Abstract
Identifying the factors that have promoted host shifts by phytophagous insects at a macroevolutionary scale is critical to understanding the associations between plants and insects. We used molecular phylogenies of the beetle genus Blepharida and its host genus Bursera to test whether these insects have been using hosts with widely overlapping ranges over evolutionary time. We also quantified the importance of host range coincidence relative to host chemistry and host phylogenetic relatedness. Overall, the evolution of host use of these insects has not been among hosts that are geographically similar. Host chemistry is the factor that best explains their macroevolutionary patterns of host use. Interestingly, one exceptional polyphagous species has shifted among geographically close chemically dissimilar plants.
Determining which factors have directed the evolution of the associations between plants and phytophagous insects has been a central interest in the field of plant–insect interactions for the last 30 years. Two main scenarios have been advanced to explain macroevolutionary patterns of host use. According to the first scenario, shifts by herbivorous insects are mediated by the similarities of secondary compounds of their hosts (1). An insect could be physiologically preadapted to a new host whose toxic compounds are similar to those of its old host. The associations between various insects and plants containing furanocoumarins have been interpreted according to this scenario (2). Macroevolutionary data, although scarce, also indicates that host shifts by phytophagous insects could sometimes be mediated by plant chemical similarity (3–5).
The second model explains the patterns of evolution of host use in terms of parallel cladogenesis. If insect lineages remain associated with their hosts over a long time, events that isolate host populations might also isolate populations of their associated insects, which may eventually result in allopatric cospeciation. The high concordance between the cladogram of the chrysomelid genus Phyllobrotica and that of its host plants may reflect parallel cladogenesis (6).
An obvious alternative to these scenarios is that insects have been shifting among hosts that are geographically available. According to this model, a shift to a particular plant species is more likely to evolve if its geographical range is coincident with the geographic distribution of the old host. Therefore, lineages of insects would evolve by attacking hosts within a biogeographic area.
The importance of host availability as a factor that influences the evolution of host use is well supported by ecological and agronomic studies. In many instances, the underlying factor is simply increased ecological opportunity (7). Geographic availability is clearly a factor in host use of plants that have invaded new regions. Local insects almost always attack new crops when they are introduced.
At a macroevolutionary level, though, the influence of host availability has been practically unexplored (5, 8). One reason for this is that its evaluation requires the reconstruction of the evolution of host shifts and phylogenetic and biogeographic information on hosts. Also, extensive host chemical information is necessary to estimate the relative importance of availability vs. chemical similarity. Thus, detailed information on a broad range of issues for two interacting groups of organisms is needed to evaluate the role of host range coincidence relative to host chemistry or phylogeny. Another difficult issue is that there might be correlations between host phylogeny, host chemistry, and host geography. Statistical techniques that consider this kind of multiple correlation involving phylogenetic trees are only beginning to be developed.
Here, we present an evaluation of the relevance of host geographical distribution in explaining the macroevolutionary patterns of host shifts by using the herbivorous genus Blepharida (Chrysomelidae: Alticinae) with molecular insect and plant phylogenies. We also investigate the importance of host geographic distribution relative to host chemistry and host cladogenesis.
The New World genus Blepharida sensu stricto includes about 40 species (9). Many are monophagous, feeding mainly on Bursera and a few other Burseraceae and Anacardiaceae. The interaction of this genus with Burseraceae started probably more than 100 million years ago which makes it one of the oldest interactions known between an herbivore and its hosts (10).
The plant genus Bursera includes about 100 species distributed from the south of the United States to Peru. Bursera diversified in the tropical dry forests of Mexico, where about 80 species occur, of which 70 are endemic (11, 12). These species reach their highest diversity in the depression of the Balsas River, which runs from the north of Oaxaca to the coast of Michoacán. This depression is bordered by two of the major mountain systems of the country, the Sierra Madre del Sur to the south and the Neovolcanic Axis to the north, and is constricted almost in the middle by another mountain system, the Sierra de Taxco, which runs to the north. Many Bursera are restricted to either the east or west of the Sierra de Taxco (13).
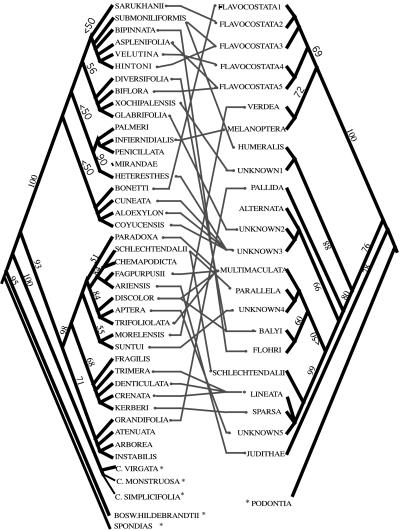
Phylogenetic hypotheses have been advanced for both Blepharida and Bursera. They were reconstructed by using the Internal Transcriber Spacer region of the nuclear ribosomal DNA (Fig. 1; refs. 3, 12) by using 23 species of Blepharida and 38 species of Bursera.
Figure 1.
Feeding associations of Blepharida beetles on Bursera hosts. Both phylogenies were reconstructed by using the nucleotide sites of the nuclear ribosomal DNA internal transcribed spacer sequences. For clarity, the hosts of polyphagous B. alternata are not indicated (but see Fig. 4 for its host plants). Asterisks indicate outgroups, and the numbers above branches are bootstrap percentages.
Bursera produces resins containing terpenes distributed in a network of canals in the cortex of the stems and throughout the leaves (14). Becerra (3) previously reported that the patterns of evolution of host use by Blepharida could be explained by the patterns of host chemical similarity, by using the molecular phylogenies of Bursera and Blepharida and a chemical characterization of Bursera. The topology of Blepharida’s phylogeny was compared with the topology of a dendrogram of Bursera species based on their chemical similarity (a chemogram). The topologies of the phylogenies of Blepharida and Bursera were also compared. These comparisons showed that there has been a greater impact of host plant chemistry than of host plant phylogeny in Blepharida’s evolution of host use. We extend this research by exploring the role of host geographic availability and its relationships with plant chemistry and plant phylogeny in the evolution of host affiliation by Blepharida.
Materials and Methods
Reconstruction of Blepharida’s Evolutionary Host Use.
To reconstruct the evolution of host use by Blepharida, we used the published DNA phylogeny of species of Blepharida (Fig. 1; ref. 3). For most beetles, we found evidence of association to a plant species by observing them feeding and mating in the field. In many cases, they were also observed ovipositing. We have confirmed this evidence of host relationship during multiple visits to natural populations of each Bursera species over 3 to 6 years, depending on the species. Distributions of Bursera species used in this study are relatively well known (13, 15). Although we may have visited only one or two study sites for narrow endemics, we have looked at multiple sites across the range for widespread species. We have ignored several isolated observations of one or a few individual herbivores seen only one time on a host. We have also followed entire life cycles of about half of the Blepharida species used in this study by rearing them in captivity on their hosts.
Measuring the Geographic Relationship of the Bursera Species.
Using previously published geographic distributions of Bursera species (13, 15) updated with more recent information from herbarium specimens, we constructed maps of distribution of 38 Bursera species. Maps were constructed by using a baseline map of Mexico on which we recorded the localities for each species.
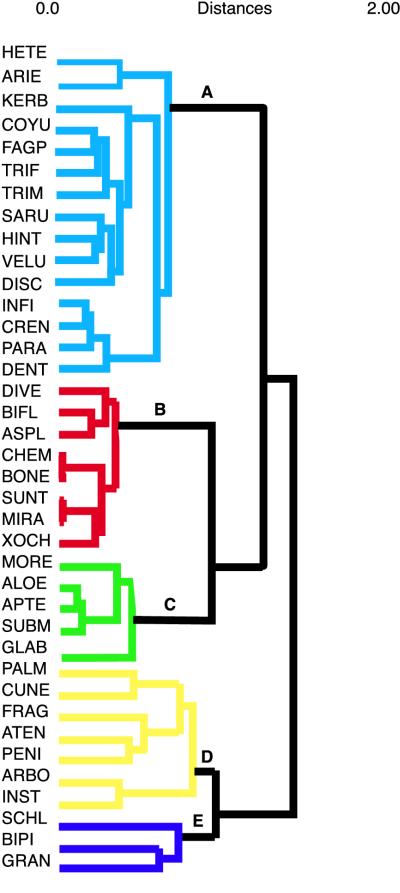
To estimate the geographic relationship of Bursera species, we constructed a dendrogram based on the similarity of their geographical ranges (a biogeogram). To construct this dendrogram, we used a transparent map of Mexico of the same dimensions as the baseline map divided in squares of 1° longitude and latitude. Each square was numbered. By setting this transparent sheet on top of the map of distribution of each species, we were able to record the numbers of the squares in which each species was present. A matrix of Euclidean distances between Bursera species was constructed on the basis of their presence or absence in each square. The dendrogram was constructed with a cluster analysis of this matrix by using Ward’s Method (16). To check for the robustness of the clusters, we also performed cluster analyses with upgma (16) and the complete linkage method.
Comparison of the Insect Phylogeny with the Biogeogram.
To investigate whether Blepharida’s evolutionary patterns of host use could be explained by the patterns of similarity of their hosts’ geographical ranges, we compared the topology of Blepharida’s phylogeny with the topology of the biogeogram.
The phylogeny of Blepharida includes B. alternata, which is a polyphagous species in the sense that it feeds on many Bursera species, unlike most others members of Blepharida investigated which feed on only one. The inclusion of polyphagous species in the analysis can sometimes cause an overall poor correlation between trees (17). Because of this, and because B. alternata’s pattern of host use is very different from most Blepharida species, we did statistical analyses both with and without B. alternata.
Factoring out Host Chemistry and Plant Phylogeny.
Because a concordance between Blepharida’s phylogeny and the biogeogram could be spurious because of a correlation between Bursera’s biogeography and its phylogeny or chemistry, we tested whether host geography could still explain the patterns of Blepharida’s host shifts after factoring out host chemical similarity. This meant looking for host shifts among similar geographic ranges which were not also shifts among chemically similar hosts. We used the dendrogram of host chemical similarity from Becerra (3) to quantify chemical similarity. The dendrogram is the result of a cluster analysis of a matrix of Euclidean distances between species constructed on the basis of the presence or absence of chemical compounds screened by gas chromatography. We also tested whether host biogeography could still explain the patterns of host shifts by Blepharida when host phylogeny was factored out.
Factoring out Host Geography from the Correlation Between Insect Phylogeny and Host Chemistry.
Becerra (3) previously reported that host chemical similarity could explain the patterns of host affiliation of Blepharida even when host phylogeny was factored out. This was done by quantifying the congruence between Blepharida’s phylogeny and a chemogram of Bursera species, while factoring out correlation due to plant phylogeny. Here we extend this previous analysis by comparing the insect phylogeny with the chemogram factoring out the effect of plant geography.
Comparison of Trees and Dendrograms and the Analyses of Partial Correlations.
To compare two trees or a tree and a dendrogram, we used three independent techniques. These were character tracing with macclade (18), tree mapping with the computer program component (17), and Farris’ distortion coefficient (19). The first method is graphical, whereas the latter two are statistical approaches that were selected because they are sensitive even when the topologies compared are only loosely congruent. This is an expected scenario for phytophagous insects that disperse rather freely among hosts.
Tree mapping measures the overlap between two trees or dendrograms by creating a reconciled tree of the plants and insects, under the assumption that their association is due to association by descent. To construct a reconciled tree, component modifies one of the cladograms or dendrograms by duplicating branches as necessary until it includes the topology of the other cladogram or dendrogram. There are two measures of fit that can be tested statistically by comparing the insect tree with many random host trees. “Leaves added” is the difference between the number of nodes in the insect and reconciled tree, and “losses” is the number of instances in which an insect is absent where it is predicted to occur on the reconciled tree.
Farris’ distortion coefficient provides a measure of the discordance of the branching topology of two trees, A and B, by estimating how distorted each clade of A is on B (for the purpose of comparing tree topologies, dendrograms are the same as cladograms, and clusters are clades). For each clade of A, one counts how many times the clade is fragmented on the B tree (which is the same as the number of fragments minus one). This number is divided by the maximum number of fragmentation, which equals the number of taxa of the clade minus one. For example, if a clade of A includes three taxa, and they are all separate in tree B, the coefficient for that cluster of A is 2/2 = 1 or maximum distortion. The distortion coefficient is the average of the values for all clades of A. Perfect congruence yields a coefficient of 0, and complete distortion, a value of 1. We compared the biogeogram (tree A) with the insect phylogeny (tree B) by looking at the fragmentation of the clades of the biogeogram in the insect phylogeny. The distortion coefficient was tested statistically by comparing the observed coefficient to the distribution of coefficients obtained by repeatedly randomizing the biogeogram. Randomization of the biogeogram followed the Markovian (or random speciation) model (18). Because of difficulties in programming the calculation of the coefficient when a plant species is a host of more than one species of insect, we excluded Blepharida flavocostata 3, Blepharida parallela, and Blepharida balyii from the distortion coefficient analyses.
To quantify the partial correlation of host use with geographic proximity while factoring out the correlation possibly due to plant chemistry, the distortion coefficient can be modified in the following way: Take each clade of the biogeogram that has some correlation with the insect phylogeny (i.e., not completely fragmented when traced on the insect tree). For such a clade with z species, take each unfragmented subclade and trace it onto the chemical dendrogram.
Each such unfragmented subclade will have some number of species, x. If two or more (2 ≤ n ≤ x) of these species are found in clusters of x or fewer species in the chemogram, these members are considered to be correlated with chemistry and thus are not considered as contributing to the partial correlation of biogeography with insect phylogeny. These members of the subclade contribute n − 1 possible fragmentations to the count of maximal possible fragmentations (z − 1). The procedure is to subtract n − 1 from z − 1 in the denominator for that clade of the biogeogram. For large unfragmented subclades (x ≥ 4), there may be more than one group of species found in clusters of the chemogram with x or fewer species that contain two or more members of the subclade. n − 1 must be subtracted from the denominator for each such group of size n. Clades of the biogeogram that indicate complete correlation with both the insect tree and the chemogram (unfragmented when traced in each topology) would have a denominator of zero. These are simply excluded from the calculation of the average partial distortion coefficient.
The distortion coefficient records correlation as lack of fragmentation relative to maximum possible fragmentation. The partial distortion technique assumes that unfragmented groupings that are also correlated on a third topology do not represent independent correlation, and they are not considered in the count of maximal possible fragmentation. Thus, the same amount of observed fragmentation is considered to be a fraction of a lower maximum possible fragmentation. This gives higher distortion coefficients, indicative of a lower correlation when only independent sources of correlation are considered.
For example, consider a clade of z = 5 species in the biogeogram, which has some correlation with insect phylogeny due to a subclade of x = 4 species, which is unfragmented in the insect phylogeny (distortion = 1/4 because only one of four possible fragmentations occurs). To calculate the partial distortion for this clade, first check the chemogram for clusters of four or fewer species having two or more of the four members of this subclade. Suppose that n = 2 of them are found in a cluster of four or fewer species in the chemogram. Subtract n − 1 = 1 from z − 1 = 4 from the denominator of the partial distortion of the clade of five species. The partial distortion will now equal 1/3, which is greater than the original distortion of 1/4 (i.e., less correlation) because the two species that were correlated with chemistry are no longer considered to be a source of potential distortion.
Results
Bursera’s Main Biogeographic Areas.
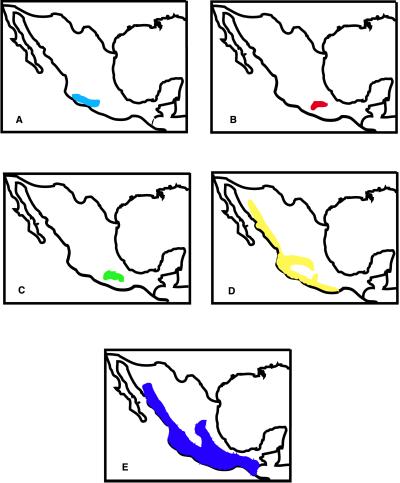
The cluster analysis with Ward’s method identified five main groups of Bursera species according to the degree of similarity of their geographical ranges (Fig. 2). Group A includes mainly those species distributed on the west side of the Balsas depression (Fig. 3A). Group B includes mostly species whose geographic range includes the lowest areas of the east side of the Balsas depression and the drier areas to the Northeast of the depression (Fig. 3B). Group C includes Bursera species that inhabit the east side of the Balsas depression, often reaching higher altitudes than species from group B and having distributions extending to the states of Puebla and Oaxaca (Fig. 3C). One species of this group, Bursera glabrifolia, is also found on the west side of the depression. In group D are the Bursera from the Pacific Coast and the southern part of the Mexican Altiplano (central highlands; Fig. 3D). Group E has three species that have wide distributions (Fig. 3E).
Figure 2.
Dendrogram of Bursera on the basis of similarity of geographical ranges (biogeogram). Letters and color coding indicate the five main biogeographical groups of plants.
Figure 3.
Bursera’s main biogeographic areas in Mexico according to our cluster analyses. Letters and colored areas indicate the geographic distribution of species groups in the biogeogram.
The topology of the dendrogram made by using Ward’s method is identical to that generated with upgma and very similar to that obtained with the complete linkage method. The groups obtained with our data and methodology are also highly concordant with well-known phytogeographic areas for Mexico (15).
Comparison of the Biogeogram with Blepharida’s Phylogeny.
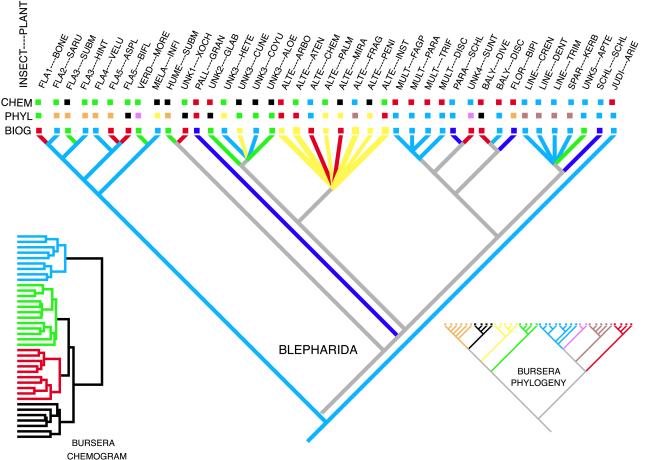
It is instructive to look at the geographic distribution of sister taxa at the tips of the insect phylogeny as well as their outgroups with unambiguous biogeographic relations (Fig. 4). Insect sister taxa are often in different biogeographic regions. Frequently these regions abut, usually involving the east and west sides of the Balsas depression (coded as blue and red or blue and green in Fig. 4). In at least one case, the abutting ranges are separated elevationally (Blepharida unknown 3 on Bursera cuneata vs. Blepharida unknown 3 on Bursera coyucensis and Bursera aloexylon). In several of these cases, the geographically abutting insect sister taxa were on phylogenetically closely related plants (e.g., B. flavocostata 4 on Bursera velutina and B. flavocostata 5 on Bursera asplenifolia). These are potential instances of plant allopatric speciation without an accompanying insect speciation or of incipient allopatric cospeciation. Yet the host plants are almost always chemically related, too. Thus, the pattern could also have resulted from chemically mediated host shifts. Another common pattern is geographically abutting insect sister taxa on chemically related but phylogenetically unrelated plants (Blepharida florhi on Bursera bipinnata and B. balyi on Bursera discolor and Bursera diversifolia). The insect sister taxa with abutting geographic ranges are more often on plants that were chemically related than phylogenetically related. This pattern suggests that the predominant mechanism is chemically mediated host shifts, occurring either before the biogeographic separation or across adjacent biogeographic regions.
Figure 4.
The five main groups of the biogeogram are traced onto Blepharida’s phylogeny. All polytomies indicate multiple host use by a single beetle species except for the polytomy that includes B. lineata, which is unresolved. The chemical, phylogenetic, and biogeographic groups of the hosts are color coded in squares above the branch tips as per Insets and Fig. 2.
Included ranges are a less common biogeographic pattern (Fig. 4). This situation occurs when one insect taxon is on a widespread plant species while its sister taxon is on one or more narrowly distributed plants whose ranges are included within the larger range (e.g., Blepharida schlechtendalii on widespread Bursera schlechtendalii and the beetle sister taxa on several Bursera with included ranges). When the directionality of the change in host use is resolved, the “included species” are always derived. These cases usually involve chemically related plants that are phylogenetically unrelated, suggesting sympatric chemically mediated host shifts followed by insect speciation.
Some evolutionary patterns of host use in Fig. 4 involve plant species in the same biogeographic regions. These always involve the use of multiple hosts by a single insect species (e.g., Blepharida lineata). These insect species usually attack several chemically and phylogenetically related hosts. This pattern suggests that Blepharida on different hosts with broadly overlapping species ranges have not diverged or speciated.
B. alternata is an exceptional species that has been able to colonize a variety of chemically and phylogenetically unrelated species of Bursera within its biogeographic region. The distribution of this beetle is primarily coastal. Although some Bursera from this biogeographic cluster occur in the central highlands, we have never collected B. alternata there. We have found it on two other Bursera species (B. excelsa and B. laxiflora) not included in this analysis that also occur in the Pacific Coast region. This species nicely illustrates the pattern we would expect if geographic range were the predominant factor determining changes in host use.
The general trends seen with character tracing were confirmed independently by the two statistical methods (Table 1). The congruence between the insect phylogeny and the plant biogeogram was not significant according to tree mapping (“leaves added,” P < 0.09; “losses,” P < 0.09) and only marginally significant with the Farris’ distortion coefficient when the polyphagous species B. alternata was not included (P = 0.055; Table 1). However, when this species was included, both tests were significant (“leaves added,” P < 0.04; “losses,” P < 0.007; Farris’ distortion coefficient (DC), P = 0.038).
Table 1.
Correlation of Blepharida’s phylogeny (topology B) with host biogeography, host phylogeny, and host chemistry (topology A)
| Topology A
|
Topology B
|
DC
|
PDC
|
||
|---|---|---|---|---|---|
| Excluding Blepharida alternata | |||||
| Discounting the correlation with plant chemistry | Discounting the correlation with plant phylogeny | Discounting the correlation with plant biogeography | |||
| Plant biogeogram | Insect phylogeny | 0.89 P = .055 | 0.91 P < .1 | 0.90 P < .1 | — |
| Plant phylogeny | Insect phylogeny | 0.89 P = .013 | 0.91 P < .1 | — | 0.88 P < .05 |
| Plant chemogram | Insect phylogeny | 0.73 P < .001 | — | 0.76 P < .05 | 0.77 P < .05 |
| Including Blepharida alternata | |||||
| Plant biogeogram | Insect phylogeny | 0.92 P = .038 | 0.92 P < .05 | 0.92 P < .05 | — |
| Plant phylogeny | Insect phylogeny | 0.90 P = .005 | 0.94 P < .1 | — | 0.92 P < .05 |
| Plant chemogram | Insect phylogeny | 0.83 P < .001 | — | 0.84 P < .05 | 0.87 P < .05 |
When B. alternata was excluded from the analyses, the correspondence between the insect phylogeny and the biogeogram was lower and not significant after factoring out host chemistry [partial DC (PDC), P < 0.1) or host phylogeny (PDC, P < 0.1; Table 1). When B. alternata was included, the modified distortion coefficient remains significant when either plant chemistry (PDC, P < 0.05) or host phylogeny (PDC, P < 0.05) was factored out.
The trends reported by Becerra (3) were confirmed by new analyses. The correlation between plant phylogeny and insect phylogeny was nonsignificant when plant chemistry was factored out, either including or excluding the B. alternata (Table 1). The probabilities associated with the randomizations in this investigation are different because in that study (3), the insect phylogeny was randomized. It is better to randomize the plant phylogeny because it is fully resolved, whereas the insect phylogeny has polytomies. In the new analysis, the simple correlation between plant phylogeny and insect phylogeny was significant and remained significant when the correlation with plant biogeography was removed. This is probably because of an overall lack of correlation between plant phylogeny and plant biogeography. We also found that plant biogeography does not affect the high correlation between plant chemistry and insect phylogeny (Table 1).
Discussion
If host use of Blepharida beetles is determined primarily by similarity in the geographic range of species in the host genus, we would expect high congruence between the insect phylogeny and the dendrogram of similarity in host geographic ranges. This expected pattern is nicely illustrated by one exceptional beetle species: B. alternata. We do not yet know the underlying factors promoting polyphagy in this species. Yet, the preeminent pattern for the genus as a whole is one of using chemically similar, sometimes phylogenetically similar, plants that often have allopatric distributions. Some beetle sister taxa have included ranges, pairing a widespread taxon with another having a more restricted included range. For these, the hosts are most often chemically related phylogenetically distant taxa. When beetles do use multiple hosts with similar geographic ranges, the hosts are usually chemically and phylogenetically related (except in the case of B. alternata).
Several authors believe that much of the diversification of extant Bursera is recent and a result of the enclosure of the Balsas depression. During the Pliocene–Pleistocene, intense orogenic activity raised the Neovolcanic axis and closed off the depression. The cold and warm cycles of the glaciations during Pleistocene may have contributed to Bursera’s diversification during this time (13). Because the ranges of some Bursera populations may have contracted and expanded, distributions could have fragmented, resulting in isolation and differentiation. Such historical events could give rise to allopatric cospeciation and result in a current pattern of related insect taxa on plants with abutting allopatric ranges or even included ranges. Although this is a plausible scenario for some allopatric Blepharida sister taxa, most are on chemically related but phylogenetically unrelated plants. None of the insect sister taxa with included ranges are on phylogenetically closely related plants, precluding the allopatric cospeciation explanation for them.
The abutting allopatric distributions and included distributions of many Blepharida sister taxa suggest that allopatric speciation in conjunction with orogenesis and climatic fluctuations may have been involved in insect speciation, too. Yet being on phylogenetically related plants appears to actually restrict Blepharida diversification. This pattern is especially clear for the allopatric abutting species that are hosts of insect sister taxa. Among these are found both related hosts with undifferentiated insects and unrelated hosts with differentiated insects. Plants with included ranges that are hosts of insect sister taxa tend to be unrelated and have well-differentiated insects on them. Plants with widely overlapping ranges that are hosts of related insects (excluding the case of B. alternata) tend to be phylogenetically related and have conspecific undifferentiated beetles.
It is common in Central Mexico to find several to many species of Bursera co-occurring. It is striking that beetles in such places rarely shift among chemically unrelated hosts despite having the ecological opportunity to do so. Co-occurrence should make beetle oviposition mistakes likely, yet a beetle in a given place primarily uses one or more hosts with similar chemistry. This may reflect the high dependence of Blepharida adults and larvae on Bursera’s chemistry. Adults mate and females feed on host plants before oviposition. Host location by adults is very probably cued on these plants’ volatile compounds (14). In many Blepharida species, larvae use their host compounds extensively for their own defense. They festoon themselves with their own feces, and readily regurgitate and defecate when disturbed by predators. These enteric discharges contain their host’s natural products (20, 21).
Up to now, there have been almost no studies that have tested the relative importance of different factors in the evolution of herbivore–host affiliation. Our study suggests that the importance of different factors may be quite variable. The single polyphagous species responds more to the geographic distribution of host plants, whereas the monophagous species have responded more strongly to plant chemistry.
Of the three factors tested, host chemistry best explains the overall patterns of host shifts by Blepharida beetles. As Bursera species evolved, they acquired new chemical compounds, often convergently in unrelated species (3). Blepharida, instead of coping with new defenses either in time or space, went on attacking species with similar chemistry.
This work was supported by National Science Foundation grants INT-9505941 and DEB-9815648, and by the National Geographic Society.
Abbreviations
- DC
Farris’ distortion coefficient
- PDC
partial DC
References
- 1.Ehrlich P R, Raven P H. Evolution. 1964;18:586–608. [Google Scholar]
- 2.Berenbaum M. Evolution. 1983;37:163–179. doi: 10.1111/j.1558-5646.1983.tb05524.x. [DOI] [PubMed] [Google Scholar]
- 3.Becerra J X. Science. 1997;276:253–256. doi: 10.1126/science.276.5310.253. [DOI] [PubMed] [Google Scholar]
- 4.Futuyma D J, McCafferty S S. Evolution. 1990;44:1885–1913. doi: 10.1111/j.1558-5646.1990.tb04298.x. [DOI] [PubMed] [Google Scholar]
- 5.Köpf A, Rank N E, Roininen H, Julkunen-Thtto R, Pasteels J, Tahvanainen J. Evolution. 1998;52:517–528. doi: 10.1111/j.1558-5646.1998.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 6.Farrell B, Mitter C. Evolution. 1990;44:1389–1403. doi: 10.1111/j.1558-5646.1990.tb03834.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernays E A, Chapman R F. Host-Plant Selection by Phytophagous Insects. New York: Chapman & Hall; 1994. pp. 261–263. [Google Scholar]
- 8.Mardulyn P, Milinkovitch M, Pasteels J M. Syst Biol. 1997;46:722–747. doi: 10.1093/sysbio/46.4.722. [DOI] [PubMed] [Google Scholar]
- 9.Furth D G. Mem. Ent. Soc. Washington. 1998. Num 21. [Google Scholar]
- 10.Furth D G. J N Y Entomol Soc. 1992;100:399–414. [Google Scholar]
- 11.Rzedowski J, Kruse H. Taxon. 1979;28:103–116. [Google Scholar]
- 12.Becerra J X, Venable D L. Am J Bot. 1999;86:1047–1057. [PubMed] [Google Scholar]
- 13.Toledo C. B.S. thesis. Mexico, D.F.: Universidad Nacional Autónoma de México; 1982. [Google Scholar]
- 14.Becerra J X. Ecology. 1994;276:1991–1996. [Google Scholar]
- 15.Kohlmann B, Sanchez-Colon S. Metodos Cuantitativos en la Biogeografia. Mexico, D. F.: Publicaciones del Instituto de Ecologia; 1984. [Google Scholar]
- 16.SAS Institute Incorporated. sas/stat User’s Guide, Version 6. NC: Cary; 1989. [Google Scholar]
- 17.Page R. Component User’s Manual. 1992. , release 2.0. (Natural History Museum, London). [Google Scholar]
- 18.Maddison D, Maddison W. macclade Version 3. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 19.Farris J S. Syst Zool. 1973;72:50–54. [Google Scholar]
- 20.Vencl F C, Morton T C. Chemoecology. 1998;8:25–32. [Google Scholar]
- 21.Evans, P. H., Becerra, J. X., Venable, D. L. & Bowers, W. L. Chem. Ecol., in press.