Summary
Photodynamic therapy (PDT) mediated with vascular acting photosensitizer Tookad (pd-bacteriopheophorbide) was investigated as an alternative modality for treating prostate cancer. Photodynamic effects on the prostate gland and its adjacent tissues were evaluated in a canine model. Interstitial prostate PDT was performed by irradiating individual lobes with a cylindrical diffuser fiber at various drug/light doses. The sensitivity of the adjacent tissues to Tookad PDT was determined by directly irradiating the surface of the bladder, colon, abdominal muscle and pelvic plexus with a microlens fiber at various drug/light doses. The prostate and adjacent tissues were harvested one-week after the treatment and subjected to histopathological examination. PDT-induced prostate lesions were characterized by marked hemorrhagic necrosis. The bladder, colon, abdominal muscle and pelvic plexus appeared to be sensitive to PDT although the Tookad PDT-induced responses in these tissues were minimal compared to that of the prostate gland at the same dose levels. Nevertheless, the protection of the adjacent tissues should be taken into consideration during the total prostate ablation process due to their sensitivity to PDT. The sensitivity of the prostatic urethra is worth further investigation. Direct intraurethral irradiation might provide an ideal means to determine the sensitivity of the prostatic urethra and might lead to transurethral PDT protocols for the management of benign prostatic hyperplasia.
Keywords: photodynamic therapy, Tookad, prostate, bladder, colon, pelvic nerve, urethra
1 Introduction
Photodynamic therapy (PDT) is gradually becoming an accepted treatment modality for both malignant and non-malignant diseases.1 While the majority of approved PDT protocols treat superficial lesions, such as actinic keratosis and Barrett’s esophagus; interstitial approaches for the ablation of solid tumor, such as prostate cancer, brain tumor, liver cancer and head and neck cancers are now being investigated worldwide.2-5
Prostate cancer is still the most common neoplasm in men in the western world. The feasibility of using interstitial PDT approaches for the treatment of prostate cancer has been studied in various animal models by us and several other groups.6-15 The healthy canine prostate has often been used as an animal model due to its resemblance both in physical size and in anatomical structure to that of the human prostate. Canine prostate PDT mediated by various photosensitizers has been investigated since the early 1980’s.12 Previous animal studies of canine models indicate that interstitial PDT has a great potential in the treatment of prostate cancer and benign prostatic hyperplasia (BPH).16,17 Recent clinical trials also demonstrate that PDT is a promising modality for the treatment of primary and recurrent localized prostate cancer.18-22 Current clinical protocols involve the transperineal insertion of multiple light delivery and monitoring probes through the conventional brachytherapy template under transrectal ultrasound guidance. In principle, PDT must destroy not only neoplastic tissue but also all remaining normal glandular epithelium in order to become an acceptable modality.2 This often requires the treatment to achieve a total ablation of the entire prostate gland. Therefore, it is critical to understand the potential response of the adjacent tissues of the prostate gland during such process. To date, the possible side effects of prostate PDT on the adjacent tissues, such as the abdominal muscle, bladder, colon, pelvic nerve, prostatic urethra and urethral sphincter, etc., have not been fully explored. The integrity of these structures and preservation of their functions are important for avoiding possible urinary and sexual dysfunctions, which often occur in conventional treatments, such as radiotherapy and radical prostatectomy.23,24 Therefore, it is important that the effects of the interstitial PDT on the adjacent tissues of the prostate gland are carefully evaluated in order to preserve these structures and their functions while still achieving the ultimate goal of a total ablation of the cancerous prostate gland.
Photosensitizer Tookad is a relatively new photosensitizing drug.25 It is a pure Pd-substituted bacteriochlorophyll derivative with a maximum absorption wavelength at 763 nm and a relatively fast clearance from the body. Its effectiveness on ablating the prostate gland and prostate cancer has been demonstrated in various animal models by our group.7-9 Its usefulness in the treatment of localized prostate cancer is currently being investigated in human trials.20 Preliminary results show that the Tookad PDT offers a deeper tissue penetration of light and therefore can generate a larger volume of tissue lesion. Its fast clearance may significantly reduce the risk of unwanted skin photosensitization.26
This study uses a canine model to evaluate the direct effects of Tookad PDT on the prostate gland and its adjacent tissues. Interstitial prostate PDT was performed by irradiating individual lobes with a diode laser (763 nm) and cylindrical diffuser fibers to activate the IV administered photosensitizer Tookad during or shortly after the drug infusion. The sensitivity of the adjacent tissues to Tookad PDT was determined by superficially irradiating the bladder, colon, abdominal muscle and pelvic plexus with a microlens fiber at various drug/light doses and drug-light intervals. The prostate and adjacent tissues were harvested one-week after the treatment and subjected to histopathological examination. Since the characteristics of Tookad PDT-induced prostatic lesions have been reported in detail in our previous reports,7-9 this manuscript will therefore focus on the evaluation of photodynamic effects on adjacent tissues of the prostate gland.
2 Experimental method
2.1 Animal models
Healthy adult male beagle dogs (3-9 years old, N = 18) were used in this study. Standard canine laporatomy procedures and prostate PDT experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committees of Denver HealthONE Alliance and Colorado State University.7 The size of the prostate gland was typically 2 × 3 cm (width × length).
2.2 Pre-medication
Benadryl (IV 0.7 ∼ 1.4 mg/kg) and Dexamethasone (SQ, 2 mg per dog) were given 24 h prior to and/or immediately prior to the start of photosensitizer infusion to counteract the effects of Cremophor EL (co-solvent of Tookad solution) on the blood pressure.7
2.3 Photosensitizer
Tookad (Pd-bacteriopheophorbide, also known as WST09, 2.5 mg/ml; Steba Biotech, France) was given to the animals at a dosage of 0.25-2 mg/kg via a slow intravenous (IV) infusion over a period of 10 min using an automatic pump. The maximal plasma concentration occurred at the end of drug infusion.9 From the onset of drug infusion, the dog was kept under the dimmed ambient lighting to prevent possible skin photosensitization.
2.4 Light source and light delivery
The PDT light source was generated by a portable 763 nm diode laser (CeraLas, Biolitec AG; Germany) with a maximum output of 4 W and a calibration port for determining delivered output power. The laser output was directly coupled into a beam splitter that allowed up to 4 irradiation fields to be treated simultaneously at identical or varied fluence rates. The irradiation light fluence rates were controlled at 150 mW/cm for interstitial irradiations delivered through a cylindrical diffuser fiber (1 or 3 cm active length, 1.3 mm outer diameter; CeramOptec GmbH, Germany) and at <=150 mW/cm2 for the surface irradiations delivered through a microlens fiber (Medlight S.A., Ecublens, Switzerland), respectively. Light irradiation was applied in the following modes:
Simultaneous mode (SM) — Light irradiation began at the onset of the drug infusion and continued after the completion of drug infusion. SM was used for the interstitial irradiation of the prostate and the superficial irradiation of the pelvic nerve.
Peak mode (PM) or drug concentration peak and light irradiation overlapping mode — In this mode, the light was applied during drug infusion and continued after the completion of drug infusion so as to center the light delivery period around the peak of the photosensitizer concentration in blood and hence, putatively, should be maximally effective. PM was used for the superficial irradiation of muscle.
Post-infusion mode (PIM) — Light irradiation began at 15 min after the completion of the drug infusion, i.e. the interval between drug infusion and light irradiation (drug infusion to light irradiation or DLI) was set at 15 min. PIM was used for the superficial irradiation of the bladder and colon.
Their relationships to the plasma drug concentration are demonstrated in Figure 1. The drug distribution data were reported in our previous publication.9
Fig. 1.
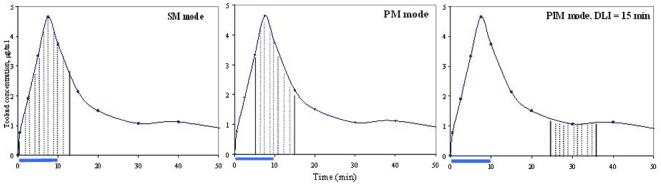
Light irradiation modes. The horizontal bar indicates the drug infusion time (10 min). The curve shows the drug distribution after IV injection of Tookad (1 mg/kg). The shadow area corresponds to the light irradiation time of 100 J at 150 mW.
2.5 PDT procedures
Interstitial prostate PDT was performed by placing the diffuser fiber tip in the middle section of the right and/or left lobe, either perpendicular (1-cm tip, n = 4) or parallel (3-cm tip, n = 2) to the prostatic urethra.
Superficial PDT was performed by pointing a microlens fiber perpendicular to the serosal surfaces of the bladder (n = 5), colon (n = 7) and abdominal wall (n = 8) to form a 1-cm diameter irradiation spot. A sterile black plastic sheet (1 mm thick) with a 1-cm diameter hole was used to provide an identical size of irradiation field and to shield surrounding tissue from light exposure. For direct pelvic nerve irradiation, the left side pelvic plexus (containing the pelvic nerve) (n = 2) was superficially irradiated with a microlens fiber through the 1-cm diameter hole of the black plastic sheet. To protect underlying prostate and adjacent colon tissues, one black plastic sheet was placed behind the pelvic plexus and another placed on the side of the colon. The detailed treatment schemes for the prostate, bladder, colon, abdominal muscle and pelvic nerve are summarized in Table 1.
Table 1.
Treatment schemes
| Drug dose (mg/kg) | Light irradiation mode | Fluence rate | Light dose | No. of treated lobe(s) or site(s) | |
|---|---|---|---|---|---|
| Prostate | 0.25 | SM | 150 mW/cm | 100 J/cm | 1 |
| 0.25 | SM | 150 mW/cm | 200 J/cm | 1 | |
| 0.5 | SM | 150 mW/cm | 100 J/cm | 1 | |
| 0.5 | SM | 150 mW/cm | 200 J/cm | 1 | |
| 1 | SM | 150 mW/cm | 200 J/cm | 2 | |
| Bladder | 2 | PIM, DLI = 15 min | 35 mW/cm2 | 20 J/cm2 | 1 |
| 2 | PIM, DLI = 15 min | 35 mW/cm2 | 40 J/cm2 | 4 | |
| Colon | 2 | PIM, DLI = 15 min | 70 mW/cm2 | 10 J/cm2 | 1 |
| 2 | PIM, DLI = 15 min | 70 mW/cm2 | 20 J/cm2 | 1 | |
| 2 | PIM, DLI = 15 min | 70 mW/cm2 | 40 J/cm2 | 3 | |
| 2 | PIM, DLI = 15 min | 70 mW/cm2 | 80 J/cm2 | 2 | |
| Muscle | 1 | PM | 150 mW/cm2 | 50 J/cm2 | 1 |
| 1 | PM | 150 mW/cm2 | 75 J/cm2 | 3 | |
| 1 | PM | 150 mW/cm2 | 100 J/cm2 | 1 | |
| 2 | PM | 150 mW/cm2 | 50 J/cm2 | 2 | |
| 2 | PM | 150 mW/cm2 | 75 J/cm2 | 1 | |
| Pelvic N. | 1 | PM | 150 mW/cm2 | 50 J/cm2 | 1 |
| 1 | SM | 150 mW/cm2 | 200 J/cm2 | 1 |
The cutaneous branches of the saphenous nerve were used as the model of peripheral nerve tissue for studying the effect of Tookad PDT on the nerve function (e.g. conductivity) and structure. Details of function study and experimental procedures have been reported elsewhere.27 Briefly, the cutaneous branch of the saphenous nerve (right pelvic limb) was exposed through an incision where it accompanied the cranial branch of the saphenous artery. Superficial treatment was performed with a microlens fiber on the surface of the saphenous nerve at a light dose of 50, 100 or 200 J/cm2 at 150 mW/cm2 after slow IV infusion of Tookad (0 - 2.0 mg/kg) over a period of 10 min.
2.6 Histopathological examination
At necropsy, the prostate, underlying rectum, bladder, and sections of treated pelvic plexus, colon and abdominal wall were removed and photographed. These tissue samples were dissected into 3 mm thick blocks and were fixed in 10% neutral buffered formalin and followed by paraffin embedding. Sections of 5 μm thickness were stained with standard H&E staining to examine PDT induced subacute histopathological changes. In addition, sections of the saphenous nerve specimen underwent osmium post-fixation, dehydration, plastic embedment, and then sectioning on an ultramicrotome at 1 μm thickness and followed by toluidine blue staining.
3 Results and Discussion
In this study, the effects of Tookad PDT on the prostate and its surrounding tissues (e.g. bladder, colon, abdominal muscle, pelvic nerve, and prostatic urethra) were investigated in healthy dogs under the standard laporatomy procedure. In addition to the interstitial irradiation of the prostate gland, sections of the bladder, colon, abdominal muscle and pelvic nerve were superficially irradiated at different drug/light doses. Tissue responses were evaluated with histopathological examinations at one week post-PDT. Ideally, the effects of PDT ablation of the prostate on adjacent tissues should be studied under a total ablation mode. The light dose at the prostate boundary and in the adjacent tissues might be monitored or calculated during the total ablation. This can only be achieved through the irradiation of the prostate under a complicated experiment setup of multiple-fiber insertion.20,21 The main goal of this animal study was to characterize PDT-induced tissue lesions under defined drug and light doses. In our single fiber per lobe setup, light was distributed at the center of each lobe during interstitial prostate irradiation and should have no direct effect on adjacent tissues. Alternatively, the direct superficial irradiation of the target tissue under a definitive dose was used to obtain valuable information on the correlation of drug/light dose and tissue lesion.
Tookad is a relatively new second-generation photosensitizer that acts by damaging vasculature and altering blood supply to the irradiated area in current PDT protocols - i.e. light is delivered to the targeted tissue while the photosensitizers are mainly circulating inside the blood vessels. This action mode is defined as the “passive vascular-targeting PDT”.28 The characteristics of Tookad PDT mediated prostatic lesions have been reported in detail in our previous reports, but to a less extent on the bladder and colon.7-9 This section will focus on the evaluation of the photodynamic effects on adjacent tissues of the canine prostate gland.
3.1 The prostate and prostatic urethra
At one week post Tookad PDT, the subacute lesions were characterized by marked hemorrhagic necrosis (Fig. 2A-2C). Homogeneous necrotic debris was seen at the center of the irradiated area. This indicated that a total ablation was achieved. Some remaining small vessels had fibrinoid necrosis and thrombosis. PDT-induced lesions were well delineated from the adjacent unaffected normal tissue. The diameter and volume of necrosis increased with the increase of the drug dose and light dose.7,9 Under the same drug/light dose, different light irradiation modes produced a similar lesion volume. Necrotic lesions (< 3 mm) could be induced at a very low drug dose (e.g. 0.25 mg/kg) at a light dose of 200 J/cm but not 100 J/cm. The nonglandular tissue (e.g. fibromuscular stroma) appeared to be damaged in the same fashion as the glandular tissue, characterized by marked degeneration. The boundary of the lesions could be easily identified under microscopic examination, suggesting a PDT response threshold. However, the cross-section view of the treated prostate showed that the lesion was not always in a perfect spheral or oval shape and some sections had poorly-defined borders (see Fig. 2C). The peripheral lesions beyond the light-irradiated zone might be caused by downstream vascular shutdown. Therefore, the conventional PDT dosimetry model might not be suitable for predicting the lesion threshold for the vascular acting PDT.
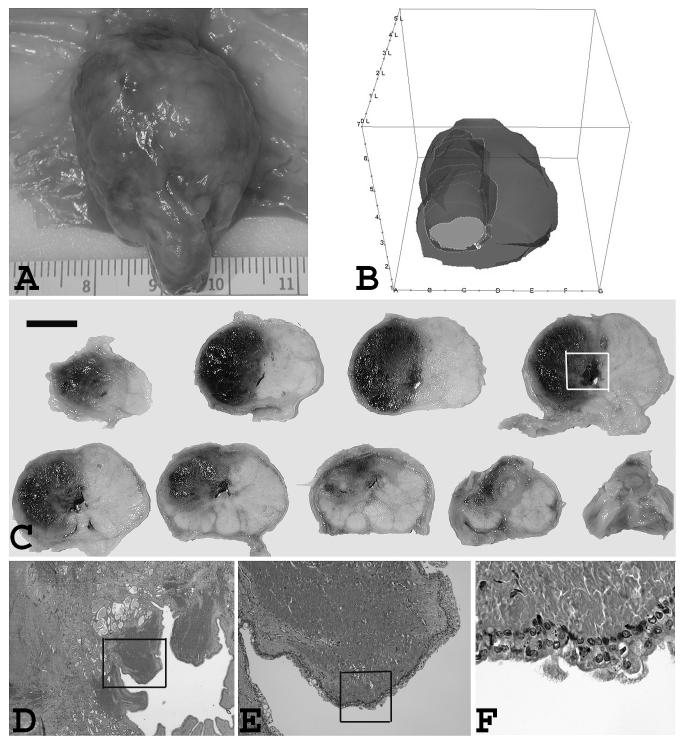
Fig. 2.
Gross and microscopic view of the prostate gland and prostatic urethra one week post PDT. (A) Ventral view of treated side and untreated side. Interstitial treatment was performed with a 3-cm diffuser at 200 J/cm and 150 mW/cm after slow IV infusion of Tookad (1 mg/kg) in right lobe. Patchy sub-capsular hyperemia was visible at the surface of treated side. (B) 3-D reconstruction of the same prostate showing PDT-induced lesion. (C) Multiple transverse sections with ventral side up. Photo was taken after formalin fixation. Dark regions correspond to the PDT-induced hemorrhagic necrosis. The PDT-induced lesion extends to the prostatic urethra. Images are displayed with the anatomic right on the left side of the image. (Bar = 1 cm) (D) Microscopic view of the marked area of tissue block No. 4 of the previous photo. Several periurethral lesion on the right prostatic urethral area with intact urethra. The urethra was open and right side was unaffected. (×20) (E) The close up view of the marked area of the previous photo. Necrosis and degeneration in the periurethral region. (×40) (F) The close up view of the marked area of the previous photo. Mild submucosa congestion and small areas of partial epithelial disruption. (×100)
At high light/drug dose levels, the PDT-induced lesion extended to the prostatic urethra and produced significant periurethral damage but only mild submucosa congestion and small areas of partial epithelial disruption were observed (Fig. 2D-2F). Post-PDT urinalysis showed only a trace of blood in the urine samples, indicating microhematuria.
Dogs showed no severe post-PDT urethral complications. None of the dogs showed urinary retention. This is indeed encouraging that the effect of Tookad PDT on the prostatic urethra was minimal, both functionally in terms of micturation and from the gross and microscopic appearances, even when the prostatic urethra is within the treatment zone and the immediately adjacent prostate tissue has been totally destroyed. Although the lack of urethral obstruction after PDT might have been related to the use of the steroid (e.g. Dexamethasone) given to suppress the side effects of Cremophor, the urethra sensitivity to the Tookad PDT is worth further investigation. Direct intraurethral light irradiation might provide an ideal means to determine the definitive damage threshold of the prostatic urethra and lead to transurethral PDT protocols for the management of benign prostatic hyperplasia (BPH).
3.2 The bladder
At one week post-PDT, there was no visible damage on either the serosal or mucosal surface of the bladder due to the scattered light while the prostate underwent the interstitial irradiation. There was neither macroscopic nor microscopic damage on the serosal surface of the bladder due to the direct surface irradiation up to 20 J/cm2 at a drug dose level of 2 mg/kg and DLI of 15 min. There was no visible damage on the serosal surface due to the direct surface irradiation up to 40 J/cm2. However, microscopic examination showed mild hemorrhage and edematous reactions at this dose level, but there was no sign of necrosis. However, at a similar dose level (e.g. 2 mg/kg, 50 J/cm and DLI of 15 min) the interstitial irradiation could produce an average lesion size of 1.5 cm in the prostate.7
3.3 The colon
At one week post PDT, the treated sections of the colon and underlying rectum were examined. There was no visible damage on serosal or mucosal surfaces of the colon due to scattered light or direct surface irradiation up to 40 J/cm2 at a drug dose level of 2 mg/kg and DLI of 15 min. The area of colon receiving 80 J/cm2 surface irradiation showed marked superficial hemorrhage. The ulceration corresponding to the irradiation field was clearly visible, but there was no apparent perforation or fistula. Under microscopic examination, only mild inflammatory reactions were seen on the colon surface directly irradiated at 40 J/cm2, and the mucosa and underlying colonic structures were well preserved. At 80 J/cm2 there was marked hemorrhagic damage accompanied by inflammation and mucosal ulceration. Full depth necrosis of various severities was observed within the irradiation field (3 × 4 mm). Microscopic examination confirmed that the treatment did not result in any form of colon perforation. However, at a similar dose level (e.g. 2 mg/kg, 100 J/cm and DLI of 15 min) the interstitial irradiation could produce an average lesion size of 2 cm in the prostate.7
3.4 Abdominal wall
At one week post-PDT, there was no visible damage on the rectus sheath due to scattered light while the prostate underwent the interstitial irradiation. Under the direct surface irradiation in the peak mode, mild hemorrhage was visible at the irradiated site one week post PDT. At the dose levels of 1 mg/kg and 50 J/cm2, the lesion depth was 3 mm and diameter was 5 mm. At the dose levels of 2 mg/kg and 50 J/cm2, the lesion depth was 5 mm and diameter 10 mm. At the highest dose level (i.e. 2 mg/kg and 75 J/cm2) the lesion was 8 mm deep and did not reach to the anterior rectus sheath. The structure of the posterior rectus sheath was intact. No ulcer or perforation lesion was detected.
Under microscopic examination, inflammatory lesions were seen in the sheath, connective and muscular tissues in all light/drug dose groups. For instance, at 1 mg/kg and 50 J/cm2, a mild necrotic lesion was seen in muscular fibers. At 2 mg/kg and 50 J/cm2, severe necrotic lesions were seen in muscular fibers. A total destruction of muscular tissue was seen in the some small areas at 75 J/cm2 and 100 J/cm2 at drug doses equal to or greater than 1 mg/kg. However, at the same irradiation mode and dose levels the interstitial irradiation could induce marked necrotic lesion in the prostate. The lesion size could reach 1.2 cm at 1 mg/kg and 50 J/cm and beyond 2.4 cm at 2 mg/kg and 100 J/cm.9
3.5 The urethral sphincter
The urethral sphincter was not directly irradiated in this study. The microscopic examination of those prostates treated interstitially confirmed that, outside the necrotic area, the external striated urethral sphincter was unaffected.
3.6 The pelvic nerve
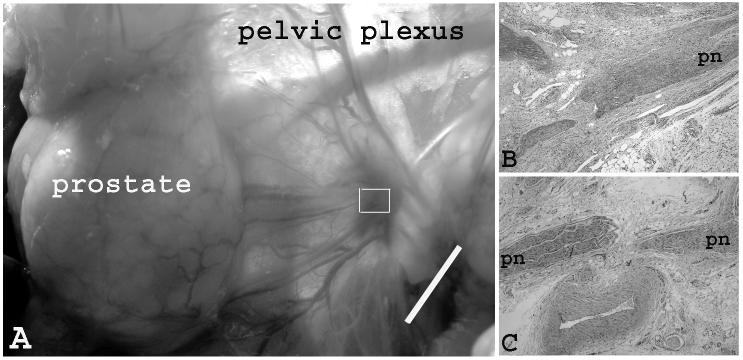
Hyperemia lesions could be seen at the irradiated site at one-week post PDT. At 50 J/cm2, the lesion size was smaller than the irradiated area (Fig. 3A). At 200 J/cm2, the lesion size was larger than the irradiated area, indicating severe vasculature damage at a higher light dose. Microscopic examination of specimens of both dose groups revealed that segmental endoneurial hemorrhage was present in the nerve fascicles. Degeneration nerve fibers and necrotic neurons were present. The adjacent connective tissue showed areas of hemorrhage and fibrosis, regional accumulations of inflammation cells in varying proportions at both light dose levels (Fig. 3B).
Fig. 3.
Gross and microscopic view of the pelvic plexus and pelvic nerve one week post PDT. (A) Hyperemia lesions induced by 1 mg/kg and 50 J/cm2. The lesion size was smaller than the size of irradiated area (i.e. 1 cm). (Bar = 1 cm) (B) Microscopic view of the marked area of the previous photo. H&E staining of longitudinal section of pelvic plexus showed the segmental endoneurial hemorrhage of the pelvic nerve fascicles (PN). Degeneration nerve fibers and necrotic neurons were present. The adjacent connective tissue showed areas of hemorrhage and fibrosis, regional accumulations of inflammation cells in varying proportions. (C). Microscopic view of the control (no drug, no light). (×20)
It is equally important to evaluate the effects of Tookad PDT on the functions of the pelvic nerve. However, due to the great technical difficulties encountered in an effort to measure the conductivity changes of the pelvic nerve prior to and post PDT, the cutaneous branches of the saphenous nerve were used as a model for studying the effect of Tookad PDT on the nerve conductivity and results were reported elsewhere.27 Briefly, in the saphenous nerve model, complex action potentials (CAPs) were recorded directly from the surface of the distal portion of the saphenous nerve. At the 1 mg/kg and 50 J/cm2, there was no change in CAP profile. At 100 J/cm2, there were significant changes in CAPs profile, but the treated nerve was still able to conduct a signal. Nevertheless, higher threshold stimulation and amplification factors were required to produce an evoked CAP. A total loss of nerve conductivity was confirmed at 200 J/cm2. This suggests that PDT might impair the nerve conduction at a higher dose level. Histopathological examination revealed that the treatment doses of 2 mg/kg and 50 J/cm2 produced very little visible change. There was some reactive and connective tissue proliferation around the treatment site, however, the saphenous nerve fiber and adjacent artery looked unchanged. Treatment with 1 mg/kg and 100 J/cm2 produced some visible changes. Mild edema along the incision line and nerve tract was obvious after one week. Treatment with 200 J and 1 mg/kg induced more damage. Vasculature surrounding the saphenous nerve appeared irritated. The nerve itself looked swollen and individual fibers were not as distinct as they were before PDT treatment. Treatment with 2 mg/kg and 200 J/cm2 produced significant changes. The irradiated nerve looked edematous, with generalized cellulitis appearing around the whole nerve track and surgical site at the one week follow up. Microscopic examination showed that some areas of epineurium had mild hemorrhage, leukocyte infiltration, and fibroplasias and vascular hypertrophy. The nerve fascicles and nerve fibers were free of lesions (Fig. 4).
Fig. 4.
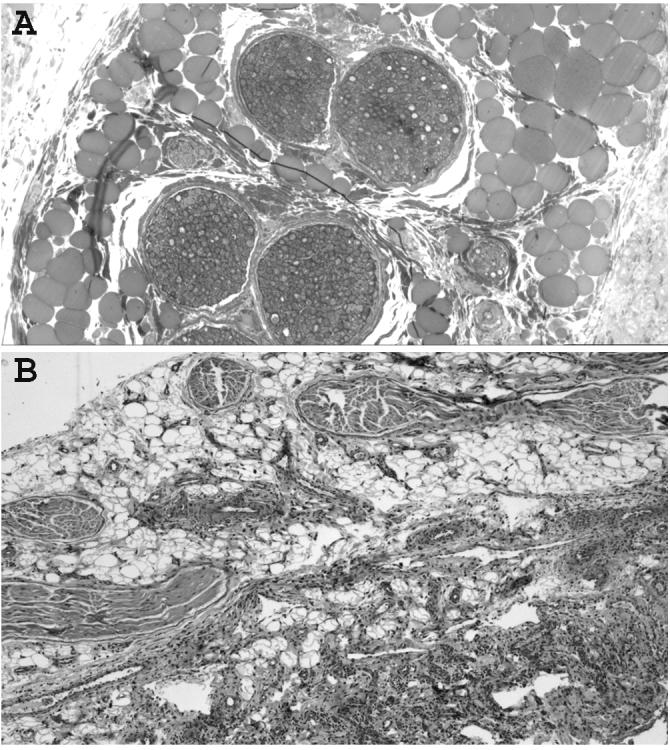
Microscopic view of the saphenous nerve one week post PDT. (A) Toluidine blue staining of the cross section of central region treated with 1 mg/kg and 200 J/cm2. Nerve fibers were free of lesions. (B) H&E staining of the longitudinal section of central region treated with 2 mg/kg and 200 J/cm2. Microscopic examination showed that some areas of epineurium had mild hemorrhage, leukocyte infiltration, and fibroplasias and vascular hypertrophy. The nerve fascicles and nerve fibers were free of lesions though. (×20)
The bladder, colon, abdominal muscle and pelvic plexus appeared to be sensitive to Tookad PDT. The colon is more sensitive than the bladder, muscle and nerve tissues. However, for reasons that are not fully understood, the Tookad PDT-induced responses in the normal bladder, colon, muscle and nerve tissues appeared to be minimal compared to that of the prostate gland at the same dose levels. For instance, at the dose levels of 2 mg/kg and 50 J/cm, Tookad PDT could produce an average lesion size of 1.5 cm in the prostate.7,9 But only microscopic lesion in bladder, colon, and nerve tissues. While maximizing the PDT effect at the peak mode, it only produced mild necrosis with a depth of 5 mm in muscle tissue.
There is considerable light scatter during interstitial prostate PDT, particularly at the higher fluences. For the largest PDT lesions, such as an interstitial irradiation of 200 J/cm, 1.5 cm radius of lesion and attenuation depth of 3 mm, and assuming a few millimeter range for fluence build-up due to light backscattering, an upper estimation of the light fluence at the PDT lesion boundary is ∼ 20 J/cm2. This is consistent with the observation of no colon or bladder or muscle damage due to scattered light. The relatively lower sensitivity of the colon/rectum, bladder and muscle should provide an additional safety margin that should allow aggressive treatment of the entire prostate with low risk of damage to these adjacent structures due to the scattered light.
Although it is impossible to measure the conductivity of the pelvic nerve, the histopathological examination indicates that the pelvic nerve is more sensitive to Tookad PDT than the cutaneous branches of the saphenous nerve. This might be attributed to the dense vasculatures inside the pelvic plexus and adjacent to the pelvic nerve. The clinical relevance of this finding certainly needs to be further studied. Nevertheless, the protection of the adjacent tissues should be taken into consideration in the total prostate ablation process.
Interestingly, our data demonstrate that although the PDT-induced lesion can reach to the prostatic urethra and produce significant periurethral damages, but only minimal urethral damage both functionally and histopathologically were observed. The sensitivity and response of the prostatic urethra to the Tookad PDT is worth further investigation. Direct intraurethral light irradiation might provide an ideal means to determine the definitive damage threshold of the prostatic urethra. Transurethral PDT protocols might be feasible for the management of BPH even though the mechanisms and clinical goals of treating BPH differ to that of prostate cancer.
5 Conclusions
Tookad-mediated vascular targeting PDT can produce marked hemorrhagic necrosis inside the prostate gland. It can provide an effective alternative for the treatment of localized prostate cancer. Tested adjacent tissues (e.g. bladder, colon, abdominal muscle, and pelvic nerve) show different sensitivities to Tookad PDT. The protection of the adjacent tissues should be taken into consideration during the total prostate ablation process.
Acknowledgements
The authors thank David Luck, Don Maul, Lawrence Whalen, Daniel Gould, Susan Kraft, Elisa French, Nadira Trncic and Liping Liang for their technical assistance. This project was supported partly by STEBA BIOTECH (France) and US NIH Grant (CA 43892).
References
- 1.Huang Z. A review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005;4:283–294. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selman SH. Photodynamic therapy for prostate cancer: One urologist’s perspective. Photodiag. Photodyn. Therapy. 2007;4:26–30. doi: 10.1016/j.pdpdt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Muller PJ, Wilson BC. Photodynamic therapy of brain tumors - a work in progress. Lasers Surg. Med. 2006;38:384–389. doi: 10.1002/lsm.20338. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z. Photodynamic therapy in China: 25 years of unique history - Part Two: clinical experience. Photodiag. Photodyn. Therapy. 2006;3:71–84. doi: 10.1016/j.pdpdt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Biel M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg. Med. 2006;38:349–355. doi: 10.1002/lsm.20368. [DOI] [PubMed] [Google Scholar]
- 6.Lee LK, Whitehurst C, Chen Q, Pantelides ML, Hetzel FW, Moore JV. Interstitial photodynamic therapy in the canine prostate. Br. J. Urol. 1997;80:898–902. doi: 10.1046/j.1464-410x.1997.00460.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Huang Z, Luck D, Beckers J, Brun PH, Wilson BC, Scherz A, Salomon Y, Hetzel FW. Preclinical studies in normal canine prostate of a novel palladium-bacteriopheophorbide (WST09) photosensitizer for photodynamic therapy of prostate cancer. Photochem. Photobiol. 2002;76:438–445. doi: 10.1562/0031-8655(2002)076<0438:PSINCP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Huang Z, Chen Q, Trncic N, LaRue SM, Brun PH, Wilson BC, Shapiro H, Hetzel FW. Effects of Pd-bacteriopheophorbide (TOOKAD)-mediated photodynamic therapy on canine prostate pretreated with ionizing radiation. Rad. Res. 2004;161:723–731. doi: 10.1667/rr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Chen Q, Luck D, Becker J, Wilson BC, Trncic N, Larue SM, Blanc D, Hetzel FW. Studies of a vascular-acting photosensitizer, Pd-bacteriopheophorbide (Tookad), in normal canine prostate and spontaneous canine prostate cancer. Lasers Surg. Med. 2005;36:390–397. doi: 10.1002/lsm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsi RA, Kapatkin A, Strandberg J, Zhu T, Vulcan T, Solonenko M, Rodriguez C, Chang J, Saunders M, Mason N, Hahn S. Photodynamic therapy in the canine prostate using motexafin lutetium. Clin. Cancer Res. 2001;7:651–660. [PubMed] [Google Scholar]
- 11.Momma T, Hamblin MR, Wu HC, Hasan T. Photodynamic therapy of orthotopic prostate cancer with benzoporphyrin derivative: local control and distant metastasis. Cancer Res. 1998;58:5425–5431. [PubMed] [Google Scholar]
- 12.Selman SH, Keck RW, Hampton JL. Transperineal photodynamic ablation of the canine prostate. J. Urol. 1996;156:258–260. [PubMed] [Google Scholar]
- 13.Selman SH, Albrecht D, Keck RW, Brennan P, Kondo S. Studies of tin ethyl etiopurpurin photodynamic therapy of the canine prostate. J. Urol. 2001;165:1795–1801. [PubMed] [Google Scholar]
- 14.Chang S, Buonaccorsi G, MacRobert A, Bown SG. Interstitial and transurethral photodynamic therapy of the canine prostate using meso-tetra-(m-hydroxyphenyl) chlorine. Int. J. Cancer. 1996;67:555–562. doi: 10.1002/(SICI)1097-0215(19960807)67:4<555::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Chang S, Buonaccorsi GA, MacRobert AJ, Bown SG. Interstitial photodynamic therapy in the canine prostate with disulfonated aluminum phthalocyanine and 5-aminolevulinic acid-induced protoporphyrin IX. The Prostate. 1997;32:89–98. doi: 10.1002/(sici)1097-0045(19970701)32:2<89::aid-pros3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Wilson BC, Shetty SD, Patterson MS, Cerny JC, Hetzel FW. Changes in in vivo optical properties and light distributions in normal canine prostate during photodynamic therapy. Radiat. Res. 1997;147:86–91. doi: 10.2307/3579447. [DOI] [PubMed] [Google Scholar]
- 17.Muschter R. Photodynamic therapy: a new approach to prostate cancer. Current Urol. Reports. 2003;4:221–228. doi: 10.1007/s11934-003-0073-4. [DOI] [PubMed] [Google Scholar]
- 18.Nathan TR, Whitelaw DE, Chang SC, Lees WR, Ripley PM, Payne H, Jones L, Parkinson MC, Emberton M, Gillams AR, Mundy AR, Bown SG. Photodynamic therapy for prostate cancer recurrence after radiotherapy: a phase I study. J. Urol. 2002;168:1427–1432. doi: 10.1016/S0022-5347(05)64466-7. [DOI] [PubMed] [Google Scholar]
- 19.Zaak D, Sroka R, Höppner M, Khoder W, Reich O, Tritschler S, Muschter R, Knüchel R, Hofstetter A. Photodynamic Therapy by Means of 5-ALA Induced PPIX in Human Prostate Cancer - Preliminary Results. Med. Laser Appl. 2003;18:91–95. [Google Scholar]
- 20.Weersink RA, Bogaards A, Gertner M, Davidson SR, Zhang K, Netchev G, Trachtenberg J, Wilson BC. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: clinical experience and practicalities. J. Photochem. Photobiol. B. 2005;79:211–222. doi: 10.1016/j.jphotobiol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Du KL, Mick R, Busch TM, Zhu TC, Finlay JC, Yu G, Yodh AG, Malkowicz SB, Smith D, Whittington R, Stripp D, Hahn SM. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg. Med. 2006;38:427–434. doi: 10.1002/lsm.20341. [DOI] [PubMed] [Google Scholar]
- 22.Moore CM, Nathan TR, Lees WR, Mosse CA, Freeman A, Emberton M, Bown SG. Photodynamic therapy using meso tetra hydroxy phenyl chlorin (mTHPC) in early prostate cancer. Lasers Surg. Med. 2006;38:356–363. doi: 10.1002/lsm.20275. [DOI] [PubMed] [Google Scholar]
- 23.Mauroy B, Demondion X, Drizenko A, Goullet E, Bonnal JL, Bisert J, Abbou C. The inferior hypogastric plexus (pelvic plexus): its importance in neural preservation techniques. Surg. Radiol. Anat. 2003;25:6–15. doi: 10.1007/s00276-002-0083-9. [DOI] [PubMed] [Google Scholar]
- 24.Ali M, Johnson IP, Hobson J, Mohammadi B, Khan F. Anatomy of the pelvic plexus and innervation of the prostate gland. Clin. Anat. 2004;17:123–129. doi: 10.1002/ca.10187. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber S, Gross S, Brandis A, Harmelin A, Rosenbach-Belkin V, Scherz A, Salomon Y. Local photodynamic therapy (PDT) of rat C6 glioma xenografts with Pd-bacteriopheophorbide leads to decreased metastases and increase of animal cure compared with surgery. Int. J. Cancer. 2002;99:279–285. doi: 10.1002/ijc.10299. [DOI] [PubMed] [Google Scholar]
- 26.Weersink RA, Forbes J, Bisland S, Trachtenberg J, Elhilali M, Brun PH, Wilson BC. Assessment of cutaneous photosensitivity of TOOKAD (WST09) in preclinical animal models and in patients. Photochem. Photobiol. 2005;81:106–113. doi: 10.1562/2004-05-31-RA-182. [DOI] [PubMed] [Google Scholar]
- 27.Dole KC, Chen Q, Hetzel FW, Whalen LR, Blanc D, Huang Z. Effects of photodynamic therapy on peripheral nerve: in situ compound-action potentials study in a canine model. Photomed. Laser Surg. 2005;23:172–176. doi: 10.1089/pho.2005.23.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen B, Pogue BW, Hoopes PJ, Hasan T. Vascular and cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr. 2006;16:279–305. doi: 10.1615/critreveukargeneexpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]