Abstract
Cardiac remodelling is commonly defined as a physiological or pathological state that may occur after conditions such as myocardial infarction, pressure overload, idiopathic dilated cardiomyopathy or volume overload. When training excessively, the heart develops several myocardial adaptations causing a physiological state of cardiac remodelling. These morphological changes depend on the kind of training and are clinically characterised by modifications in cardiac size and shape due to increased load. Several studies have investigated morphological differences in the athlete’s heart between athletes performing strength training and athletes performing endurance training. Endurance training is associated with an increased cardiac output and volume load on the left and right ventricles, causing the endurance-trained heart to generate a mild to moderate dilatation of the left ventricle combined with a mild to moderate increase in left ventricular wall thickness. Strength training is characterised by an elevation of both systolic and diastolic blood pressure. This pressure overload causes an increase in left ventricular wall thickness. This may or may not be accompanied by a slight raise in the left ventricular volume. However, the development of an endurancetrained heart and a strength-trained heart should not be considered an absolute concept. Both forms of training cause specific morphological changes in the heart, dependent on the type of sport. (Neth Heart J 2008;16:129-33.)
Keywords: ventricular remodelling, heart, sports, hypertrophy, Prinzmetal angina, acetylcholine, multifocal spasm
Cardiac remodelling is commonly defined as a physiological or pathological state that may occur after conditions such as myocardial infarction, pressure overload, idiopathic dilated cardiomyopathy or volume overload.1
The athlete’s heart is a physiological condition that can be defined as a morphological consequence of systematic training in athletes with the following features: increase in maximal cardiac output, increase in stroke volume, decrease in resting heart rate and electrocardiographic changes in conduction and repolarisation. A broad variety of cardiovascular adaptations can be achieved after either dynamic or isometric exercise, or a combination of both.2
First, we address the question how exercise-induced hypertrophy of the heart differs from cardiac hypertrophy of pathological origin. Second, we review the morphological changes in the heart that are typical for both endurance training and strength training.
Remodelling: physiological vs. pathological
Cardiac remodelling is clinically manifested by changes in cardiac size, shape and function in response to cardiac injury or increased load.1 Various causes of cardiac remodelling share several molecular, biochemical, and mechanical pathways. The process of cardiac remodelling is largely influenced by haemodynamic load, neurohumoral activation and additional factors such as endothelin, cytokines, nitric oxide production and oxidative stress.1
The heart is considered a supplier of a stable flow of blood, which responds to an increase in demand by raising stroke volume and heart rate.3 Volume overload and increased filling pressures lead to increased stretching force on the myocardium.4 Due to the Frank-Starling mechanism the heart muscle is able to increase contractile force when the ventricular wall is stretched. Cardiac remodelling can be a physiological or pathological condition:1
physiological remodelling is a compensatory change in the proportions and function of the heart; this type of remodelling can be seen in athletes;
pathological remodelling may occur after conditions such as myocardial infarction (pressure overload), inflammatory myocardial disease, with idiopathic dilated cardiomyopathy, or with volume overload.
The cardiac cell that is particularly involved in the remodelling process is the cardiomyocyte.
Stretching caused by the increase of haemodynamic load causes the heart to undergo a hypertrophic response. Cardiomyocytes expand by synthesis of new contractile proteins and the assembly of new sarcomeres in-parallel.3 This will increase the contractile force per cell. This form of remodelling is homogeneous and leads to an increase in the myocyte of the myocardium.5 This hypertrophic response induced by exercise is referred to as physiological remodelling.
When the heart is subject to chronic increases in demand (such as myocardial infarction) a different form of remodelling takes place. The majority of this category of remodelling is irreversible. The type of cardiac remodelling, due to conditions such as chronic overload, is associated with an inhomogeneous, disproportionate contribution of cardiac fibroblasts that produce interstitial fibrillar collagen. Collagen is principally synthesised by fibroblasts but also by vascular smooth muscle cells in response to a variety of pathological stimuli, including increased oxidative and mechanical stress, ischaemia and inflammation.6 Fibroblast stimulation increases collagen synthesis and causes fibrosis of both the infarcted and noninfarcted regions of the ventricle.7 This can lead to a loss of cardiomyocytes by apoptosis or necrosis and eventually these cardiomyocytes are replaced by fibroblasts and extracellular collagen.3 Fibrosis increases the stiffness of the myocardium, which interferes with filling of the heart.8 Loss of myocytes is an important mechanism in the development of cardiac failure.9 The apoptosis of cardiomyocytes will reduce the contractile force and diminish myocardial wall thickness. This is termed dilated cardiomyopathy. When the heart is exposed to a pressure overload and fails to undergo hypertrophy, this can also lead to ventricular dilatation.3,10 The increased stiffness of the myocardium and the diminished contractility are consequences of pathological remodelling and a strong predictor of heart failure.10
Remodelling: endurance vs. strength training
Morganroth et al. were the first to uncover two different morphological forms of athletes hearts: a strengthtrained heart and an endurance-trained heart.11
Endurance sports such as cycling, running and triathlon induce physical loads with high dynamic and high static components. In training and competition, endurance-trained athletes sustain long intervals with high cardiac output, high heart rate, high stroke volume and a moderate increase in mean arterial blood pressure.2,12 Dynamic exercise imposes a volume load on the left ventricle. The cardiac output of trained endurance athletes may increase from 5 to 6 l/min at rest to up to 40 l/min during maximal exercise.13 Besides an increase in cardiac output, the blood pressure increases as well, although not to the same extent as during strength training. Consequently, the endurance-trained heart needs to adapt to both volume and pressure load. The heart responds by increasing left ventricular internal diameter and left ventricular wall thickness.14
Ventricular dilation is caused by volume overload. Hereby, new sarcomeres are added in-series to existing sarcomeres. Volume overload-induced cardiac hypertrophy is known as eccentric hypertrophy (figure 1). In endurance training, the volume load is a predominant factor; therefore, the endurance-trained heart develops eccentric hypertrophy.15
Figure 1.
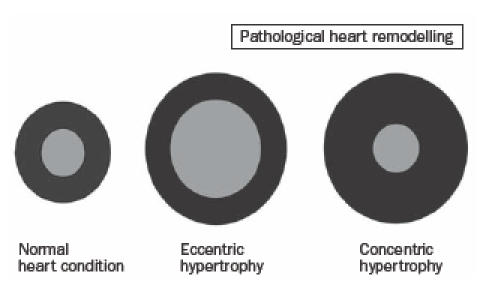
Pathological heart remodelling.
Cardiovascular adaptations in power athletes vary from those in endurance-trained athletes. Strength training is considered a static exercise. In static exercises the stroke volume of the heart is not affected but the overload is characterised by marked elevations in systolic and diastolic blood pressure and a modest increase in cardiac output, heart rate and oxygen consumption.16,17 High level resistance training is associated with sudden and large pressor responses. The blood pressure response during weightlifting can increase to levels as high as 320/250 mmHg.18
Due to an increased afterload, high intraventricular pressure is needed to open the aortic valve. During the ejection phase, the high levels of afterload and intraventricular pressure lead to an increase in myocardial wall stress, which is the major stimulus for cardiac hypertrophy in the pressure-overloaded heart.19
There is a substantial increase in systolic blood pressure with high-intensity isometric activity.18 The heart responds to a pressure overload in strength training by adding new sarcomeres in-parallel to existing sarcomeres. As a consequence, the wall thickness increases. This pathological condition is called concentric hypertrophy (figure 1).
Combined with a reduced compliance due to increased stiffness of the ventricle, this leads to diastolic dysfunction. Maron et al. found that the early filling phase of diastole is significantly prolonged and associated with a decreased rate and volume of rapid filling.20 Figures 2 and 3 depict ultrasound images of hearts with concentric and eccentric hypertrophy.
Figure 2.
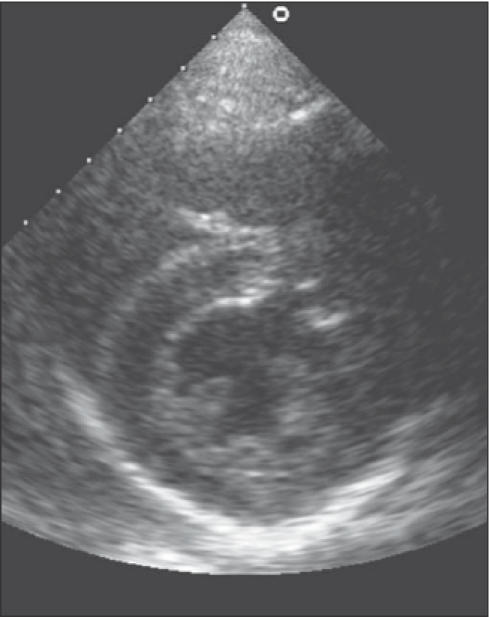
Ultrasound showing concentric hypertrophy of the heart.
Figure 3.
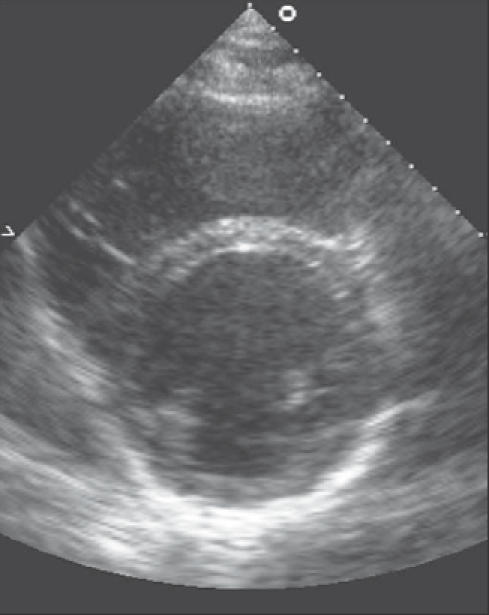
Ultrasound showing eccentric hypertrophy of the heart.
Results
Pellicia et al. described a variety of morphological changes in the left ventricular chambers of athletes performing endurance sports.21,22 These findings seemed to be associated with the haemodynamic overload. They concluded that endurance sports had a strong impact on left ventricular wall thickness and left ventricular cavity size. The authors measured left ventricular dimensions above the upper limit of normal (>55 mm).21,22Hoogsteen et al. reported that in cyclists the onset of the process of dilatation of the left ventricle and left atrium takes place at a relatively early stage. The process of dilatation seems to continue during the athlete’s career. There is also evidence that in cyclists the left ventricular mass is increased at an early age. Over time, the athletes show no significant changes in diastolic function of the left ventricle.23
Several studies have described morphological characteristics that differed between various endurance sports. Hoogsteen et al. found myocardial adaptations that differed between endurance sports, including cycling, marathon and triathlon. These differences in cardiac adaptation are induced by various pressure loads and exercise-related volumes.16 Pluim et al. found that runners show predominantly increased left ventricular wall thickness, whereas cyclists particularly demonstrate dilatation of the left ventricle.14
Hoogsteen et al. investigated the effects of cardiac remodelling on different groups of these highly trained endurance athletes; a prevalence of eccentric remodelling was found, being most apparent in cyclists.16,23 The combination of volume loading and pressure loading creates a physiological trigger that induces differentiation in left ventricular systolic and diastolic parameters such as dilatation, increased left ventricular mass and diastolic profile.3,16
These findings differ from the remodelling of the strength-trained heart. Martin et al. found that the effects of isometric exercise on left ventricular performance depend on two factors: the intensity of isometric exercise and the muscular mass involved in the contraction.24 Spirito et al. investigated the haemodynamic responses to isometric exercise in 947 elite athletes and concluded that athletes performing isometric exercise show increased myocardial mass with or without a slight increase in left ventricular volume.25 Kasikcioglu et al. presented comparable results.19 However, left ventricular end-systolic wall stress proved to be lower in the athletes than in the control group. This is believed to be due to the cardiovascular adaptation factors that affect left ventricular hypertrophy. 19 No further research on this matter has been conducted. Fisman et al. reported similar results and also make mention of a thicker interventricular septum in hearts of weightlifters than in hearts of sedentary individuals.17
Spirito et al. found that power disciplines do have a disproportionately larger impact on left ventricular wall thickness than on cavity size.25 Pelicia et al. reported an increase in absolute wall thicknesses; however, they rarely exceeded the upper normal limits (<12 mm).26 When comparing hearts of athletes performing strength and endurance training, Fisman et al. found an increased left ventricular mass in both groups with a particularly high absolute increase of mean value for weightlifters (weightlifting mean LV mass 220 g vs. runners mean LV mass 214 g).17
Vinereanu et al. found minor structural differences in left ventricular volumes and mass between both training groups.15 These findings are supported by Pluim et al. who performed a meta-analysis on cardiac structure and function of runners, cyclists, strengthtrained athletes and control subjects. The left ventricular mass did not differ between endurance-trained athletes and strength-trained athletes, but was significantly larger than the left ventricular mass of control subjects. The left ventricular internal diameter showed a significant difference between the three groups of athletes and control subjects. The mean relative wall thickness was significantly lower in endurance-trained athletes than in strength-trained athletes (0.39 mm vs. 0.44 mm).14
Pluim et al. concluded that the development of an endurance-trained heart and a strength-trained heart should not be considered an absolute concept. Strength-trained athletes who were expected to develop pure concentric left ventricular hypertrophy also showed an increase in left ventricular diameter. Endurance-trained athletes, who were considered to develop pure eccentric left ventricular hypertrophy, demonstrated an increase in left ventricular enddiastolic diameter and also show a more pronounced increase in wall thickness.14
In every form of endurance training, blood pressure increases (pressure load), in addition to the increase in cardiac output (volume load), just as in every form of strength training, heart rate, cardiac output and blood pressure increase.14
Conclusion
In hearts of athletes performing either strength or endurance training, typical structural and morphological adaptations have been reported.
Due to an increased dynamic load, the heart of the endurance athlete responds predominantly with eccentric hypertrophy. Endurance-trained hearts are subject to increased volume and pressure loading, leading to specific myocardial changes such as left ventricular dilatation and increased left ventricular mass. Strength training is associated with a marked elevation in systolic and diastolic blood pressure. The heart of the strength-trained athlete responds to sudden and large pressure overload with concentric left ventricular hypertrophy, in some cases accompanied by increases in left ventricular diameters. However, the development of an endurance-trained heart and a strengthtrained heart should not be considered an absolute concept. Both strength training and endurance training cause left ventricular hypertrophy, but left ventricular wall thickness is found to be higher in strength training, while dilatation of the left ventricle is a prominent feature of endurance trained hearts.
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodelling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodelling. On behalf of an International Forum on Cardiac Remodelling. J Am Coll Cardiol 2000;35:569-82. [DOI] [PubMed] [Google Scholar]
- 2.Hoogsteen J. Cardiologic aspects of endurance athletes [Thesis]. Leiden University, Leiden, the Netherlands, 2004. [Google Scholar]
- 3.Wakatsuki T, Schlessinger J, Elson EL. The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci 2004; 29:609-17. [DOI] [PubMed] [Google Scholar]
- 4.Anversa P, Olivetti G, Capasso JM. Cellular basis of ventricular remodelling after myocardial infarction. Am J Cardiol 1991;68: 7D-16D. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol 2000;15:264-72. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Alexander RW, O’Rourke RA, Roberts R, King SB, Wellens HJJ. In Hurst’s the heart. 10th edition, p 119 McGraw-Hill, USA, 2001. [Google Scholar]
- 7.Volders PGA, Willems IEMG, Cleutjens JPM Arends JW, Havenith MG, Daemen MJ. Interstitial collagen is increased in the noninfarcted human myocardium after myocardial infarction. J Mol Cell Cardiol 1993;25:1317-23. [DOI] [PubMed] [Google Scholar]
- 8.Matsubara LS, Matsubare BB, Okoshi MP, Cicogna AC, Janicki JS. Alterations in myocardial collagen content affect rat papillary muscle function. Am J Physiol Heart Circ Physiol 2000;279: 1534-9. [DOI] [PubMed] [Google Scholar]
- 9.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med 1997; 336(16):1131-41. [DOI] [PubMed] [Google Scholar]
- 10.Lips DJ, de Windt LJ, van Kraaij DJ, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur Heart J 2003;24:883-96. [DOI] [PubMed] [Google Scholar]
- 11.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med 1975;82:521-4. [DOI] [PubMed] [Google Scholar]
- 12.Schaier J, Stein P, Keteyian S, Fedel F, Ehrman J, Alam M. Left ventricular response to submaximal exercise in endurance trained athletes and sedentary adults. Am J Cardiol 1992;70:930-3. [DOI] [PubMed] [Google Scholar]
- 13.Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol 1968;25:619-25. [DOI] [PubMed] [Google Scholar]
- 14.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart: a meta-analysis of cardiac structure and function. Circulation 2000;101:336-44. [DOI] [PubMed] [Google Scholar]
- 15.Vinereanu D, Florescu N, Sculthorpe N, Tweddel AC, Stephens MR, Fraser AG. Left ventricular long-axis diastolic function is augmented in the hearts of endurance-trained compared with strength-trained athletes. Clin Sci 2002;103:249-57. [DOI] [PubMed] [Google Scholar]
- 16.Hoogsteen J, Hoogeveen A, Schaffers H, Wijn PFF, van Hemel NM, van der Wall EE. Myocardial adaptation in different endurance sports: an echocardiographic study. Int J Cardiovasc Imaging 2004;20:19-26. [DOI] [PubMed] [Google Scholar]
- 17.Fisman EZ, Embon P, Pines A, Tenenbaum A, Drory Y, Shapira I, et al. Comparison of left ventricular function using isometric exercise Doppler echocardiography in competitive runners and weightlifters versus sedentary individuals. Am J Cardiol 1997;79: 355-9. [DOI] [PubMed] [Google Scholar]
- 18.MacDougall JD, Tuxen D, Sale DG, Moroz JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol 1985; 58:785-90. [DOI] [PubMed] [Google Scholar]
- 19.Kasikcioglu E, Oflaz H, Akhan H, Kayserilioglu A, Mercanoglu F, Umman B, et al. Left ventricular remodelling and aortic distensibility in elite power athletes. Heart Vessels 2004;19:183-8. [DOI] [PubMed] [Google Scholar]
- 20.Maron BJ. Hypertrophic cardiomyopathy. Lancet 1997;350:127-33. [DOI] [PubMed] [Google Scholar]
- 21.Pellicia A, Di Paolo FM, Maron BJ. The athlete’s heart: remodelling, electrocardiogram and preparticipation screening. Cardiol Rev 2002;10:85-90. [DOI] [PubMed] [Google Scholar]
- 22.Pellicia A, Maron BJ, Sparato A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med 1991;324:295-301. [DOI] [PubMed] [Google Scholar]
- 23.Hoogsteen J, Hoogeveen A, Schaffers H, Wijn PFF, van der Wall EE. Left atrial and ventricular dimensions in highly trained cyclists. Int J Cardiol Imaging 2003;19:211-7. [DOI] [PubMed] [Google Scholar]
- 24.Martin CE, Shaver JA, Leon DF, Thompsom ME, Reddy PF, Leonard JJ. Autonomic mechanisms in hemodynamic responses to isometric exercise. J Clin Invest 1974;54:104-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spirito P, Pelliccia A, Proschan M, Granata M, Spataro A, Bellone P, et al. Morphology of the ‘athlete’s heart’ assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol 1994;74:802-6. [DOI] [PubMed] [Google Scholar]
- 26.Pelliccia A, Spataro A, Caselli G, Maron BJ. Absence of left ventricular wall thickening in athletes engaged in intense power training. Am J Cardiol 1993;72:1048-54. [DOI] [PubMed] [Google Scholar]


