Abstract
Background
The GlucoWatch® G2™ Biographer (“GW2B”, Cygnus, Inc.) provides near continuous monitoring of glucose values in near real time. This device is equipped with two types of alarms to detect hypoglycemia. The hypoglycemia alarm is triggered when the current glucose measurement falls below the level set by the user. The “down alert” alarm is triggered when extrapolation of the current glucose trend anticipates hypoglycemia to occur within the next 20 minutes.
Methods
We used data from an inpatient accuracy study to assess the performance of these alarms. During a 24-h clinical research center stay, 89 children and adolescents with T1DM (3.5–17.7 years old) wore 174 GW2Bs and had frequent serum glucose determinations during the day and night.
Results
Sensitivity to detect hypoglycemia (reference glucose ≤60 mg/dL) during an insulin-induced hypoglycemia test was 24% with the hypoglycemia alarm alone and 88% when combined with the down alert alarm. Overnight sensitivity from 11pm–6am was 23% with the hypoglycemia alarm alone and 77% when combined with the down alert alarm. For 16% of hypoglycemia alarms, the reference glucose was above 70 mg/dL for 30 minutes before and after the time of the alarm. For the two alarm types combined, the corresponding false positive rate increased to 62%.
Conclusions
The down alert alarm substantially improves the sensitivity of the GW2B to detect hypoglycemia at the price of a large increase in the false alarm rate. The utility of these alarms in the day to day management of diabetic children remains to be determined.
Introduction
In pediatric patients with type 1 diabetes mellitus (T1DM), hypoglycemia is a major management challenge, particularly at night. In the DCCT, the risk of severe hypoglycemia in adolescents with T1DM was greatly increased compared with the risk in adults, regardless of the intensity of treatment.1 In very young children with T1DM, there are heightened concerns that hypoglycemia will cause permanent neurologic sequelae.2, 3 Across all age groups, the possibility of a severe hypoglycemic event occurring at school, at play, or at night while sleeping is one of the greatest fears of patients and parents alike.4 A device with the ability to continually monitor the blood glucose and to reliably detect hypoglycemia in real time would therefore be a valuable tool in the management of T1DM in children.
The GlucoWatch® G2™ Biographer (“GW2B”; Cygnus, Inc., Redwood City, CA) provides a near real-time glucose measurement every 10 minutes.5 Since glucose values are obtained frequently, it allows for the prediction of future hypoglycemia, a new tool in diabetes management, which is not possible with standard glucose monitoring when 4–6 discrete glucose values are obtained each day. The GW2B is equipped with an alarm that sounds when either 1) the measured glucose drops below the level set by the user (hypoglycemia alarm) or 2) the trend of consecutive glucose values projects that this glucose level will be reached within 20 minutes (down alert alarm). Because of the physiological lag between blood glucose and interstitial fluid as well as the instrument lag of the system, the hypoglycemia alarm reflects the blood glucose level approximately 20 minutes earlier. The down alert alarm, on the other hand, should reflect the blood glucose level at the time of the alarm because the 20 minute lag is counterbalanced by projecting the glucose value 20 minutes ahead.
The accuracy of the GW2B has been reported in several studies,6–10 but data in children are limited.11 We conducted a five-center CRC-based study that assessed the accuracy of the GW2B in children with T1DM by comparing the sensor glucose measurements with serum glucose measurements made by a central laboratory. Results showed lower sensor accuracy in the hypoglycemic range than in the hyperglycemic range, with a median relative absolute difference (RAD) between the sensor and reference values of 16% overall and 38% when the reference glucose was ≤70 mg/dL.12 Sensitivity of the hypoglycemia alarm was 23% when the alarm was set at 60 mg/dL.13 Sensitivity increased at higher alarm settings but at the expense of a high false positive rate. We concluded that this high false positive rate limits the utility of the hypoglycemia alarm function.
The down alert alarm has received little attention in prior reports on GW2B performance. We investigated whether the down alert alarm performance is superior to that of the hypoglycemia alarm by analyzing the data collected in this inpatient accuracy study and comparing the sensitivity and false positive rates of both alarms.
Methods
This study was conducted by the Diabetes Research in Children Network (DirecNet) at five academic centers in the United States. The study protocol consisted of a 24-hour inpatient stay in a clinical research center during which the accuracy of the GW2B and the Continuous Glucose Monitoring System, CGMS™ (“CGMS”; Medtronic MiniMed, Northridge, CA) was assessed by comparing sensor glucose values with reference serum glucose values measured at a central laboratory.12, 14 The central laboratory used a hexokinase method which has been suggested as the reference method for measuring glucose. 15, 16 The study protocol included a 60-minute meal-induced hyperglycemia test and a 90-minute insulin-induced hypoglycemia test during which blood samples were drawn for reference glucose measurements every 5 minutes. At other times, samples were drawn 1) hourly between 7 a.m. and 9 p.m.; 2) every half hour between 9 p.m. and 7 a.m.; 3) when the sensors were calibrated; and 4) when there were symptoms of hypoglycemia. The analyses of the current study consisted of an evaluation of the GW2B hypoglycemia and down alert alarms during the insulin-induced hypoglycemia test and overnight between 11 p.m. and 6 a.m. The subject eligibility criteria, study protocol and procedures, and informed consent process have been described in detail in prior manuscripts;12, 14 only methods pertinent to this study are described herein.
The insulin-induced hypoglycemia test was performed only on subjects age 7 years or older. Subjects were excluded from the analysis if the reference glucose at the end of the test was between 61 and 70 mg/dL and still dropping. A GW2B sensor’s data were included only if there were at least 4 readings during the test and at least one of which occurred on or after the reference glucose nadir. These criteria were met for 66 GW2Bs worn by 46 subjects ranging in age from 7.1 to 17.7 years (mean 12.6); 46% were female and 93% were Caucasian. The analysis included all GW2B sensor readings from the initial insulin bolus injection until 30 minutes after the reference glucose nadir. The subjects had a median of 14 reference glucose values during the test (range 6–19). Of the total 530 possible GW2B sensor readings during the test, 501 (95%) were available for analysis and 29 (5%) were missed due to skips.
A GW2B sensor’s data were included in the overnight analysis if there existed a continuous 3 hour period from 11 p.m. to 6 a.m., during which the subject had at least 6 reference glucose values and at least 15 (~80% of the 18 expected based on 1 reading every 10 minutes) sensor glucose values. These criteria were met for 135 GW2Bs worn by 81 subjects ranging in age from 3.5 to 17.7 years (mean 10.1); 48% were female and 88% were Caucasian. There were 4,289 GW2B sensor readings available for analysis, with a median of 32 glucose values per GW2B (range 16–43). The subjects had a median of 15 overnight reference glucose values (range 6–19).
Statistical Methods
Because the actual hypoglycemic alarm settings varied by subject, the GW2B alarms were retrospectively reevaluated to simulate a hypothetical alarm setting of 60 mg/dL. The GW2B was considered to have given a hypoglycemia alarm whenever the sensor glucose reading was ≤60 mg/dL. For the down alert alarm, a linear slope was calculated based on the difference in glucose values from the current and previous readings and a down alert alarm was issued if that slope predicted a glucose reading ≤60 mg/dL within 20 minutes. More specifically, if gc and gp denote the current and previous GW2B sensor glucose readings (ignoring skips), respectively, and tc and tp denote the times (in minutes) those readings were given, the GW2B was considered to have issued a down alert alarm at time tc if each of the following conditions was met:
tc − tp ≤ 50 minutes.
/minute (rate of change at least 0.5% of previous glucose value per minute).
(linear extrapolation predicts a reading below 60 mg/dL in the next 20 minutes).
Insulin-induced hypoglycemia test
A subject was considered to have achieved hypoglycemia if the nadir reference glucose value was ≤60 mg/dL. Each GW2B was evaluated for whether or not it would have given either a hypoglycemia alarm and/or down alert alarm according to the algorithms described above. Time from alarm to true hypoglycemia was defined as the time of the first alarm to the first time the reference glucose was ≤60 mg/dL. For subjects wearing two GW2Bs during the hypoglycemia test, this was evaluated separately for each one.
Overnight (11 p.m. to 6 a.m.)
The reference glucose level was estimated between the half-hour measurements by linear interpolation. Results were similar using cubic spline interpolation (data not shown). No interpolation was calculated between reference glucose measurements taken more than 40 minutes apart.
Hypoglycemic episodes were defined as periods with at least two interpolated reference glucose readings ≤60 mg/dL and no readings >70 mg/dL (the episode was considered to have ended when the interpolated reference glucose was >70 mg/dL). Distinct episodes were required to be separated by a period of at least 30 minutes with all reference glucose values >70 mg/dL. Similar episodes were also defined for two values ≤70 mg/dL ending with a value >80 mg/dL. Each episode was evaluable for sensitivity if the GW2B sensor gave at least 50% of the expected readings (based on 1 reading every 10 minutes) during the period. Sensitivity was defined as the percentage of episodes during which the GW2B would have given at least 1 alarm and was calculated separately for each alarm type (hypoglycemia alarm vs. down alert alarm). A combined analysis was also conducted looking at the first GW2B alarm of either type.
Analysis of false positives was restricted to the first overnight alarm separately for each type and for each GW2B. A combined analysis was also conducted looking at the first alarm of either type. An alarm was considered a false positive if the nadir (interpolated) reference glucose was >60 mg/dL continuously for a period of ±30 minutes. False positive rates were also calculated based on the nadir >70 mg/dL.
Results
Insulin-induced Hypoglycemia Test
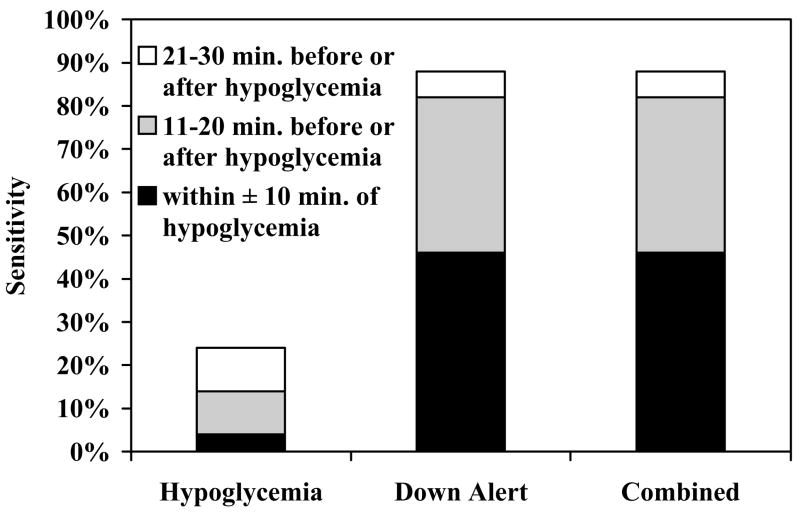
Hypoglycemia (nadir reference glucose ≤60 mg/dL) was achieved by 34 (74%) subjects wearing 50 GW2Bs during the test. A hypoglycemia alarm occurred for 12 of the 50 GW2Bs for a sensitivity rate of 24%. However, a down alert alarm occurred for 44 of the 50 GW2Bs, improving sensitivity to 88%.
The 12 subjects who did not achieve hypoglycemia during the test wore a total of 16 GW2Bs. A false positive hypoglycemia alarm occurred for 1 (6%) and a false positive down alert alarm occurred for 9 (56%) of the 16 GW2Bs (Table 1/Figure 1). The 9 false positives were from 6 subjects whose nadir reference glucose ranged from 68–113 mg/dL with a median value of 79 mg/dL.
Table 1. GW2B Alarms during Insulin-Induced Hypoglycemia Test.
Alarms based on a setting of 60 mg/dL. Data from 66 GW2B sensors worn by 46 subjects.
| Reference Glucose Nadir ≤ 60 mg/dL | Reference Glucose Nadir > 60 mg/dL | |
|---|---|---|
| Number of Subjects | 34 | 12 |
| Number of GW2B sensors | 50 | 16 |
| Hypoglycemia Alarma | ||
| anytime during test | 12 (24%) | 1 (6%) |
| within ± 30 min. of hypoglycemiab | 12 (24%) | N/A |
| within ± 20 min. of hypoglycemiab | 7 (14%) | N/A |
| within ± 10 min. of hypoglycemiab | 2 (4%) | N/A |
| Down Alert Alarma | ||
| anytime during test | 44 (88%) | 9 (56%) |
| within ± 30 min. of hypoglycemiab | 44 (88%) | N/A |
| within ± 20 min. of hypoglycemiab | 41 (82%) | N/A |
| within ± 10 min. of hypoglycemiab | 23 (46%) | N/A |
| Combineda,c | ||
| anytime during test | 44 (88%) | 9 (56%) |
| within ± 30 min. of hypoglycemiab | 44 (88%) | N/A |
| within ± 20 min. of hypoglycemiab | 41 (82%) | N/A |
| within ± 10 min. of hypoglycemiab | 23 (46%) | N/A |
Only consider the first alarm during the test for each GW2B.
Defined as first time reference glucose is ≤ 60 mg/dL.
Hypoglycemia alarm or the down alert alarm, whichever occurs first.
Figure 1.
Sensitivity during Insulin-induced Hypoglycemia Test by Alarm Type. Sensitivity defined as percent of hypoglycemic cases when the GW2B alarmed.
Overnight
There were 10 total episodes of hypoglycemia (reference glucose ≤60 mg/dL) experienced by 9 subjects wearing 13 total GW2Bs between 11 p.m. to 6 a.m. Only 1 (8%) of the 13 GW2Bs worn by these 9 subjects gave a hypoglycemia alarm (sensor glucose ≤60 mg/dL) during the episode and only 3 (23%) gave an alarm within ±30 minutes of the episode. However, 6 (46%) of the GW2Bs issued a down alert alarm during the episode and 10 (77%) issued a down alert alarm within ±30 minutes. There were no episodes during which a hypoglycemia alarm occurred that was not preceded by a down alert alarm. Adding the hypoglycemia alarms to the down alert alarms, therefore, did not improve sensitivity in the combined analysis (Table 2).
Table 2. Sensitivity of GW2B Alarms during Overnight (11 p.m. to 6 a.m.) Episodes of Hypoglycemia.
Alarms based on a setting of 60 mg/dL.
| Episodesa ≤ 60 mg/dL | Episodesa ≤ 70 mg/dL | |
|---|---|---|
| Number of subjects | 9 | 20 |
| GW2Bs worn during an episode | 13 | 35 |
| Hypoglycemia Alarm | ||
| during episode | 1 (8%) | 11 (31%) |
| from 30 min before to end of episode | 1 (8%) | 12 (34%) |
| ± 30 min either side of episodeb | 3 (23%) | 12 (34%) |
| Down Alert Alarm | ||
| during episode | 6 (46%) | 25 (71%) |
| from 30 min before to end of episode | 10 (77%) | 28 (80%) |
| ± 30 min either side of episodeb | 10 (77%) | 29 (83%) |
| Combinedc | ||
| during episode | 6 (46%) | 25 (71%) |
| from 30 min before to end of episode | 10 (77%) | 28 (80%) |
| ± 30 min either side of episodeb | 10 (77%) | 29 (83%) |
Determined by reference glucose values (see Methods).
Period beginning 30 minutes prior to the start of the episode until 30 minutes after the end of the episode.
Hypoglycemia alarm or the down alert alarm, whichever occurs first.
To investigate the performance of the GW2B alarms in subjects who did not experience hypoglycemia, we restricted the analysis to subjects who had at least 14 reference glucose values and where there were at least 21 (50%) of the GW2B sensor readings available overnight. The reference glucose stayed above 60 mg/dL all night for 52 such subjects wearing 86 GW2Bs. Only 13 (15%) of these GW2Bs falsely gave a hypoglycemia alarm, but 39 (45%) issued a down alert alarm sometime during the night and 40 (47%) gave at least one alarm of either type. Of the 71 GW2Bs worn by 43 subjects where the reference glucose stayed above 70 mg/dL all night, only 2 (3%) falsely gave a hypoglycemia alarm, but 25 (35%) issued a down alert alarm sometime during the night. Both of the GW2Bs with a false hypoglycemia alarm also gave a false down alert alarm, so the total number of GW2Bs giving a false alarm of either type was also 25 (35%).
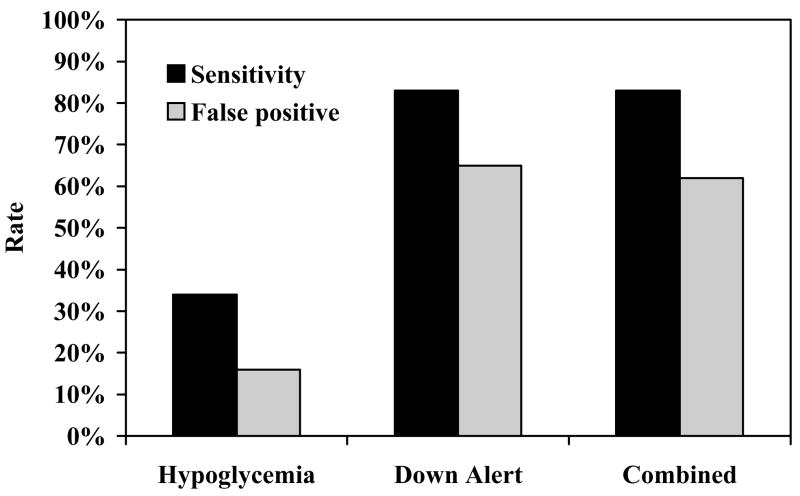
Twenty-five (19%) of the 135 GW2Bs issued at least one hypoglycemia alarm overnight. However, for 16% of the first hypoglycemia alarms in each GW2B, the interpolated reference glucose was continuously above 70 mg/dL for 30 minutes prior to and 30 minutes following the alarm. Seventy (52%) of the 135 GW2Bs issued at least one down alert alarm, but reference glucose was unavailable at the time for 2 of these cases. In the remaining 68 GW2Bs, the corresponding false alarm rate was 65%. There was only one GW2B that gave a hypoglycemia alarm, but did not also give a down alert alarm. Adding the hypoglycemia alarms to the down alert alarms in the combined analysis, therefore, had minimal effect on the false alarm rate (62%; Table 3/Figure 2).
Table 3. False Alarm Rates for GW2B Overnight (11 p.m. to 6 a.m.).
Based on the first overnight alarm at a setting of 60 mg/dL.
| Alarm Type | Na | Reference Glucose > 60 mg/dLb,c | Reference Glucose > 70 mg/dLb,c |
|---|---|---|---|
| Hypoglycemia Alarm | 25 | 60% | 16% |
| Down Alert Alarm | 68 | 78% | 65% |
| Combinedd | 69 | 77% | 62% |
Number of GW2Bs giving at least one alarm overnight. Only consider the first alarm for each GW2B.
Linearly interpolated value between half-hourly measurements.
Reference glucose continuously high for ± 30 minutes from time of the alarm.
Hypoglycemia alarm or the down alert alarm, whichever occurs first.
Figure 2.
Overnight Sensitivity and False Positive Rates by Alarm Type. Sensitivity defined as percent of hypoglycemic episodes where the GW2B alarmed within ± 30 min. False positive rate defined as the percent of alarms where the reference glucose was continuously > 70 mg/dL from 30 minutes prior to 30 minutes after the alarm.
Discussion
A critically important function of real-time continuous glucose monitors is the detection and prevention of hypoglycemia. This study evaluated the accuracy of the algorithms used for the GW2B hypoglycemia and down alert alarms in children and adolescents with T1DM. The down alert alarm algorithm offers the possibility of increasing sensitivity by using the current glucose trend to anticipate hypoglycemia rather than waiting for a low sensor reading. The alarm function algorithms were evaluated from data collected both overnight and during an insulin-induced hypoglycemia test.
Our results demonstrate that the down alert alarm does improve sensitivity substantially. Overnight, sensitivity to detect episodes ≤70 mg/dL improved from 31% with the hypoglycemia alarm alone to 71% when both alarm types were used together. However, the corresponding impact on the false alarm rate was also substantial, increasing from 16% to 62%. Since nearly all hypoglycemia alarms in this dataset were preceded by a down alert alarm, the addition of the hypoglycemia alarm to the down alert alarm is redundant with regard to sensitivity and false alarm rate. In contrast, less than 40% of down alert alarms were accompanied by a subsequent hypoglycemia alarm. Thus, by more than doubling the number of alarms, the addition of the down alert function to the GW2B improves sensitivity, but at the price of an elevated false positive rate.
For some users this high false alarm rate may not be acceptable. For parents of children with T1DM, however, detection of more hypoglycemic episodes during overnight sleep may be a valuable feature that is worth the negative aspects of false alarms. For example, if the blood glucose at the time of the initial down alert alarm is ≤70 mg/dL and the child was treated for hypoglycemia at the time of the alert, 83% of potential hypoglycemic episodes would have been prevented. However, the child would also have been unnecessarily treated for hypoglycemia for approximately two-thirds of these alarms had a confirmatory home glucose meter measurement not been obtained before acting. An added problem is the danger that families may ignore the alarms once they realize that the majority are false.
The reduced sensitivity and increased false positive rate of the alarm function limits the ability to monitor for hypoglycemia. We previously reported that the GW2B has greater accuracy for hyperglycemia than hypoglycemia.12 Therefore, for many users, the greatest value of the GW2B may be for studying trends and not for the detection of hypoglycemia. For future generations of the GW2B and other real-time continuous glucose monitors, consideration should be given whether to include both a down-alert alarm and hypoglycemia alarm and if so, whether there should be an option to activate one or both.
Acknowledgments
Appreciation is expressed for the work performed by the CRC Nurses at the five clinical centers.
This research has been supported by the following NIH/NICHD Grants: HD041919-0; HD041915; HD041890; HD041918-01; HD041908-01; and HD041906-01.
Clinical Centers also received funding through the following GCRC Grant Numbers M01 RR00069; RR00059; RR 06022 and RR00070-41.
Appendix
Writing Committee: Eva Tsalikian, Craig Kollman, Nelly Mauras, Stuart Weinzimer, Bruce Buckingham, Dongyuan Xing, Roy Beck, Katrina Ruedy, William Tamborlane, Rosanna Fiallo-Scharer
The DirecNet Study Group
Clinical Centers
Listed in alphabetical order with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.
-
Barbara Davis Center for Childhood Diabetes, Denver, CO
H. Peter Chase, MD (PI); Rosanna Fiallo-Scharer, MD (I); Jennifer H. Fisher, ND, RN (C)
-
Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA
Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Linda F. Larson, RN (C)
-
Nemours Children’s Clinic, Jacksonville, FL
Tim Wysocki, PhD, ABPP (PI); Nelly Mauras, MD (I); Kristen M. Gagnon, MS, RD (C); Pauline Todd, RN (C)
-
Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA
Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Jennifer M. Block, RN, CDE (C); Elizabeth L. Kunselman, RN, CDE (C)
-
Department of Pediatrics, Yale University School of Medicine, New Haven, CT
William V. Tamborlane, MD (PI); Stuart A. Weinzimer, MD (I); Elizabeth A. Doyle, MSN (C)
Coordinating Center
Jaeb Center for Health Research, Tampa, FL
Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Dongyuan Xing, MPH
Data and Safety Monitoring Board
Dorothy M. Becker, MBBCh; Christopher Cox, PhD; Christopher M. Ryan, PhD; Neil H. White, MD, CDE; Perrin C. White, MD
University of Minnesota Central Laboratory
Michael W. Steffes, MD, PhD; Jean M. Bucksa, CLS; Maren L. Nowicki, CLS
National Institutes of Health
Gilman D. Grave, MD; Barbara Linder MD, PhD; Karen K. Winer, MD
Footnotes
These data were presented at the 2003 Diabetes Technology and Therapeutics Annual Meeting in San Francisco, CA, November 6–8.
LifeScan, Milpitas, CA, provided the One Touch® Ultra® Blood Glucose Monitoring Systems and the blood glucose test strips.
The GlucoWatch® G2TM Biographers were purchased from Cygnus, Inc. at a discounted price.
References
- 1.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:228–229. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 2.Rovet JF, Ehrlich RM, Hoppe M. Intellectual deficits associated with early onset of insulin-dependent diabetes mellitus in children. Diabetes Care. 1987;10:510–515. doi: 10.2337/diacare.10.4.510. [DOI] [PubMed] [Google Scholar]
- 3.Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: A 7-year prospective study. J Pediatr. 1999;134:503–506. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- 4.Marrero DG, et al. Fear of hypoglycemia in the parents of children and adolescents with diabetes: maladaptive or healthy response? Diabetes Educator. 1997;23:281–286. doi: 10.1177/014572179702300306. [DOI] [PubMed] [Google Scholar]
- 5.Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res. 2002;18:S49–53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- 6.Pitzer KR, et al. Detection of hypoglycemia with the GlucoWatch Biographer. Diabetes Care. 2001;24:881–885. doi: 10.2337/diacare.24.5.881. [DOI] [PubMed] [Google Scholar]
- 7.Garg SK, et al. Correlation of fingerstick blood glucose measurements with GlucoWatch Biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708–1714. doi: 10.2337/diacare.22.10.1708. [DOI] [PubMed] [Google Scholar]
- 8.Tamada JA, et al. Noninvasive glucose monitoring: comprehensive clinical results. JAMA. 1999;282:1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 9.Tierney MJ, et al. Effect of acetaminophen on the accuracy of glucose measurements obtained with the GlucoWatch Biographer. Diabetes Technol Ther. 2000;2:199–207. doi: 10.1089/15209150050025140. [DOI] [PubMed] [Google Scholar]
- 10.Tierney MJ, et al. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Biolectron. 2001;16:621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 11.Eastman RC, et al. Use of the GlucoWatch biographer in children and adolescents with diabetes. Pediatric Diabetes. 2002;3:127–134. doi: 10.1034/j.1399-5448.2002.30302.x. [DOI] [PubMed] [Google Scholar]
- 12.The Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the GlucoWatch Biographer in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:791–800. doi: 10.1089/152091503322526996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of the GlucoWatch G2 Biographer and the Continuous Glucose Monitoring System during hypoglycemia. Experience of the Diabetes Research in Children Network. Diabetes Care. 2004;27:722–726. doi: 10.2337/diacare.27.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the CGMS in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:781–789. doi: 10.1089/152091503322526987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neese JW, et al. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national reference method. HEW Publication No. (CDC) 77-8330. Atlanta: Centers for Disease Control; 1976. [Google Scholar]
- 16.Passey RB, et al. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974) Clin Chem. 1977;23:131–139. [PubMed] [Google Scholar]