Abstract
To understand the structure, role, and regulation of individual Ca2+ pumps in plants, we have used yeast as a heterologous expression system to test the function of a gene from Arabidopsis thaliana (ECA1). ECA1 encoded a 116-kDa polypeptide that has all the conserved domains common to P-type Ca2+ pumps (EC 3.6.1.38). The amino acid sequence shared more identity with sarcoplasmic/endoplasmic reticulum (53%) than with plasma membrane (32%) Ca2+ pumps. Yeast mutants defective in a Golgi Ca2+ pump (pmr1) or both Golgi and vacuolar Ca2+ pumps (pmr1 pmc1 cnb1) were sensitive to growth on medium containing 10 mM EGTA or 3 mM Mn2+. Expression of ECA1 restored growth of either mutant on EGTA. Membranes were isolated from the pmr1 pmc1 cnb1 mutant transformed with ECA1 to determine if the ECA1 polypeptide (ECA1p) could be phosphorylated as intermediates of the reaction cycle of Ca2+-pumping ATPases. In the presence of [γ-32P]ATP, ECA1p formed a Ca2+-dependent [32P]phosphoprotein of 106 kDa that was sensitive to hydroxylamine. Cyclopiazonic acid, a blocker of animal sarcoplasmic/endoplasmic reticulum Ca2+ pumps, inhibited the formation of the phosphoprotein, whereas thapsigargin did not. Immunoblotting with an antibody against the carboxyl tail showed that ECA1p was associated mainly with the endoplasmic reticulum membranes isolated from Arabidopsis plants. The results support the model that ECA1 encodes an endoplasmic reticulum-type Ca2+ pump in Arabidopsis. The ability of ECA1p to restore growth of mutant pmr1 on medium containing Mn2+, and the formation of a Mn2+-dependent phosphoprotein suggested that ECA1p may also regulate Mn2+ homeostasis by pumping Mn2+ into endomembrane compartments of plants.
Calcium is not only an important intracellular signal for many stimuli-induced responses in eukaryotes (1), it is also essential for the functioning of the secretory system. A variety of signals can trigger the opening of Ca2+-specific channels on the plasma membrane and endomembranes, causing massive Ca2+ influx and accumulation in the cytoplasm. The fluctuation in cytosolic Ca2+ directly elicits responses by altering the function of Ca2+-binding proteins and their targets. The increase in cytosolic Ca2+ is transient as Ca2+ pumps and antiporters at the plasma membrane (PM) or internal membranes become activated and restore cytosolic Ca2+ to basal levels. In addition to a role of Ca2+ in signaling, lumenal Ca2+ concentration ([Ca2+]) is emerging as an important player in the secretory system. For example, pmr1 mutant defective in a Golgi Ca2+ pump secretes proteins that in wild-type cells are retained in the endoplasmic reticulum (ER) (2, 3). In mammalian cells, the correct folding and assembly of proteins depend on chaperones, like a Ca2+-binding protein, calnexin (4). Thus intralumenal Ca2+ supplied by pumps would be required for the normal operation of the secretory system in plants (5). Although Ca2+ pumps play an important role in both signaling and secretion, the properties and regulation of individual Ca2+ pumps that fine tune cytosolic [Ca2+] and supply lumenal Ca2+ are not well understood in plants (1).
The literature illustrates the diversity of Ca2+ pumps on the plasma membrane and endomembranes from a variety of plants; however, biochemical distinction among the pumps has been difficult for several reasons. The pumps share many similarities as P-type ATPases, and there is a lack of distinguishing features such as specific inhibitor sensitivity. Furthermore, each pump type is not necessarily restricted to one particular organelle or membrane. A few biochemical traits are useful in discriminating between two major types of Ca2+ pumps in plants. The PM-type pump is energized by GTP or ATP and is stimulated by calmodulin. This type of pump can be located on endomembranes, like the vacuole (6, 7), as well as the plasma membrane (7, 8). Another pump is associated mainly with the ER. This ER-type pump hydrolyzed ATP preferentially and was inhibited by cyclopiazonic acid (CPA), but not stimulated by calmodulin (7). Although PM-type Ca2+-ATPases have been partially purified by calmodulin-affinity chromatography, several related calmodulin-stimulated Ca2+ pumps could be copurified simultaneously. Thus, a molecular approach to study individual pumps is necessary.
In spite of the multiplicity of Ca2+ pumps, none of the genes encoding Ca2+ pump homologs isolated so far have been functionally characterized. For example, Wimmers et al. (9) isolated a gene (LCA) encoding a protein that shares ≈50% identity with animal sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA). The deduced polypeptide of LCA has 1048 amino acids (116 kDa), 8 transmembrane (TM) domains, and all of the highly conserved domains of P-type translocating ATPases. Another gene (PEA1) isolated from Arabidopsis thaliana encoded a polypeptide that is 40–44% identical to various mammalian PM-type Ca2+-ATPase (10). PEA1p (plastid envelope ATPase) was located to the chloroplast inner envelope, although its function is unclear. Recently, a related gene was identified in cauliflower. Based on sequence identity with tryptic peptides, BCA1 appeared to encode a calmodulin-stimulated Ca2+ pump previously purified from endomembranes (11).
Here we demonstrate that a gene from A. thaliana (ECA1) complemented yeast mutants defective in Ca2+ pumps by restoring their growth on EGTA. The protein encoded by the ECA1 gene formed a phosphoprotein that has characteristics of a phosphorylated intermediate of Ca2+-pumping ATPases. Thus we demonstrate a plant gene encoding a functional ER-type Ca2+-ATPase.
MATERIALS AND METHODS
Yeast Strains and Their Growth Media.
Saccharomyces cereviciae strains W303–1A (MATa, leu2, his3, ade2, trp1, ura3), pmr1 AA542 (MATa, pmr1::HIS3, leu2, ade2, trp1, ura3), pmr2 K633 (MATa pmr2::HIS3, leu2, ade2, trp1,ura3), triple mutant K616 (MATa pmr1::HIS3 pmc1::TRP1 cnb1::LEU2, ura3) were used (12). Wild-type and mutant strains were grown for 24 h in standard YPD medium (yeast extract/peptone/dextrose) for transformation except for strains AA542 and K616 which were supplemented with 10 mM CaCl2. Transformants were selected on synthetic complete medium minus uracil (SC-URA). The medium consisted of 6.7 g/liter yeast nitrogen base without amino acids, 2 g/liter of drop-out mix without uracil and 2% glucose as a carbon source (13).
DNA Manipulations.
Screening cDNA libraries and DNA sequencing. An A. thaliana (L. cv. Columbia) leaf cDNA library in λZAP (14) was screened with a partial cDNA clone encoding a putative Ca2+-ATPase from tobacco (15). A 1.9-kb clone was isolated and used to probe a size-fractionated cDNA library (3–6 kb) prepared from hypocotyls (16). Five positive clones were isolated and in vivo excised into pBluescript. Restriction mapping and partial sequencing of these clones showed that they fell into two groups, named ECA1 (3.3 kb) and ECA2 (2.5 kb). ECA1 cDNA was restricted and subcloned into pUC18 and pUC19. Both strands were sequenced by the dideoxynucleotide chain-termination method (17) with T7 polymerase (Pharmacia). The DNA sequences were analyzed using the Wisconsin Package gcg program as well as Mac dnasis.
Construction of expression plasmid.
Taking advantage of an EcoRI site 6 bp before the initiation codon, ATG, the entire open reading frame of ECA1 plus a partial 3′-untranslated region was excised with EcoRI from pBluescript. The 3.2-kb fragment was subcloned into a yeast expression vector, p426Gal1, at the EcoRI site between the yeast galactokinase gene (Gal1) promoter and CYC1 termination sequence (18). The orientation and junctions of the construct, designated as pECA1, were verified by sequencing.
Yeast Transformation and Growth.
The wild-type and mutants strains of S. cereviciae were transformed with p426 vector alone or with pECA1 by the LiOAc/PEG methods (19) and selected for uracil prototrophy by plating on SC-URA medium. The Ura+ colonies were picked and grown for 2–3 days in SC-URA medium for complementation studies.
Isolation of Yeast Membranes.
Transformants were inoculated into 20 ml of SC-URA medium and incubated overnight. The culture was diluted 10-fold into SC-URA/Gal medium and grown overnight to an OD600 of 1–1.8. The cells were pelleted at 4,000 × g for 1 min, washed with 10 ml of distilled water, and pelleted. Membranes were isolated using the glass bead method with some modification (20). Cells were suspended in 10 ml of glass beads buffer (GBB; 10% sucrose/25 mM Hepes-KOH, pH 7.5/3 mM EGTA/2 mM DTT) and repelleted. Typically 1–2 ml cells were resuspended in 1 volume of GBB plus 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM benzamidine, 5 μg/ml pepstatin and leupeptin, and 0.5 mg/ml BSA. An equal volume of glass beads (Sigma) was added and the mixture was vortex mixed for 2 min at maximum speed. The lysate was centrifuged at 5,000 × g for 5 min and the supernatant was saved. The pellet was suspended in 1 volume of GBB plus protease inhibitors, vortex mixed, and centrifuged as above. The supernatants were pooled and layered onto a 25/45% sucrose step gradient containing 20 mM Hepes-KOH (pH 7.0), 1 mM DTT, 0.2 mM PMSF, and 5 mM benzamidine and centrifuged at 108,000 × g for 2 h. Membranes at the 25/45% sucrose interface were collected and stored at −80°C. Protein concentration was determined with the Bio-Rad reagent.
Formation of Phosphoenzyme (PE).
[32P]Phosphoprotein formation was assayed according to Chen et al. (21) with some modification. The reaction mixture (200 μl) contained 25 mM Hepes-1,3-bis[Tris (hydroxymethyl)methylamino]propane (BTP) (pH7.0), 100 mM KCl, and 5–20 μg membrane protein. To test cation dependence, EGTA was added to a final concentration of 500 μM to reduce the free Ca2+ concentration to <10 nM (22). Then various divalent salt was added to a total concentration of 1 mM MgSO4, 540 μM CaCl2, or 500 μM MnCl2 so that the final free concentration was 989 μM, 45 μM, or 3 μM, respectively, as computed with the maxchelator program (23). The reaction was started by adding [γ-32P]ATP (1–2 mCi/reaction; 1 Ci = 37 GBq) (Amersham; 3,000 Ci mmol−1) to a final concentration of 2 or 100 nM, and terminated after 15 or 120 sec by adding 0.2 ml of a stop solution containing 50 mM NaH2PO4, 2 mM ATP and 20% trichloroacetic acid (TCA). The TCA precipitate was pelleted, washed with a solution containing 10% TCA, 25 mM NaH2PO4, and 1 mM ATP, and pelleted again. The pellet was suspended in 30 μl of sample buffer and subjected to SDS/PAGE (pH 6.0) and autoradiography (21).
Fractionation of Membranes from Arabidopsis.
Five grams of A. thaliana L. cv. Columbia plants grown in B5 liquid culture for 4 weeks was homogenized with a mortar and pestle in 50 mM Hepes-BTP (pH 7.4), 250 mM sorbitol, 3 mM EGTA, 2 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 0.5% BSA. The homogenate was centrifuged at 12,000 × g for 10 min, and the resulting supernatant was centrifuged at 110,000 × g for 50 min. The microsomal pellet was resuspended in the above solution without BSA and layered onto a 12-step gradient with 1.2 ml each of 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, and 45% sucrose. The sucrose solutions contained 25 mM BTP (pH 7.0), 2 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 2 mM benzamidine. After centrifugation at 110,000 × g for 16 h (Beckman SW28), 0.75-ml fractions were collected and stored at −80°C.
Immunostain.
Membranes from yeast or from the sucrose gradient fractions (25 μl) of Arabidopsis were mixed with an equal vol. of a 2× sample buffer. The proteins were separated on a 7% SDS/polyacrylamide gel, blotted onto Immobilon-P and probed with several antibodies (24). Polyclonal antibody (anti-ACA3 1374) was generated against a glutathione S-transferase fusion protein containing the last 27 residues of ECA1 polypeptide (ECA1p). Monoclonal antibody 11A1 recognized calnexin (X. Li and H.S., unpublished data).
Chemicals.
Erythrosin B and CPA were obtained from Sigma, and thapsigargin was purchased from LC Service (Woburn, MA). All other chemicals were reagent grade.
RESULTS
ECA1 Encodes a P-type Cation Pump Homolog.
Arabidopsis cDNA libraries were screened with a partial cDNA encoding a putative Ca2+-ATPase from tobacco (15). Five positive clones were obtained and sequence analysis showed that a 3.3-kb clone, named ECA1, was nearly full-length and identical to ACA3 (J.F.H., unpublished data). The translation initiation codon of ECA1 was identified at position 72, as this ATG was preceded by a stop codon and was contiguous to a consensus sequence (CAUGG) of translation initiation sites (25). ECA1 contained an open reading frame of 1,061 amino acids. The deduced polypeptide had a molecular mass of 116,359 Da, and all the highly conserved domains characteristic of P-type cation pumps (Fig. 1). Ten TM domains were predicted by hydrophobic moment analysis (26) and by analogy to SERCA pumps (27). ECA1p shared 67% and 53% identity to a tomato Ca2+-ATPase, LCA1p (9), and to a mammalian muscle SERCA 2a pump (27), respectively; but less than 32% identity to PEA1, a putative Ca2+-ATPase on the chloroplast envelope (10). A hydrophilic domain between transmembrane domain 4 (TM4) and TM5 of ECA1p included a potential aspartyl phosphorylation site within the motif CSDK, and ATP-binding domains conserved in P-type ion pumping ATPases (Fig. 1). TM5 and -6 of ECA1p shared 82% and 88% identity to the corresponding TM domains of the rabbit SERCA pump. Significantly, six amino acids within TM4, -5, -6, and -8 (see Fig. 1) required for high-affinity Ca2+ transport in animal SERCA pumps (28) are all conserved in ECA1p. Sequence analyses therefore strongly suggested ECA1 encoded a P-type Ca2+ pump (EC 3.6.1.38).
Figure 1.
Alignment of the deduced amino acid sequences of ECA1 from Arabidopsis and of rabbit SERCA 2a (SER) (27) illustrates the conserved domains of P-type Ca2+-ATPases. The potential phosphorylation site (Asp-383) and two regions (533–536 and 727–740) that form the putative ATP-binding domain (underlined in bold) are located in the central hydrophilic loop. Each TM region is indicated with a line above the ECA1 sequence and a line below the corresponding SERCA TM domain. Potential Ca2+-binding sites (E341, E800, N825, T828, D829, E961) within predicted TM4, -5, -6, and -8 in ECA1 correspond to residues (marked with an asterisk below the residue) required for Ca2+ transport in rabbit SERCA pump (28). Residues sharing identity or similarity are denoted by | and :, respectively. Spaces shown as periods were introduced to maximize the alignment. The alignment was performed with gap in the Wisconsin Package gcg program.
Complementation of Yeast Mutants Defective in Ca2+ Pumps by pECA1.
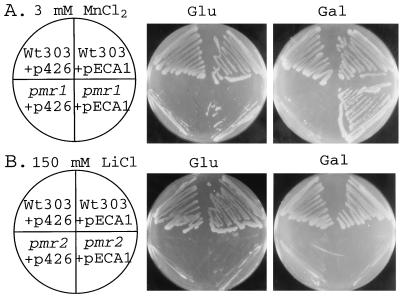
To test the function of ECA1p in yeast mutants defective in either Na+ pumps or Ca2+ pumps, the 3.2-kb ECA1 cDNA was subcloned into a yeast expression vector, p426Gal1, under the control of a galactose-inducible promoter. Wild-type yeast transformed with vector (p426) alone grew in a medium containing 3 mM Mn2+ (Fig. 2A) or 10 mM EGTA (Fig. 3A). However, pmr1 mutants in which a Golgi Ca2+ pump was disrupted were sensitive to Mn2+ toxicity (Fig. 2A) and to EGTA (Fig. 3A) as shown before (2, 29). When pmr1 mutants were transformed with pECA1, growth on SC-URA medium containing either Mn2+ (Fig. 2A) or EGTA (Fig. 3A) was restored in the presence of galactose, but not of glucose. In contrast, pECA1 was unable to restore growth of pmr2 mutant on 150 mM LiCl (Fig. 2B) or 1 M NaCl (data not shown). PMR2 gene cluster encodes putative Na+ and Li+ efflux pumps on the plasma membrane that are required for tolerance of these ions (30). Thus ECA1p was more effective as a Ca2+ pump than as a Li+ or Na+ efflux pump under these experimental conditions.
Figure 2.
pECA1 restored the growth of yeast pmr1 mutant on medium with Mn2+ but not of pmr2 mutant on Li-containing medium. Wild-type (W303), pmr1 (AA542), and pmr2 (K633) cells were transformed with either a high-copy plasmid vector (p426) alone or the vector containing the Arabidopsis ECA1 (pECA1). The cells were streaked on SC-URA plates containing glucose (Glu) or galactose (Gal) plus either 3 mM MnCl2 (A) or 150 mM LiCl (B) and incubated for 4 days at 30°C.
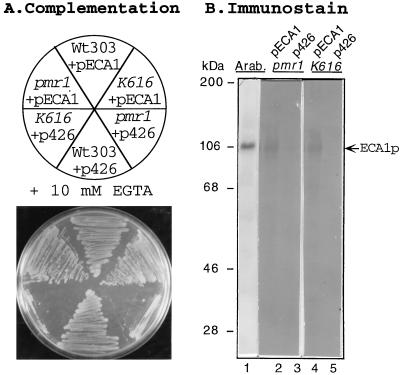
Figure 3.
Complementation of growth on EGTA by pECA1 is accompanied by expression of the ECA1 polypeptide in pmr1 and pmr1 pmc1 cnb1 mutants. Wild-type yeast (W303), pmr1 (AA542), and pmr1 pmc1 cnb1 (K616) were transformed with control vector (p426) or pECA1 as in Fig. 2. (A) The cells were streaked on SC-URA plates containing galactose and 10 mM EGTA at pH 6.2 and incubated for 3 days at 30°C. (B) To detect the ECA1 polypeptide, microsomal membranes were isolated from pmr1 and pmr1 pmc1 cnb1 mutants transformed with p426 alone (lanes 3 and 5) or pECA1 (lanes 2 and 4). Protein (5 μg) was separated by SDS/PAGE, blotted, and immunostained with antibody against ECA1p (1:50,000). Lane 1 was loaded with microsomal membranes isolated from Arabidopsis (1:500).
ECA1 expression also complemented the Mn2+ and EGTA sensitivity of a triple mutant (K616) which lacked both endogenous Ca2+ pumps (PMR1 and PMC1) and calcineurin (CNB1) function. PMC1 encodes a vacuolar Ca2+ pump required by cells for growth on medium containing high [Ca2+] (12). Disruption of the gene encoding the B subunit of the calcineurin gene (cnb1) was necessary as the pmr1 pmc1 double mutant is inviable unless calcineurin inhibition of a vacuolar H+/Ca2+ antiporter (Vcx1p) was relieved (29). Like the pmr1 mutant, the triple mutant transformed with the vector alone grew poorly on a medium containing Mn2+ (data not shown) or very low Ca2+ (+10 mM EGTA) (Fig. 3A). However, the triple mutant transformed with pECA1 became tolerant of both EGTA (Fig. 3A) and 3 mM Mn2+ (data not shown) supporting the idea that ECA1 encoded a functional Ca2+ pump.
Complementation of growth on EGTA-containing media was accompanied by the expression of ECA1p in transformants. A polyclonal antibody against the carboxyl tail of ECA1p recognized a 106-kDa polypeptide in microsomal membranes isolated from pECA1-transformants (Fig. 3B, lanes 2 and 4), but not from yeast transformed with a control vector (Fig. 3B, lanes 3 and 5). The antibody was highly specific, as it recognized one major polypeptide in microsomal membranes from Arabidopsis plants (Fig. 3B, lane 1). The similar size of proteins expressed in Arabidopsis plants and in the pECA1-transformed mutants indicated that the coding region in ECA1 was full-length, although the deduced polypeptide was about 10 kDa larger than its apparent size estimated by SDS/PAGE.
ECA1p Forms a Ca2+-Dependent PE.
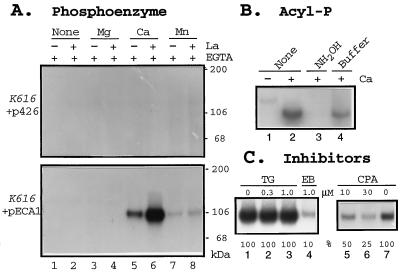
If ECA1p is a P-type Ca2+-pumping ATPase, it should form a phosphorylated intermediate as a part of the reaction cycle (31). To test this, microsomes isolated from pECA1-transformed or p426-transformed K616 were incubated with [γ-32P]ATP under various conditions. One major phosphoprotein of 106 kDa was formed in membranes isolated from pECA1-transformed K616 (Fig. 4A Lower), but not from p426-transformed K616 (Fig. 4A). Phosphorylation was dependent on the presence of Ca2+, but not Mg2+. La3+enhanced the steady-state level of the phosphoprotein several fold (Fig. 4A, lanes 5 and 6). The denatured PE was sensitive to hydroxylamine (Fig. 4B), indicating the hydrolysis of an acyl phosphate bond (32) probably to Asp-383 (Fig. 1). Together, these results provide compelling evidence that ECA1p is a P-type Ca2+-dependent ATPase.
Figure 4.
Formation of a Ca2+-dependent phosphoprotein is inhibited by CPA. (A) Ca2+-dependent PE. Membranes were isolated from triple mutants, K616, transformed with either pECA1 (Lower) or with vector alone (Upper). Membranes were incubated with 2 nM [32P]ATP for 2 min with 0.5 mM EGTA alone (none), or with added divalent cations to give a final free concentration of 990 μM Mg2+, 45 μM Ca2+, or 3 μM Mn2+ in the presence (+) or absence (−) of 50 μM La3+. The reaction was stopped with TCA, and the proteins were analyzed by SDS/PAGE and autoradiography. (B) Sensitivity of PE to hydroxylamine. Membranes were isolated from pECA1-transformed triple mutants. PE formed without La3+ was terminated with TCA (as in A). The TCA pellet was either untreated (lanes 1 and 2) or incubated with 1 ml of 0.5M hydroxylamine (NH2OH) in 100 mM Mes-KOH buffer at pH 6.0 (lane 3) or with buffer alone (lane 4) for 15 min. Protein was precipitated with 0.2 ml 50% TCA. (C) Inhibition of PE formation by erythrosin B (EB) and CPA, but not by thapsigargin (TG). Membranes isolated from triple mutant transformed with pECA1 were incubated with DMSO (lanes 1 and 7) or inhibitors for 20 min before assay. PE formation was assayed with 45 μM Ca2+, and 100 nM ATP for 15 sec without La3+. PE was quantitated by PhosphorImager.
It is interesting that CPA, a blocker of animal SERCA pumps, inhibited the phosphorylation of ECA1p, whereas another SERCA ATPase inhibitor, tharpsigargin (33), had little or no effect (Fig. 4C). CPA decreased the initial rate of PE formation by 50% at concentrations (10 μM) that block the activity of animal SERCA-type ATPase (34). The initial rate of PE formation was inhibited 90% by 1 μM erythrosin B (Fig. 4C, lane 4), a halogenated derivative of fluorescein that binds to nucleotide-binding sites with high affinity and specificity (35). Erythrosin B is a potent inhibitor of both PM-type and ER-type Ca2+-ATPases from plants (36). These results suggested ECA1 encoded a Ca2+ pump that was related to the ER-type.
The ability of pECA1 to restore growth of pmr1 or K616 mutants on Mn2+-containing medium (Fig. 2) suggested the possibility that this ATPase might pump Mn2+ in addition to Ca2+. If so, binding of Mn2+ to the ATPase should stimulate the formation of a phosphorylated intermediate as seen above with Ca2+. To test this idea, EGTA was added to the reaction mixture to chelate divalent cations. When Mn2+ was added to give a final free concentration of about 3 μM, a phosphoprotein of 106 kDa was formed (Fig. 4A, lanes 7 and 8). La3+ slightly enhanced the steady-state levels of Mn2+-dependent phosphoprotein. The results supported the idea that ECA1p is a divalent cation pump with specificity for Mn2+ as well as Ca2+.
ECA1p Is Mainly Localized on ER Membranes.
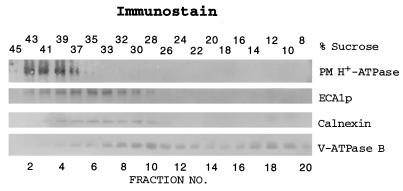
To determine the subcellular location of ECA1p, membranes were isolated from Arabidopsis plants and fractionated by a sucrose density gradient. ECA1p migrated to a density of 33–35% sucrose, similar to that of calnexin, an ER chaperone (Fig. 5) (4). The peak of ECA1p was separated from the PM (39–41%) and the vacuolar membrane (26–30%), which were detected by immunoblotting with antibodies to the PM H+-ATPase and to the V-ATPase subunit B, respectively. When Mg2+ was present in the homogenization buffer and in the sucrose gradient, the peak of ECA1p was shifted to 38% sucrose in parallel with calnexin (data not shown). These results demonstrated that ECA1p was mainly localized in the ER. Because ECA1p was distributed in membranes at a density higher than that of calnexin, ECA1p could also be localized on other organelles, such as the Golgi.
Figure 5.
ECA1p was mainly localized on the ER of Arabidopsis plants. Membrane (2–6 μg protein) fractionated by a sucrose gradient was separated by SDS/PAGE, blotted, and probed with either polyclonal antibodies against ECA1p (1:500 dilution), anti-CTF2 (37) against the C-terminal end of PM H+-ATPase, AHA2 (1:10,000), or monoclonal antibodies 11A1 against oat calnexin (1:100) and 2E7 (24) against the B subunit of oat V-ATPase (1:500). One experiment is representative of two experiments.
DISCUSSION
A Plant Gene, ECA1, Encodes a P-Type Ca2+-ATPase.
Using yeast as a heterologous expression system (38), we provide the first direct evidence that a cloned plant gene encodes a functional Ca2+-ATPase. Overexpression of an Arabidopsis gene, ECA1, restored the growth of yeast mutants (pmr1 or K616) defective in Ca2+ pumps on a medium containing submicromolar levels of Ca2+ (Fig. 3). Wild-type yeast contains two Ca2+ pumps. Pmr1p pumps Ca2+ (39) into the Golgi compartment (2, 3) to support a variety of secretory functions required for normal growth rates. Pmc1p is required for efficient Ca2+ sequestration into the vacuole and is necessary for tolerance of high external [Ca2+] (12). Wild-type yeast grows well on a medium with submicromolar concentrations of Ca2+, apparently because the two Ca2+ pumps can sequester Ca2+ and thus increase [Ca2+] in endomembrane compartments. Growth of pmr1 or triple mutants on medium with 10 mM EGTA was poor, probably because [Ca2+] in the Golgi and secretory vesicles was insufficient to support normal growth rates (2, 12). Restoration of growth of pECA1-transformed triple mutant or pmr1 on EGTA medium (Fig. 3) would suggest that endolumenal [Ca2+] has been sufficiently increased to support normal growth rates. Thus the function of ECA1p in yeast resembled closely the function of the Golgi Ca2+ pump, Pmr1p, although the two proteins shared only 37% identity. The results suggested that pECA-transformed cells expressed an active Ca2+ pump on the endomembranes of either pmr1 or the triple mutant. This idea is supported by in vitro studies. As Ca2+ pumping by ECA1p was masked by the activity from the vacuolar H+/Ca2+ antiporter (data not shown), we determined whether ECA1p could be phosphorylated in a manner similar to PE intermediates formed during the reaction cycle of Ca2+-pumping ATPases.
The mechanism of P-type ion pumping ATPases involves a conformational change of two alternate phosphorylated intermediates (E1E2) as part of the reaction cycle. The reaction cycle is essential for coupling ATP hydrolysis to ion transport. Briefly, the sequence of reactions for a SERCA pump (31) is as follows: (i) two Ca2+ bind to the high-affinity binding sites (Km = 0.2–2 μM) on the cytoplasmic face of E1 thus activating the ATP-binding site; (ii) ATP binds and transfers its terminal phosphate to an aspartyl residue to form an acyl phosphate bond (E1∼P); (iii) a conformational change (E2-P) occurs so that the bound Ca2+ ions face the exoplasmic surface; (iv) Ca2+ are released to the exterior as the Km of the Ca2+ binding site in E2P is 1–3 mM; and (v) the acyl phosphate is hydrolyzed to regenerate E1. The sensitivity to hydroxylamine indicated that the phosphate was linked to the ECA1 protein by an acyl phosphate linkage (Fig. 4B). The phosphoprotein was identified as a Ca2+-ATPase based on its dependence on Ca2+, and the increased PE level in the presence of La3+ (Fig. 4A). La3+ can substitute for Ca2+ in activating enzyme phosphorylation by binding to the high-affinity Ca2+ binding sites of a SERCA pump (40). The resulting PE undergoes very slow hydrolytic cleavage. The slow turnover of the Ca2+ pump results in an increase in PE levels when La3+ is present under appropriate conditions. Thus the properties of the phosphorylated ECA1p are characteristic of the phosphorylated intermediate of E1E2 Ca2+-pumping ATPases.
ECA1p Is an ER-Type Ca2+-ATPase.
Several lines of evidence indicate that ECA1 encodes an ER-type Ca2+ pump: (i) its amino acid sequence shares 53% identity to SERCA pump (27) and less than 32% identity to PM-type Ca2+ ATPase (10); (ii) the phosphoprotein formed by ECA1p is sensitive to CPA, a specific SERCA inhibitor (Fig. 4); and (iii) ECA1 encodes a 116-kDa polypeptide that is mainly localized on the ER of Arabidopsis plants (Fig. 5). Thus ECA1p resembled a carrot ER-associated Ca2+ pump that was partially inhibited by CPA and insensitive to calmodulin (7). Preliminary results showed that ECA1p did not bind to calmodulin. It is interesting that the carboxyl tail of ECA1p contained a motif, KXKXX (Fig. 1), that functions as an ER-retention sequence of type I proteins (41). To distinguish between multiple genes encoding ER-type and PM-type Ca pumps in plants, we have named the first gene encoding a functional ER-type Ca-ATPase from Arabidopsis as ECA1.
Surprisingly, the localization of ECA1p on the ER membranes in Arabidopsis differs from that of a putative Ca2+-ATPase (LCAp) from tomato (9). ECA1 and LCA encode related homologs of one another as the two polypeptides share 67% identity and 80% similarity. Immunoblotting with antibodies against a region (580–624) of the LCAp hydrophilic domain showed that this polypeptide was localized on both the PM and the vacuolar membrane, but not the ER. Although alternative splicing of a single gene is a possibility in tomato (42), several genes encode ER-type Ca2+ pump homologs in A. thaliana (F.L. and H.S., unpublished data).
Role in Mn2+ Transport?
The ability of ECA1p to restore the growth of pmr1 mutant in Mn-containing medium suggested that this Ca2+ pump may also catalyze Mn2+ transport (Fig. 2). Recently, Pmr1p has been implicated in supplying Mn2+, in addition to Ca2+, into the Golgi compartment (43). pmr1 mutants contain 3–4 times more Mn2+ than did wild-type cells. The high level of Mn2+ is associated with an increased sensitivity to Mn added in the growth medium. Thus Pmr1p could affect cellular Mn2+ homeostasis by pumping Mn2+ into the Golgi apparatus and by extruding excess Mn2+ by vesicular transport out of the cell or to the vacuole. Mn2+ is required in the Golgi to activate Mn2+-dependent enzymes involved in protein processing and secretion (44), and Mn2+ may also function in intracellular signaling (22). Although direct evidence for Mn2+ transport has not been demonstrated with Pmr1p, a rabbit SERCA ATPase does catalyze active 54 Mn2+ transport into sarcoplasmic reticulum vesicles (45). In plants, manganese is an essential nutrient that is required by the oxygen-evolving complex in photosynthesis and for activating many enzyme-catalyzed reactions (46). Because the free cytoplasmic [Mn2+] of 0.2 μM (47) is less than the average [Mn2+] of 100 μM in plant cells (46), Mn2+ must be actively sequestered into endomembrane compartments. The Mn2+-dependent formation of a PE would support the idea that ECA1p could translocate Mn2+, as well as Ca2+, into the lumen of the ER or the Golgi. Recently, a mutant in which the ACA3 gene (GenBank accession no. U93845; identical to ECA1) was disrupted by transferred DNA (T-DNA) insertion has been isolated from Arabidopsis (48). It will be interesting to test whether this mutant is sensitive to high levels of Mn2+.
Summary.
We have demonstrated that a yeast mutant, K616, defective in two Ca2+ pumps provides an expression system to study individual Ca2+ pumps from plants and perhaps other sources. The triple mutant shows no background activity of Ca2+-dependent PE formation (Fig. 3A), indicating the cell was devoid of other P-type Ca2+ pumps. This expression system will allow us to identify functionally and characterize putative Ca2+ or divalent cation pumps that are encoded by multiple genes from Arabidopsis, and thus to understand better the biological role of each pump in the plant.
Acknowledgments
We thank Paul M. Hasegawa for providing a partial cDNA clone encoding a Ca-ATPase from tobacco, and Richard D. Vierstra and the Arabidopsis Biological Resource Center (Ohio State University) for providing Arabidopsis cDNA libraries. We acknowledge Douglas M. Fambrough, Rajini Rao, and Giuseppe Inesi for many stimulating discussions. Most of this work is supported by a grant from the Department of Energy (DE-FG02-95ER20200) to H.S. and by the Maryland Agricultural Experiment Station (MD-J-151). K.W.C. is supported in part by Basic O’Conor Research Grant 95-1137 from the March of Dimes Birth Defects Foundation and in part by National Institutes of Health Grant GM53082. Part of this research is supported by grants to J.F.H. from the U.S. Department of Agriculture (92-37304-7889) and the U.S. Department of Energy (DE-FG03-94ER20152).
ABBREVIATIONS
- ECA1
an ER-type Ca2+-ATPase gene
- ECA1p
ECA1 polypeptide
- TM
transmembrane
- PMSF
phenylmethylsulfonyl fluoride
- BTP
1,3-bis[Tris (hydroxymethyl)methylamino]propane
- CPA
cyclopiazonic acid
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- ER
endoplasmic reticulum
- PE
phosphoenzyme
- SC-URA
synthetic complete medium minus uracil
- TCA
trichloroacetic acid
- PM
plasma membrane
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. U96455).
References
- 1.Bush D S. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- 2.Rudolph H K, Antebi A, Fink G R, Buckley C M, Dorman T E, LeVitre J, Davidow T E, Mao J, Moir D. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 3.Antebi A, Fink G R. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergerson J M, Brenner M B, Thomas D Y, Williams D B. Trend Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 5.Battey N H, Blackbourn H D. New Phytol. 1993;125:307–311. doi: 10.1111/j.1469-8137.1993.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 6.Askerlund P. Plant Physiol. 1996;110:913–922. doi: 10.1104/pp.110.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang I, Ratterman D M, Sze H. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasi-Caldogno F, Carnelli A, de Michelis M I. Plant Physiol. 1995;108:104–113. doi: 10.1104/pp.108.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wimmers L E, Ewing N N, Bennett A B. Proc Natl Acad Sci USA. 1992;89:9205–9209. doi: 10.1073/pnas.89.19.9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Berkelman T, Franklin A E, Hoffman N E. Proc Natl Acad Sci USA. 1993;90:10066–10070. doi: 10.1073/pnas.90.21.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malmstrom S, Askerlund P, Palmgren M G. FEBS Lett. 1997;400:324–328. doi: 10.1016/s0014-5793(96)01448-2. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham K W, Fink G R. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose M D, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 14.Callis J, Raasch J A, Vierstra R D. J Biol Chem. 1990;265:12486–12493. [PubMed] [Google Scholar]
- 15.Perez-Prat E, Narasimhan M L, Binzel M L, Botella M A, Chen Z, Valpuesta V, Bressan R A, Hasegawa P M. Plant Physiol. 1992;100:1471–1478. doi: 10.1104/pp.100.3.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieber J J, Rothenberg M, Roman G, Feldmann K A, Ecker J R. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 17.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mumberg D, Muller R, Funk M. Nucleic Acid Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker D M, Guarente L. Methods Enzymol. 1991;194:186–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- 20.Serrano R. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen F H, Ratterman D M, Sze H. Plant Physiol. 1993;102:651–661. doi: 10.1104/pp.102.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loukin S, Kung C. J Cell Biol. 1995;131:1025–1037. doi: 10.1083/jcb.131.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bers D, Patton C, Nuccitelli R. In: Methods in Cell Biology. Nuccitelli R, editor. Vol. 40. New York: Academic; 1994. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- 24.Ward J M, Reinders A, Hsu H-T, Sze H. Plant Physiol. 1992;99:161–169. doi: 10.1104/pp.99.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutcke E A, Chow K C, Mickel F S, Moss K A, Kern H F, Sceele G A. EMBO J. 1987;6:43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberg D, Sweet R M, Terwilliger T C. Proc Natl Acad Sci USA. 1984;81:140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandl C J, Green N, Korczak B, MacLennan D H. Cell. 1986;44:597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- 28.Clarke D M, Loo T W, Inesi G, MacLennan D H. Nature (London) 1989;339:476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham K W, Fink G R. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieland J, Nitsche A M, Strayle J, Steiner H, Rudolph H K. EMBO J. 1995;14:3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Meis Methods Enzymol. 1988;157:190–206. doi: 10.1016/0076-6879(88)57075-1. [DOI] [PubMed] [Google Scholar]
- 32.Schatzmann H J. Annu Rev Physiol. 1989;51:473–485. doi: 10.1146/annurev.ph.51.030189.002353. [DOI] [PubMed] [Google Scholar]
- 33.Sagara Y, Inesi G. J Biol Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- 34.Siedler N W, Jona I, Vegh M, Martinosi A. J Biol Chem. 1989;426:17816–17823. [PubMed] [Google Scholar]
- 35.Mignaco J A, Barrabin H, Scofano H M. J Biol Chem. 1996;271:18423–18430. doi: 10.1074/jbc.271.31.18423. [DOI] [PubMed] [Google Scholar]
- 36.Briskin D P. Plant Physiol. 1990;94:397–400. doi: 10.1104/pp.94.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewitt N D, Hong B, Sussman M R, Harper J F. Plant Physiol. 1996;112:833–844. doi: 10.1104/pp.112.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frommer W B, Ninnemann O. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:419–444. [Google Scholar]
- 39.Sorin A, Rosas G, Rao R. J Biol Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 40.Squier T C, Bigelow D J, Fernandez-Belda F J, DeMeis L, Inesi G. J Biol Chem. 1990;265:13713–13720. [PubMed] [Google Scholar]
- 41.Nilsson T, Jackson M, Peterson P A. Cell. 1989;58:707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- 42.Ferrol N, Bennett A B. Plant Cell. 1996;8:1159–1169. doi: 10.1105/tpc.8.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapinskas P J, Cunningham K W, Liu X F, Fink G R, Culotta V C. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman R J, Swaroop M, Murtha-Riel P. Biochemistry. 1994;33:9813–9819. doi: 10.1021/bi00199a001. [DOI] [PubMed] [Google Scholar]
- 45.Chiesi M, Inesi G. Biochemistry. 1980;19:2912–2918. doi: 10.1021/bi00554a015. [DOI] [PubMed] [Google Scholar]
- 46.Loneragan J F. In: Manganese in soil and plants. Graham R D, Hannam R J, Uren N C, editors. Dordrecht, The Netherlands: Kluwer; 1988. pp. 113–124. [Google Scholar]
- 47.Quiquampoix H, Loughman B C, Ratcliffe R G. J Exp Bot. 1993;44:1819–1827. [Google Scholar]
- 48.Krysan P J, Young J C, Tax F, Sussman M R. Proc Natl Acad Sci USA. 1996;93:8145–8150. doi: 10.1073/pnas.93.15.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]