Abstract
Although the biological roots of aggression have been the source of intense debate, the precise physiological mechanisms responsible for aggression remain poorly understood. In most species, aggression is more common in males than females; thus, gonadal hormones have been a focal point for research in this field. Although gonadal hormones have been shown to influence the expression of aggression, in many cases aggression can continue after castration, indicating that testicular steroids are not completely essential for the expression of aggression. Recently, the mammalian neuropeptide arginine vasopressin (AVP) has been implicated in aggression. AVP plays a particularly important role in social behavior in monogamous mammals, such as prairie voles (Microtus ochrogaster). In turn, the effects of social experiences may be mediated by neuropeptides, including AVP. For example, sexually naïve prairie voles are rarely aggressive. However, 24 h after the onset of mating, males of this species become significantly aggressive toward strangers. Likewise, in adult male prairie voles, central (intracerebroventricular) injections of AVP can significantly increase intermale aggression, suggesting a role for AVP in the expression of postcopulatory aggression in adult male prairie voles. In this paper, we demonstrate that early postnatal exposure to AVP can have long-lasting effects on the tendency to show aggression, producing levels of aggression in sexually naïve, adult male prairie voles that are comparable to those levels observed after mating. Females showed less aggression and were less responsive to exogenous AVP, but the capacity of an AVP V1a receptor antagonist to block female aggression also implicates AVP in the development of female aggression.
Arginine vasopressin (AVP) is a nonapeptide hormone primarily synthesized in the paraventricular nucleus and the supraoptic nucleus of the hypothalamus. In the brain, AVP predominantly binds to a G protein-coupled, V1a receptor subtype having seven transmembrane domains. V1 receptors depend on phosphatidyl inositol hydrolysis and intracellular calcium mobilization as part of the second messenger system for transcriptional activation and gene expression. In addition, there are numerous areas within the brain that receive AVP fiber projections from the paraventricular nucleus, including the amygdala, hippocampus, and posterior pituitary. Additional sites of AVP synthesis include the locus ceruleus and the bed nucleus of the stria terminalis, which sends neural projections to the lateral septum and the lateral habenular nucleus, among others (see refs. 1–4).
AVP and related peptides have well documented effects on a variety of systems associated with the physiological and behavioral defense of homeostasis. For example, AVP is necessary for water retention and affects blood pressure and other aspects of cardiovascular function (5). In rats, AVP influences the regulation of the hypothalamic-pituitary-adrenal axis, and it is likely that the central release of AVP is sensitive to stressful experiences (6, 7). In addition, AVP can enhance avoidance learning and some forms of memory (8). AVP also can influence the expression of territorial aggression in hamsters (9) and mate guarding in male prairie voles (Microtus ochrogaster) (10).
Although experiments conducted to determine the effects of AVP in adult animals are common, less is known regarding the role of this peptide during development (11, 12). Prairie voles offer an attractive model system both for the study of aggression and for the analysis of the developmental effects of AVP. In adult prairie voles, AVP affects several of the behavioral characteristics that define monogamy (13, 14), including high levels of social contact (15), male parental care (16, 17), the development of partner preferences (9, 10), and mating-induced aggressive behavior (10, 18). In addition, intracerebroventricular injection of AVP in adult male prairie voles has been shown to increase exploratory activity in an elevated plus maze (19).
AVP cell bodies and receptors are present well before birth (20), suggesting that this system is functional during the postnatal period (21). In this paper, we hypothesized that the behavioral systems, which are regulated by AVP in adulthood, might also be responsive to developmental changes in AVP. This hypothesis was tested in prairie voles by manipulating the functional levels of AVP during the early postnatal period and by assessing subsequent behavioral changes in adult animals.
Methods
Hormone Treatment.
Prairie voles used in this study were fourth generation descendants of a stock originally captured near Urbana, IL. The animals were housed under a 14:10 light/dark cycle, in polycarbonate mouse cages. Purina rabbit chow and water were provided ad libitum. On the first postnatal day, prairie vole pups were sexed, weighed, and toe-clipped for identification. Males and females were randomly assigned to one of six different groups (n = 8–10 per group). Experimental animals were generated from 10 independent breeding pairs over six generations. Only one male and one female pup per litter received hormone treatments, and like-sex siblings received either saline (controls) or no treatment (stimulus animals) in each generation. Over the first seven postnatal days, each animal either received (a) 120 ng of AVP; (b) 12 ng of AVP; (c) 1.2 ng of AVP; (d) 0.5 ng of [d(CH2)5Tyr(Me)]AVP [an AVP antagonist (AVPA)], each in 50 μl of saline; or (e) 50 μl of saline; or (f) was left untreated and unhandled. To limit the number of groups in this experiment, only one type and dose of AVP antagonist was chosen. The AVP antagonist used here is known to be selective for the V1a receptor subtype found in the brain and has been used at this dose level to successfully block the behavioral effects of both mating and AVP in adult prairie voles (10, 15). Although the AVP antagonist used in the aforementioned studies was administered directly into the intracerebral ventricles of adult animals, it was assumed in our study that peripheral injections of 0.05 ng of AVP antagonist would be suitable given the size of the neonates (1/10 of adult size) and their altricial state. It is known that the blood–brain barrier in altricial species is not fully formed at birth (22) and there is mounting physiological and behavioral evidence (23–30) to suggest that AVP can pass and perhaps play a facilitatory role in the blood–brain barrier of neonatal and adult rats, respectively (31–34). In our study, all of the pharmacological agents were injected intraperitoneally once per day by using a 30-gauge, 0.5-inch hypodermic needle affixed to a 100-μl Hamilton syringe. Treated animals were returned to their litters after each injection. All animals were weaned and paired with a same-sex sibling on postnatal day 31.
Behavior Tests and Analysis.
At 90 days of age, each subject was tested for activity and general exploration in an elevated plus maze (5-min test as described in refs. 18 and 19). Plus-maze activity levels were calculated as a percentage of the entries into the open arms divided by the sum of the open- and closed-arm entries. Twenty-four hours later, each subject was placed for 5 min in a clean polycarbonate mouse cage (12 × 18 × 28 cm) with an unfamiliar, same-sex conspecific of approximately the same body weight. All agonistic behaviors (lunges, bites, and lateral displays as defined in ref. 10) were recorded, and the total frequency of these behaviors is presented here as an index of aggression. Treatment effects were analyzed by using Kruskal–Wallis tests, and pairwise comparisons were made by using Mann–Whitney U tests with a level of significance of P < 0.05. The percentage data for animals displaying at least one act of aggression toward an unfamiliar, same-sex conspecific were analyzed by using the Yate’s Corrected χ2 test with a level of significance assigned at P < 0.05.
Results
Males.
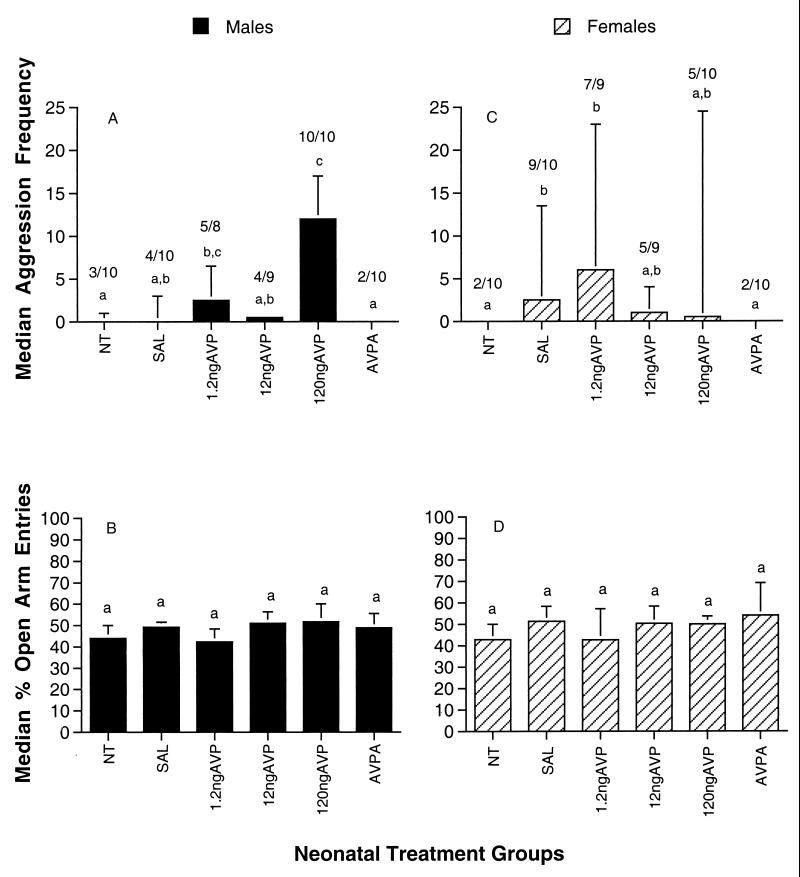
Aggression frequencies in adult, sexually naïve, male prairie voles were significantly affected by neonatal treatment with AVP (χ2 = 19.2; df = 5; P = 0.002). Males that were injected neonatally (Fig. 1A) with 120 ng of AVP were significantly more aggressive than were males receiving no treatment, saline, 12 ng of AVP, or the AVP antagonist, AVPA (pairwise comparisons; Mann–Whitney U test; P < 0.05). Further, the percentage of animals within the 120-ng AVP dose group that displayed any form of aggression toward an unfamiliar male conspecific was significantly higher (Yate’s corrected χ2; P < 0.05) when compared with the control groups. Males that received 1.2 ng of AVP also were more aggressive than males that were not treated or those receiving AVPA, but the behavior of the 1.2-ng AVP group did not differ significantly from the other AVP-treated males.
Figure 1.
Median aggression frequency (+75% quartile range) exhibited by sexually naïve, adult male (A) and female (C) prairie voles in a 5-min test with a comparable like-sex stimulus animal. Experimental animals received either no treatment (NT); one daily intraperitoneal injection of 50 μl of physiological saline (SAL); or 1.2 ng of AVP, 12 ng of AVP, 120 ng of AVP, or 0.5 ng of AVPA, each suspended in 50 μl of saline over the first 7 postnatal days of life. Columns sharing letters in common are not significantly different (Mann–Whitney U; P > 0.05). The ratio of animals showing aggression within each group is noted above each bar. Median percentage entries into the open arm of an elevated plus maze by sexually naïve, adult male (B) and female (D) experimental prairie voles during a 5-min test. There were no group differences on this measure (Mann–Whitney U; P > 0.05).
Subsequent behavioral tests of these animals supported the assumption that the capacity of neonatal AVP to increase aggression was not restricted to male–male aggression, because aggression also was seen in encounters with females. Additionally, in males, aggression induced by 120 ng of AVP continued to be shown throughout the lives of the animals, and was not further increased by sexual experience. Tendencies to display exceptionally low levels of aggression also were consistent throughout the lives of AVPA animals. In contrast, exploratory behavior (Fig. 1B) was not significantly affected by treatments with either AVP or AVPA (Kruskal–Wallis; χ2 = 3.8; df = 5; P = 0.58).
Females.
In a concurrent study of female prairie voles, only the lowest dose of postnatal AVP tended to increase the expression of aggression in sexually naïve, adult females (Fig. 1C; Kruskal–Wallis; χ2 = 13.1; df = 5; P = 0.022). As in males, neonatal treatments did not alter exploratory behavior (Fig. 1D). Further pairwise comparisons revealed that females in the 1.2-ng AVP dose group were significantly more aggressive relative to the untreated and AVPA-treated control groups (Fig. 1C). Saline-treated females also showed an increase in aggression in comparison to untreated or AVPA-treated females, but did not differ from AVP-treated females. Although a few individual females exhibited intense levels of aggression within several of the AVP-treated groups, females in general were less aggressive than their male counterparts.
Discussion
It has been proposed that hormones, which are essential for normal physiological functions in the adult, may play an important role during development by fine tuning their respective receptors or neuroendocrine pathways (35–37). The results from our study suggest that in male prairie voles the neural substrates underlying the expression of aggression are sensitive to exposure to AVP during the early postnatal period and that AVP may be a component of the developmental mechanisms regulating aggression later in life. Furthermore, the heightened level of aggression observed in AVP-treated animals was secondary neither to increased activity patterns nor to any other observed behavioral impairments. Aggression is rare in sexually naïve, adult male prairie voles; however, after mating, prairie voles exhibit a long-lasting, permanent increase in aggression (38, 39). AVP is implicated in the induction of aggression both during development and in adulthood, although whether the same cellular mechanisms are responsible for increasing aggression in both cases remains to be determined. For example, it is possible that both mating (40) and developmental exposure to AVP sensitizes AVP receptors to become more responsive to subsequent exposure to this peptide.
The vasopressinergic system in prairie voles appears to be sexually dimorphic (41), with a higher concentration of immunoreactive AVP localized in the lateral septum and lateral habenular nucleus of males. Our study confirms that male and female prairie voles responded differently to AVP challenges during early postnatal development. For example, in female prairie voles, we observed that postnatal treatment with either the lowest dose of AVP or the injections with the saline vehicle was associated with increased aggression in adulthood. On the contrary, males responded with increased aggression only at the highest dose of AVP given. One possible explanation is that females are more sensitive to handling stress, perhaps as mediated through corticosterone during early postnatal development, whereas males are more sensitive to AVP. Interestingly, both males and females exhibited virtually no aggression when treated with AVPA, suggesting that some level of endogenous AVP production as a result of handling may be directly or indirectly associated with the development of aggression in males and females, respectively. Alternatively, among those groups of females that were aggressive, only a few individual females were exceptionally aggressive, accounting for the high level of variability we observed within the respective groups and, thus, may require a larger sample size to be used in future studies.
It is generally assumed that perinatal androgens play a role in mammalian sexual differentiation. However, a sex difference with respect to the behavioral effects of AVP suggests that some aspects of the sexual differentiation of the system responsible for aggression may be present at birth. The developmental effects of androgens on aggression remain to be studied in prairie voles. However, in adult prairie voles, castration does not inhibit aggression, and aggression appears to be independent of gonadal steroids (N. Hastings and C.S.C., unpublished observations).
Recent experiments with prairie voles have implicated adrenal steroids, not gonadal hormones, in the process of behavioral masculinization in this species (42, 43). For example, postnatal treatments with corticosterone, rather than testosterone, were associated with increased mounting in female prairie voles, and neonatal castration did not reduce the tendency of males of this species to mount in response to adult androgens.
Our study suggests the more general hypothesis that peptides may mediate the effects of environmental cues on subsequent adult patterns of aggression. Mechanisms such as these could confer selective advantages by allowing individual experiences capable of releasing AVP to produce long-lasting adjustments in physiological and behavioral systems. For example, postnatal fluid restriction (drought) could increase the endogenous production of AVP and, thus, increase subsequent aggressive behavior. Under harsh environmental conditions, a more aggressive behavioral response to other animals, especially potential competitors, might be advantageous. Alternatively, under more benign conditions, endogenous AVP might remain low, permitting lower levels of aggression, especially in reproductively naïve animals.
The clinical implications of developmental exposure to AVP or related peptides are largely unknown. Analogues of AVP such as desmopressin or lysine vasopressin have been used to treat nocturnal enuresis (bed wetting) in normal children (44) or to enhance learning and memory in mentally disabled children (45, 46), respectively. Particularly disturbing is the fact that nicotine, a potent releaser of AVP (47–50), can freely pass both the placental and blood–brain barriers of fetuses and their mothers alike (51, 52). Given the recent rise in cigarette use among young girls and pregnant teenagers in the United States (53), it is important to understand the links among nicotine, vasopressin, and various conduct disorders observed in children of smoking mothers (54–59).
Given the structural similarity between oxytocin and AVP, these peptides can affect each other’s receptors. Oxytocin (Pitocin) is widely used to facilitate parturition and in some cases is used as a lactational aid. Developmental exposure to oxytocin might act as either an agonist or an antagonist on the vasopressinergic system. The long-term effects of such manipulations have not been fully examined (30).
Acknowledgments
We thank Bruce Cushing, Nancy Cushing, Edward Lee, Kinnari Patel, and Sophia Fuentes for their help with this project. All work was conducted within the guidelines established by the Animal Care and Use Committee of the University of Maryland. This research was supported by grants from the National Institutes of Health to C.S.C.
Abbreviations
- AVP
arginine vasopressin
- AVPA
an AVP antagonist [d(CH2)5Tyr(Me)]AVPI
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Barberis C, Tribollet E. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- 2.Boer G J, Buijs R M, Swaab D F, De Vries G J. Peptides. 1980;1, Suppl. 1:203–209. [Google Scholar]
- 3.Ervin M G, Kullama L K, Ross M G, Leake R D, Fisher D A. Reg Pept. 1993;45:203–208. doi: 10.1016/0167-0115(93)90207-o. [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski N L, Lolait S J, Bradley D J, O’Carroll A M, Brownstein M J, Young W S. Endocrinology. 1992;131:533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 5.Berecek K H. In: Central Neural Mechanisms in Cardiovascular Regulation. Kunos G, Ciriello J, editors. Boston: Birkhauser; 1991. pp. 1–34. [Google Scholar]
- 6.Aguilera G, Lightman S L, Kiss A. Endocrinology. 1993;132:241–248. doi: 10.1210/endo.132.1.8380375. [DOI] [PubMed] [Google Scholar]
- 7.Yates F E, Russell S M, Dallman M F, Hedge G A, McCann S M, Dhariwal A P S. Endocrinology. 1971;88:3–15. doi: 10.1210/endo-88-1-3. [DOI] [PubMed] [Google Scholar]
- 8.Engelmann M, Wotjak C T, Neumann I, Lugwig M, Landgraf R. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 9.Ferris C F, Albers H E, Wesolowski S M, Goldman B D, Luman S E. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- 10.Winslow J T, Hastings N, Carter C S, Harbaugh C R, Insel T R. Nature (London) 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 11.Van Tol H H M, Snijdewint F G M, Boer G J, Burbach J P H. Neurosci Lett. 1986;65:1–6. doi: 10.1016/0304-3940(86)90110-2. [DOI] [PubMed] [Google Scholar]
- 12.Varlinskaya E I, Petrov E S, Robinson S R, Smotherman W P. Behav Neurosci. 1994;108:395–409. doi: 10.1037//0735-7044.108.2.395. [DOI] [PubMed] [Google Scholar]
- 13.Carter C S, DeVries A C, Getz L L. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 14.Kleiman D. Quart Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 15.Cho, M. M., DeVries, A. C., Williams, J. R. & Carter, C. S. Behav. Neurosci., in press. [DOI] [PubMed]
- 16.Wang Z X, Zhou L, Hulihan T, Insel T R. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z X, Ferris C F, DeVries G J. Proc Natl Acad Sci USA. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel T R, Preston S, Winslow J T. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 19.Dharmadhikari A, Lee Y S, Roberts R L, Carter C S. Ann NY Acad Sci. 1997;807:260–272. doi: 10.1111/j.1749-6632.1997.tb51982.x. [DOI] [PubMed] [Google Scholar]
- 20.DeVries G F, Villalba C. Ann NY Acad Sci. 1997;807:273–286. doi: 10.1111/j.1749-6632.1997.tb51926.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Young L J, Kiu Y, Insel T R. J Comp Neurol. 1997;378:535–546. [PubMed] [Google Scholar]
- 22.Johanson C E. Brain Res. 1980;190:3–16. doi: 10.1016/0006-8993(80)91155-5. [DOI] [PubMed] [Google Scholar]
- 23.Bluthe R M, Schoenen J, Dantzer R. Brain Res. 1990;519:150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 24.Chen X F, Chen Z F, Liu R Y, Du Y C. Peptides. 1988;9:717–721. doi: 10.1016/0196-9781(88)90111-8. [DOI] [PubMed] [Google Scholar]
- 25.Dantzer R, Bluthe R M, Koob G F, Le Moal M. Psychopharmacology. 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 26.De Vries G J, Buijs R M, Swaab D F. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 27.De Wied D, Elands J, Kovacs G. Proc Natl Acad Sci USA. 1991;88:1494–1498. doi: 10.1073/pnas.88.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popik P, Van Ree J M. Behav Neural Biol. 1993;59:63–68. doi: 10.1016/0163-1047(93)91173-k. [DOI] [PubMed] [Google Scholar]
- 29.Strupp B J, Bunsey M, Bertsche B, Levitsky D A, Kesler M. Behav Neurosci. 1990;104:268–276. doi: 10.1037//0735-7044.104.2.268. [DOI] [PubMed] [Google Scholar]
- 30.Swabb D F, Boer G J. J Dev Physiol. 1983;5:67–75. [PubMed] [Google Scholar]
- 31.Banks W A, Kastin A J. Psychoneuroendocrinology. 1985;10:385–399. doi: 10.1016/0306-4530(85)90079-4. [DOI] [PubMed] [Google Scholar]
- 32.Brust P. J Neurochem. 1986;46:534–541. doi: 10.1111/j.1471-4159.1986.tb13000.x. [DOI] [PubMed] [Google Scholar]
- 33.Brust P, Shaya E K, Jeffries K J, Dannals R F, Ravert H T, Wilson A A, Conti P S, Wagner H N, Gjedde A, Ermisch A, Wong D F. J Neurochem. 1992;59:1421–1429. doi: 10.1111/j.1471-4159.1992.tb08456.x. [DOI] [PubMed] [Google Scholar]
- 34.Ermisch A, Ruhle H J, Landgraf R, Hess J. J Cereb Blood Flow Metab. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- 35.Csaba G. Experentia. 1986;42:750–759. doi: 10.1007/BF01941521. [DOI] [PubMed] [Google Scholar]
- 36.Boer G J, Snijdewint G M, Swaab D F. Prog Brain Res. 1988;73:245–264. doi: 10.1016/S0079-6123(08)60508-7. [DOI] [PubMed] [Google Scholar]
- 37.Handelmann G E. Prog Brain Res. 1988;73:523–533. doi: 10.1016/S0079-6123(08)60524-5. [DOI] [PubMed] [Google Scholar]
- 38.Getz L L, Carter C S, Gavish L. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- 39.Gavish L, Carter C S, Getz L L. Anim Behav. 1983;31:511–517. [Google Scholar]
- 40.Insel T R, Wang Z X, Ferris C F. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamshad M, Novak M A, DeVries G J. J Neuroendocrinology. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 42.Roberts R L, Zullo A S, Gustafson E A, Carter C S. Horm Behav. 1996;30:576–582. doi: 10.1006/hbeh.1996.0060. [DOI] [PubMed] [Google Scholar]
- 43.Roberts R L, Zullo A S, Carter C S. Physiol Behav. 1997;62:1379–1383. doi: 10.1016/s0031-9384(97)00365-x. [DOI] [PubMed] [Google Scholar]
- 44.Moffatt M E. Dev Behav Ped. 1997;18:49–56. doi: 10.1097/00004703-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Andersen L T, David R, Bonnet K, Dancis J. Life Sci. 1979;24:905–910. doi: 10.1016/0024-3205(79)90340-0. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberg J, Chazan-Gologorsky S, Hattab J, Belmaker R H. Biol Psychiatry. 1984;19:1137–1141. [PubMed] [Google Scholar]
- 47.Andersson K, Siegel R, Fuxe K, Eneroth P. Acta Physiol Scand. 1983;118:35–40. doi: 10.1111/j.1748-1716.1983.tb07237.x. [DOI] [PubMed] [Google Scholar]
- 48.Husain M K, Frantz A G, Ciarachi F, Robinson A G. J Clin Endocrinol Metab. 1975;41:1113–1117. doi: 10.1210/jcem-41-6-1113. [DOI] [PubMed] [Google Scholar]
- 49.Matta S G, Foster C A, Sharp B M. Endocrinology. 1993;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- 50.Seyler L E, Pomerleau O F, Fertig J B, Hunt D, Parker K. Pharmocol Biochem Behav. 1986;24:159–162. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
- 51.Riah O, Courriere P, Dousset J C, Todeschi N, Labat C. Cell Mol Neurobiol. 1998;18:311–318. doi: 10.1023/A:1022501131709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spector R, Goldberg M J. J Neurochem. 1982;38:594–596. doi: 10.1111/j.1471-4159.1982.tb08669.x. [DOI] [PubMed] [Google Scholar]
- 53.Matthews T J. Natl Vit Stat Rep. 1998;47:1–9. [Google Scholar]
- 54.Milberger S, Biederman J, Faraone S V, Chen L, Jones J. Am J Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- 55.Pomerleau O F. Am J Med. 1992;93(1A):2S–7S. doi: 10.1016/0002-9343(92)90619-m. [DOI] [PubMed] [Google Scholar]
- 56.Pomerleau O F, Downey K K, Stelson F W, Pomerleau C S. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 57.Rantakallio P, Laara E, Isohanni M, Moilanen I. Int J Epidem. 1992;21:1106–1113. doi: 10.1093/ije/21.6.1106. [DOI] [PubMed] [Google Scholar]
- 58.Wakschlag L S, Lahey B, Loeber R, Green S, Gordon R, Leventhal B L. Arch Gen Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- 59.Weitzman M, Gortmaker S, Sobol A. Pediatrics. 1992;90:342–349. [PubMed] [Google Scholar]