, 1.16, 0.56, 1.40, and 0.52 kb, respectively. PCR was performed in a 50-μl reaction volume that contained 1× Ex Taq buffer (Takara Shuzo, Otsu, Japan), 200 μmol dNTPs, 25 pmol of each primer pair, and 1 unit of Ex Taq polymerase (Takara). For the first amplification, the outer primer pairs (1, 3, and 5) were used for the rbcL, cox I, and aadA sequences, respectively. Thirty-five cycles of PCR (rbcL and cox I: 1 min at 94°C, 1 min at 64°C, and 1 min at 72°C; aadA: 1 min at 94°C, 2 min at 55°C, and 3 min at 72°C) were carried out. One microliter of the first PCR product was used as the template for the second PCR. The second PCR was performed with the inner primer pairs (2, 4, and 6) for rbcL, cox I, and aadA, respectively, under the same conditions as the first PCR.
Results
Serial Observation of the Preferential Disappearance of mt− Chloroplast Nucleoids in a Living Zygote.
The conventional DNA-specific fluorochrome 4′-6-diamidino-2-phenylindole is a very sensitive dye that is used to detect DNA molecules in cells, but it cannot penetrate living cells or detect mitochondrial nucleoids. The use of SYBR Green I eliminates these two problems and permits simultaneous observation of the cell nuclei and cp and mitochondrial nucleoids in living C. reinhardtii (20).
In this study, SYBR Green I staining was used to visualize the preferential digestion of fluorescent mt− cp nucleoids in living zygotes. The preferential disappearance commenced about 40 min after zygote formation and was completed within 10 min (Fig. 1). During the preferential disappearance, all the cp nucleoids in mt−-derived chloroplasts simultaneously became smaller and disappeared completely, without swelling, whereas the mt+ cp nucleoids and mitochondrial nucleoids of both parents remained unchanged. The two chloroplasts remained at a distance from each other during the preferential disappearance of the mt− cp nucleoids.
Figure 1.
Preferential disappearance of mt− cp nucleoids visualized in a living zygote of C. reinhardtii. The living zygote was stained with SYBR Green I, and the preferential disappearance was observed under B irradiation. Phase-contrast (a) and fluorescent images of identical zygotes before (b) and after (c) the preferential disappearance are shown. To distinguish the mt+ and mt− chloroplasts, small mt− gametes were used. (b and c) The mt+ (Left) and mt− (Right) chloroplasts are emitting red autofluorescence. Cell nucleus (N), cp nucleoids (big fluorescent spots; large arrow), and mitochondrial nucleoids (small fluorescent spots; small arrow) all are visualized with SYBR Green I staining. The cp nucleoids in the mt− chloroplasts (b, white arrowheads) disappeared completely within 10 min (b and c).
Labeling of mt+ and mt− cpDNA Molecules by Transformation with Bacterial aadA Gene Sequences by Using a Particle Gun.
To investigate the changes in cpDNA molecules during the preferential disappearance of mt− cp nucleoids, it was necessary to label the mt+ and mt− cpDNA molecules in individual zygotes separately. Transformed strain L03c was used for this purpose. The cpDNA of L03c strain was transformed with the bacterial aadA gene, by using a particle gun (17). The behaviors of mt+ and mt− cpDNA were monitored separately by PCR amplification of the aadA sequences by using zygotes resulting from the cross between L03c gametes and wild-type 137c gametes of the opposite mating type.
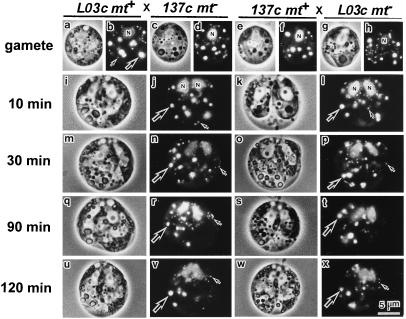
To determine whether the preferential disappearance of fluorescent mt− cp nucleoids occurred normally, crosses between L03c and wild-type gametes were examined by using SYBR Green I fluorescent microscopy (Fig. 2). The insertion of aadA sequences into cpDNA apparently had no effect on cell morphology or the activity of L03c gametes (Fig. 2 a– h). Moreover, the preferential disappearance of the fluorescent mt− cp nucleoids occurred 60–90 min after mating, whereas the fluorescent mt+ nucleoids were preserved. The two cell nuclei fused 120 min after zygote formation, just as in the cross between wild-type gametes (Fig. 2 i– x). These results suggested that the cross between L03c and wild-type gametes reflects the events that occur in the cross between wild-type gametes precisely.
Figure 2.
Phase-contrast (a, c, e, g, i, k, m, o, q, s, u, and w) and SYBR Green I fluorescent (b, d, f, h, j, l, n, p, r, t, v, and x) images of living gametes (a– h) and zygotes (i– x). The cross between L03c (harboring bacterial gene aadA in cpDNA) mt+ and 137c (wild type) mt− and the reciprocal cross were examined. Cell nuclei (N), mitochondrial nucleoids (small arrow), and cp nucleoids (large arrow) are visible in the gametes [137c mt+ (a and b) and mt−(c and d), L03c mt+ (e and f), and mt− (g and h)] and zygotes 10 (i– l), 30 (m– p), 90 (q– t), and 120 (u– x) min after mating. The fluorescent mt− cp nucleoids were preferentially digested within 60–90 min, whereas the mitochondrial nucleoids were preserved biparentally in both crosses.
Optical Isolation and Molecular Analysis of a Single Gamete or Zygote.
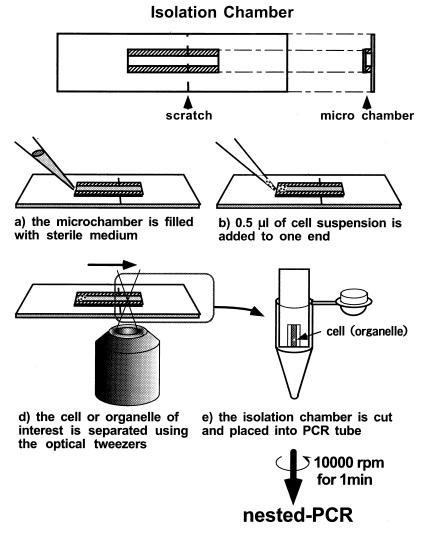
The use of optical tweezers is a novel technique for manipulating living cells or organelles in suspension without physical contact or damage. Fig. 3 summarizes the optical isolation method developed in this research, which can harvest cells or organelles of interest from a heterogeneous suspension within 5–10 min. Harvesting takes place under the direct microscopic visualization of cells or organelles. During the process, the cells or organelles can be observed in detail by phase-contrast or fluorescent microscopy with UV, B, or G excitation. Therefore, it is possible to distinguish between gametes and zygotes before and after the preferential disappearance of mt− cp nucleoids.
Figure 3.
The optical isolation process and the “isolation microchamber.” The microchamber is formed by separating a large (5 × 30 × 0.15 mm, length × width × thickness) and a small (9 × 3 × 0.15 mm) coverslip with two thin strips of adhesive tape (9 × 1 × 0.1 mm). The inside dimensions of the chamber were 9 × 1 × 0.1 mm. A small scratch was made on the chamber with a diamond knife. The bottoms of 5-cm diameter Petri dishes were cut out with a knife and replaced with thin, plastic film (≈100-μm thick). Filter paper was cut and placed in the dishes, and roughly 200 μl of distilled water was added dropwise to keep the inside of the dish moist. Then, the chamber was placed inside the dish and attached to the plastic film by using one drop of distilled water. First, the chamber was filled with sterile buffer containing 1.5% sucrose and 0.1% BSA (a). The BSA was added to prevent cells from adhering to the glass chamber. Then, 0.5 μl of cell suspension was carefully applied to one end of the chamber (b). The cells were observed with a microscope, and a single cell was trapped with the optical tweezers and transferred to the opposite end of the chamber (c). The transfer was processed automatically with a microscopic stage control system (MCU26 X, Y, Z-Axes Motor Control; Zeiss) at a velocity of 1–60 μm/sec. When the cell moved past the scratch in the chamber, the chamber was cut immediately, and the piece containing the cell of interest was placed in a PCR tube (d). The PCR tube was centrifuged for 5 sec to drop the cell into the tube. With this technique, it was possible to procure a living cell or intact organelles in a suspension within only 5–10 min.
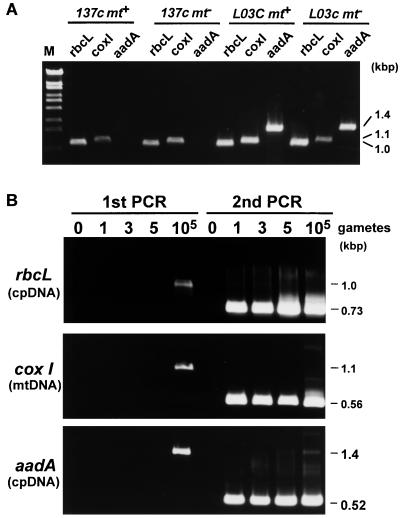
To investigate how many cells are required to detect cpDNA and mtDNA molecules, rbcL (cpDNA), cox I (mtDNA), and aadA gene sequences were amplified by PCR by using one, three, and five gametes isolated with the optical tweezers (Fig. 4). The first PCR was insufficient to detect the genes from 1–5 gametes, and a visible band was obtained only by using 105 gametes. When the second PCR was performed, all three genes could be detected as visible bands, even when only one gamete was used.
Figure 4.
PCR amplification of rbcL (cpDNA), cox I (mtDNA), and aadA (bacterial sequence introduced into the cpDNA of strain L03c) genes from 105 gametes of 137c (wild type) mt+, 137c mt−, L03c mt−, and L03c mt− gametes with the outer primer pairs (A). Nested PCR amplification of rbcL, cox I, and aadA sequences from one, three, or five gametes obtained optically and immobilized by deflagellation (B). The requisite number of gametes was isolated from the cell culture with the optical tweezers and subjected directly to nested-PCR analysis in which the rbcL, cox I, and aadA sequences were amplified by two rounds of PCR by using nested primers.
Detecting the Nuclease Activity That Causes Uniparental Inheritance of cpDNA by Using a Single Zygote.
All the bands shown in Fig. 5 were detected from a single gamete or zygote harvested with optical tweezers. The rbcL (cpDNA), cox I (mtDNA), and aadA sequences were amplified, and each experiment was repeated five times to ensure its reproducibility. Fig. 5A shows the results obtained from wild-type and L03c gametes. The rbcL and cox I sequences, which were used as control genes, were amplified from all of the single gametes. The aadA sequence was not detected in any of the wild-type gametes, whereas it was clearly detected in mt+ and mt- L03c gametes in all cases.
Figure 5.
Nested-PCR amplification of rbcL (cpDNA), cox I (mtDNA), and aadA sequences from one optically isolated gamete or zygote 10, 30, 90, and 120 min after zygote formation. One gamete of 137c mt+, 137c mt−, L03c mt+, or L03c mt− (A) or one zygote resulting from the crosses L03c mt+ × 137c mt− or 137c mt+ × L03c mt− (B) was isolated by using the optical tweezers and subjected immediately to nested PCR analysis. One typical zygote was isolated from each of the cell cultures 10, 30, 90, and 120 min after mating. To ensure reproducibility, each experiment was repeated five times (I– V). The lanes marked M are loaded with marker.
Next, single zygotes were isolated from cell cultures 10, 30, 90, and 120 min after zygote formation and subjected to nested-PCR analysis. From each cell culture, single zygotes were isolated optically and analyzed. At 10 min, the zygote had equal-sized mt+ and mt− cp nucleoids. At 30 min, the zygote had normal mt+ cp nucleoids and slightly smaller mt− cp nucleoids. At 90 min, the zygote had only mt+ cp nucleoids. At 120 min, the cell nuclei in the zygote had fused. When L03c mt+ gametes were crossed with wild-type mt− gametes, rbcL, cox I, and aadA gene sequences were detected in all the zygotes examined. On the contrary, when the L03c mt− gametes were crossed with wild-type gametes, the aadA sequences were amplified only in younger zygotes (10 and 30 min after zygote formation). After the fluorescent mt− cp nucleoids disappeared, the aadA sequences were no longer detected in the zygotes (90 and 120 min after zygote formation).
UV irradiation of mt+ gametes just before zygote formation significantly inhibited the preferential disappearance of mt− cp nucleoids whereas the irradiation of mt− gametes had almost no effect, which is consistent with the previous reports (1, 9). When the zygotes whose mt+ parents were pretreated with UV irradiation were analyzed, the aadA sequences in mt− cpDNA were clearly amplified even 120 min after zygote formation (data not shown).
Discussion
The preferential disappearance of fluorescent cp nucleoids in C. reinhardtii zygotes was reported first in 1982 (8). However, the molecular mechanism for the disappearance of the mt− cp nucleoids remains controversial (9, 10). In this study, we demonstrated that mt− cpDNA molecules are digested rapidly during the preferential disappearance of fluorescent mt− cp nucleoids. This suggests that highly effective nucleases are activated to digest the mt− cpDNA molecules just after zygote formation. The transmission pattern of organelle genes in C. reinhardtii appears to be determined at an extremely early stage of zygote maturation.
Fig. 1 shows serial observations of the preferential disappearance of mt− cp nucleoids in a living zygote. During the process, all the mt− cp nucleoids simultaneously became smaller approximately 40 min after zygote formation (Fig. 1b) and disappeared completely within 10 min (Fig. 1c). These results suggest that at least one enzyme is activated or synthesized only in the mt− chloroplast, resulting in the disappearance of mt− cp nucleoids. The two chloroplasts remained separated during the process. This suggests a possible role of the chloroplast membrane in localizing the high activity of the putative enzyme(s) that causes the disappearance of the cp nucleoids.
To learn more about the changes in cpDNA molecules during the preferential disappearance of fluorescent mt− cp nucleoids, we labeled the cpDNA molecules with bacterial aadA gene sequences by particle gun transformation (refs. 16 and 17; Fig. 2). Then we procured gametes and zygotes with or without mt− cp nucleoids, using the newly developed optical isolation method (Fig. 3). The result, shown in Fig. 4, demonstrates that cpDNA and mtDNA molecules can be detected clearly by using only a single gamete after two rounds of PCR amplification. Because each Chlamydomonas gamete contains only one chloroplast, this indicates that the gene sequences of a single chloroplast can be detected.
With this technique, we collected zygotes with and without mt− cp nucleoids and showed that the aadA sequences in mt− cpDNA molecules were no longer detected in zygotes lacking fluorescent mt− cp nucleoids (90 and 120 min after zygote formation) (Fig. 5). From this, we concluded that at least one highly effective nuclease is activated in the mt− chloroplast just after zygote formation, and this determines the non-Mendelian transmission pattern of cpDNA. The mt− cpDNA molecules are digested completely during the 10 min in which the fluorescent mt− cp nucleoids disappear. There is a discrepancy in the timing of DNA digestion observed in biochemical [6 h after zygote formation (4)] and cytological [60 min after zygote formation (8)] studies. This might result from the fact that the biochemical studies examined a cell population (including unmated gametes and zygote with and without mt− cp nucleoids) whereas the cytological study dealt with individual cells.
UV irradiation of mt+ gametes just before zygote formation significantly inhibited the digestion of the cpDNA whereas the irradiation of mt− gamete had almost no effect. This result suggests that the gene expression or protein synthesis by mt+ parent is important in the digestion of mt− cpDNA. At the time of writing, reports indicated that at least 200 polypeptides are synthesized de novo just after zygote formation. Six of these [94(α), 94(β), 94(γ), 52, 50, and 38 kDa] appear to be essential for the preferential digestion of mt− cpDNA (23). Ezy-1 polypeptide, which is thought to be the 52-kDa polypeptide, localizes in the chloroplast (24). These polypeptides might be involved in the activation or synthesis of the unknown nuclease(s).
The nuclease activity is likely to be nonspecific, because it digests exogenous DNA sequences such as aadA. A previous attempt to purify nucleases in C. reinhardtii cells identified nuclease C, a nonspecific nuclease that requires Ca2+ for full activation (11, 12). The preferential disappearance of mt− cp nucleoids is also effectively inhibited by the addition of EGTA to the cell culture (25). We think that further investigation of nuclease C will be an important key to understanding the molecular mechanism for maternal inheritance.
On the other hand, the amplification of mt+ cpDNA molecules and mtDNA was not affected during the preferential digestion of mt− cpDNA, indicating that these molecules are protected from the digestion by nuclease(s). The molecular mechanism that is responsible for the selective protection of mt+ cpDNA and mtDNA is still an open question for further study. It is possible that methylation of DNA molecules might play an important role in the protection, in a way that is analogous to the bacterial restriction-methylation system (26). Indeed, increased methylation of cpDNA molecules is detected during zygote maturation (26–28). In our experiment, however, treatment of zygotes with the methylation inhibitor 5-azaCyd had no effect on the preferential disappearance of fluorescent mt− cp nucleoids or on the preferential digestion of cpDNA molecules (data not shown). This result combined with the results of genetic studies using 5-azaCyd (29) and the hypermethylation mutant me-1 (30) suggest that methylation of cpDNA molecules is unlikely to be involved in the protection. Instead, because the preferential digestion of cp nucleoids is complete before the fusion of the mt+ and mt− chloroplasts, it is reasonable to assume that some modification to the chloroplast membrane might play an important role in protecting mt+ cpDNA and mtDNA of both mating types.
The preferential disappearance of fluorescent chloroplast or mitochondrial nucleoids of uniparental origin is not restricted to C. reinhardtii zygotes; it also occurs in ferns and higher plants. In the fern Pteris vittata, the cp nucleoids of male origin disappear during maturation of the sperm, whereas the plastid without cp nucleoids remains visible until the final stage of sperm development (31). In higher plants, such as Triticum aestivum, Lillium longiform, and Nicotiana tabacum, the fluorescent cp and mitochondrial nucleoids in generative cells disappear during pollen mitosis (32). Perhaps ferns and higher plants also have a similar nuclease to digest uniparental cp or mtDNA molecules.
Molecular analysis of a cell procured under direct observation is becoming increasingly important in various clinical and biological studies when it is difficult to prepare a pure cell population (21, 33–35). Conventional biochemical analysis of a complex cell population would merely produce confusion. The technique introduced in this report allows the researcher to obtain a single living cell, or even one chloroplast or mitochondria, from a population under direct visualization. Recently, much more attention has been paid to heterogeneity in the chloroplast (36, 37) and mitochondrial genomes (38) of higher plants. With this technique, it might be possible to explore the complexity of the chloroplast and mitochondrial genome in individual organelles. In the future, this technique combined with reverse transcription–PCR and microassays of enzyme activity will be very useful in various molecular studies.
Acknowledgments
We thank Dr. M. Goldschmidt-Clermont for generous permission to use plasmid pUCEBaadA 53(NN) for the chloroplast transformation. This work was supported by a research fellowship to Y.N. (5024) from the Japan Society for the Promotion of Science by Young Scientists and a Grant-in-Aid of Specially Promoted Research (06101002) and Scientific Research in Priority Areas (1163206) to T.K. from the Japanese Ministry of Education, Science and Culture.
Abbreviations
- cpDNA
chloroplast DNA
- mt+ and mt−
mating type positive and negative, respectively
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kuroiwa T. Int Rev Cytol. 1991;128:1–62. [Google Scholar]
- 2.Gillham N W. Organelle Genes and Genomes. New York: Oxford Univ. Press; 1994. pp. 149–152. [Google Scholar]
- 3.Birky C W., Jr Proc Natl Acad Sci USA. 1995;92:11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sager R, Lane D. Proc Natl Acad Sci USA. 1972;69:2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boynton J E, Harris E H, Burkhart B D, Lamerson P M, Gillham N W. Proc Natl Acad Sci USA. 1987;84:2391–2395. doi: 10.1073/pnas.84.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillham N W. Organelle Heredity. New York: Raven; 1978. [Google Scholar]
- 7.Kuroiwa T, Suzuki T, Ogawa K, Kawano S. Plant Cell Physiol. 1981;22:381–396. [Google Scholar]
- 8.Kuroiwa T, Kawano S, Nishibayashi S, Sato C. Nature (London) 1982;298:481–483. doi: 10.1038/298481a0. [DOI] [PubMed] [Google Scholar]
- 9.Harris E H. Chlamydomonas Sourcebook. San Diego: Academic; 1989. [Google Scholar]
- 10.Boynton J E, Gillham N W, Harris E H. Advances in Plant Gene Research. New York: Springer; 1990. [Google Scholar]
- 11.Ogawa K, Kuroiwa T. Plant Cell Physiol. 1985;26:481–491. [Google Scholar]
- 12.Ogawa K, Kuroiwa T. Plant Cell Physiol. 1985;26:493–503. [Google Scholar]
- 13.Ashkin A, Dziedzic J M. Science. 1987;235:1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- 14.Ashkin A, Shütze K, Dziedzic J M, Euteneuer U, Schliwa M. Nature (London) 1990;348:346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- 15.Shütze K, Clement-Segewald A, Ashkin A. Fertil Steril. 1994;61:783–786. [PubMed] [Google Scholar]
- 16.Goldschmidt-Clermont M. Nucleic Acids Res. 1991;19:4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikura K, Takaoka Y, Sekine M, Yoshida K, Shinmyo A. J Biosci Bioeng. 1999;87:307–314. doi: 10.1016/s1389-1723(99)80037-1. [DOI] [PubMed] [Google Scholar]
- 18.Snell W. J Mol Biol. 1982;25:47–66. [Google Scholar]
- 19.Nakamura S, Itoh S, Kuroiwa T. Plant Cell Physiol. 1986;27:775–784. [Google Scholar]
- 20.Nishimura Y, Higashiyama T, Suzuki L, Misumi O, Kuroiwa T. Eur J Cell Biol. 1998;77:124–133. doi: 10.1016/S0171-9335(98)80080-0. [DOI] [PubMed] [Google Scholar]
- 21.Richert J, Krantz E, Lôrz H, Dresselhaus T. Plant Sci. 1996;114:93–99. [Google Scholar]
- 22.Witman G B, Carlson K, Berliner J, Rosenbaum J L. J Cell Biol. 1972;54:507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura S, Sato C, Kuroiwa T. Plant Sci. 1988;56:129–136. [Google Scholar]
- 24.Ferris P J, Goodenough U W. Cell. 1993;74:801–811. doi: 10.1016/0092-8674(93)90460-8. [DOI] [PubMed] [Google Scholar]
- 25.Kuroiwa T. Microbiol Sci. 1985;2:267–272. [PubMed] [Google Scholar]
- 26.Burton W G, Gravowy C T, Sager R. Proc Natl Acad Sci USA. 1979;76:1390–1394. doi: 10.1073/pnas.76.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royer H D, Sager R. Proc Natl Acad Sci USA. 1979;76:5794–5798. doi: 10.1073/pnas.76.11.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sano H, Gravowy C T, Sager R. Proc Natl Acad Sci USA. 1981;78:3118–3122. doi: 10.1073/pnas.78.5.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng T-Y, Chiang K-S. Proc Natl Acad Sci USA. 1984;81:3438–3442. doi: 10.1073/pnas.81.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolen B L, Grant D M, Swinton D, Boynton J E, Gillham N W. Cell. 1982;28:335–343. doi: 10.1016/0092-8674(82)90351-8. [DOI] [PubMed] [Google Scholar]
- 31.Kuroiwa H, Sugai M, Kuroiwa T. Protoplasma. 1988;146:89–100. [Google Scholar]
- 32.Miyamura S, Kuroiwa T, Nagata T. Protoplasma. 1987;141:149–159. [Google Scholar]
- 33.Karrer E E, Lincoln J E, Hogenhout S, Bennet A B, Bostock R M, Martineau B, Lucas W J, Gilchrist D G, Alaxander D. Proc Natl Acad Sci USA. 1995;92:3814–3818. doi: 10.1073/pnas.92.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emmert-Buck M R, Bonner R F, Smith P D, Chuaqui R F, Zhuang, Zhengping, Goldstein S R, Weiss R A, Liotta L A. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 35.Simone N L, Bonner R F, Gillespie J W, Emmert-Buck M R, Liotta L A. Trends Genet. 1998;14:272–276. doi: 10.1016/s0168-9525(98)01489-9. [DOI] [PubMed] [Google Scholar]
- 36.Furg J E. Nature (London) 1998;398:115–116. [Google Scholar]
- 37.Suzuki H, Ingersoll J, Stern D B, Kindle K L. Plant J. 1997;11:635–648. doi: 10.1046/j.1365-313x.1997.11040635.x. [DOI] [PubMed] [Google Scholar]
- 38.Backert S, Nielsen B L, Börner T. Trends Plant Sci. 1997;2:477–483. [Google Scholar]