Abstract
Intragenic complementation has been observed at the argininosuccinate lyase (ASL) locus. Intragenic complementation is a phenomenon that occurs when a multimeric protein is formed from subunits produced by different mutant alleles of a gene. The resulting hybrid protein exhibits enzymatic activity that is greater than that found in the oligomeric proteins produced by each mutant allele alone. The mutations involved in the most successful complementation event observed in ASL deficiency were found to be an aspartate to glycine mutation at codon 87 of one allele (D87G) coupled with a glutamine to arginine mutation at codon 286 of the other (Q286R). To understand the structural basis of the Q286R:D87G intragenic complementation event at the ASL locus, we have determined the x-ray crystal structure of recombinant human ASL at 4.0 Å resolution. The structure has been refined to an R factor of 18.8%. Two monomers related by a noncrystallographic 2-fold axis comprise the asymmetric unit, and a crystallographic 2-fold axis of space group P3121 completes the tetramer. Each of the four active sites is composed of residues from three monomers. Structural mapping of the Q286R and D87G mutations indicate that both are near the active site and each is contributed by a different monomer. Thus when mutant monomers combine randomly such that one active site contains both mutations, it is required by molecular symmetry that another active site exists with no mutations. These “native” active sites give rise to the observed partial recovery of enzymatic activity.
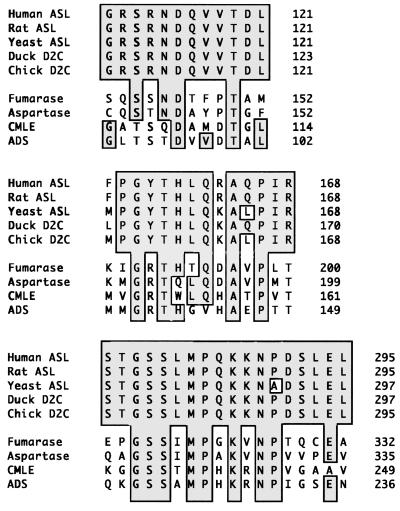
Argininosuccinate lyase (ASL; EC 4.3.2.1) participates in the urea cycle, the major pathway for the detoxification of ammonia, where it catalyzes the reversible breakdown of argininosuccinic acid into arginine and fumarate. ASL also belongs to a superfamily of metabolic enzymes, all of which function as tetramers and catalyze homologous reactions with fumarate as a product. Other members of the superfamily include δ crystallin (1–4), fumarase (5), aspartase (5), adenylosuccinase (6), and 3-carboxy-cis,cis-muconate lactonizing enzyme (7). Although, across the superfamily very little sequence similarity is observed, three regions of highly conserved amino acid sequence (5) (Fig. 1) have been identified and implicated in the common catalytic mechanism. Within the superfamily, ASL is most closely related to the avian eye lens protein, δ crystallin. Comparison of the protein sequences for ASL with those of various δ crystallins indicate 64–72% amino acid sequence identity (2–4). Certain δ crystallins, the δ II isoform, (8–11) have been shown to exhibit lyase activity approximately equivalent to that found in purified human ASL.
Figure 1.
The three regions of high sequence conservation among members of the ASL superfamily. ASL, argininosuccinate lyase; D2C, δ II crystallin; fumarase, E. coli fumarase C; aspartase, E. coli aspartase; CMLE, P. putida 3-carboxy-cis,cis-muconate lactonizing enzyme; ADS, B. subtilis adenylosuccinase.
Mutations in human ASL result in the clinical condition argininosuccinic aciduria, the second most common urea cycle disorder (12). The disease displays considerable variation in its clinical pathology with three distinct phenotypes: neonatal, subacute, and late onset (12). In an effort to understand the genetic defects that underlie argininosuccinic aciduria, McInnes, O’Brien, and their colleagues have identified, to date, 11 unique ASL mutations (13–15). McInnes et al. (16) also have demonstrated extensive intragenic complementation between ASL-deficient cell strains. Intragenic complementation is a phenomenon that occurs when a multimeric protein is formed from subunits produced by two differently mutated alleles of a gene. Thus a partially functional hybrid protein is produced from two distinct types of mutant subunits, neither of which can, on their own, give rise to appreciable enzymatic activity.
The genetic defects in the cell strains participating in the most successful complementation event have now been identified (13). The strains were found to have either a glutamine to arginine mutation at codon 286 (Q286R) or an aspartate to glycine mutation at codon 87 (D87G). Complementation between these alleles was demonstrated by constructing plasmids expressing normal ASL, the Q286R mutant, and the D87G mutant and transfecting them into COS cells either individually (i.e., normal, Q286R, D87G) or together (i.e., both the Q286R and D87G plasmids) and measuring the rate of conversion of 14C-labeled fumarate into 14C-labeled argininosuccinate. The individual mutant D87G ASL and Q286R ASL tetramers showed little (≈5%) or no (<0.01%) activity, respectively, whereas the COS cells transfected with both the D87G and Q286R ASL were found to exhibit approximately 30% wild-type ASL activity (13).
In 1964, Crick and Orgel (17) suggested that complementation in a dimeric protein between two monomers Ab and aB with different inactive regions (denoted by lowercase a and b) aggregate to form an inactive site ab and an active site AB, which results in a partial restoration of activity of ≈50%. However, they dismissed this scenario from their general theory of complementation, assuming that because a residual amount of activity remained, such a protein would not be detected as bearing mutations. In an effort to explain the observed nonlinearity of complementation maps, it was instead suggested that the “misfolding” of one mutant subunit was somehow compensated for by an unaltered portion of an adjacent mutant subunit. The three-dimensional structure of human ASL described herein has enabled us to study the concept of intragenic complementation found in certain ASL-deficient strains and provides experimental proof of Crick and Orgel’s initial hypothesis of intragenic complementation (17). Evidence for complementation according to the scheme of statistical regeneration of wild-type active sites also has been observed in vitro for the homodimeric proteins thymidylate synthase (18), ribulose-bisphosphate carboxylase/oxygenase (19), glutathione reductase (20), and mercuric reductase (21) and the homotrimeric enzyme aspartate transcarbamoylase (22). As intragenic complementation has been implicated in other diseases involving mutations in homomultimeric proteins such as propionic acidemia (23, 24) and methylmalonic aciduria (25), a logical extension to heteromultimeric proteins also may apply, making intragenic complementation a much overlooked source of phenotypic and biochemical variation in genetic disease.
EXPERIMENTAL METHODS
Crystallization and Data Collection.
Recombinant human ASL was expressed in Escherichia coli, purified, and crystallized as described previously (26). All crystals were obtained by the hanging drop vapor diffusion method. Crystals have been grown from a 10–15 mg/ml protein solution in 50 mM phosphate buffer, 5 mM DDT at pH 7.1 with 1.1 M phosphate as the precipitating agent. Intensity data were measured at room temperature using a Siemens multiwire detector mounted on a Rigaku RU-200 rotating anode generator (37 kV, 70 mA). Intensity data were processed and scaled using the program xds (27, 28). On preliminary inspection, the space group was determined to be either P3121 or its enantiomorph, P3221. On the basis of density calculations two monomers were estimated to be present in the asymmetric unit (Vm= 2.9 Å3·Da−1) giving rise to a solvent content of ≈43%. A summary of the data collection statistics is presented in Table 1.
Table 1.
Crystal and diffraction data
| Data collection | |
|---|---|
| Space group | P3121 |
| Cell dimensions | a = b = 104.59 Å, c = 183.32 Å, γ = 120° |
| Resolution | 4.0 Å |
| Total number of reflections | 23,049 |
| Unique reflections | 8,659 |
| Completeness* | 92.3% |
| Rsym(I)† | 11.6% |
Completeness quoted for all data, 69.4% of the data was >1σ(I).
Rsym(I) = Σ (I − 〈I〉)/Σ I where I are the intensity measurements for symmetry-related reflections, and 〈I〉 is the mean intensity for the reflection.
Structure Solution and Refinement.
The structure of human ASL was solved by molecular replacement using the program xplor (29) with the coordinates of enzymatically inactive turkey δ I crystallin (TDIC) as the search model (30). Because the sequence of TDIC is unknown, Simpson et al. (30) have used the sequence of chicken δ I crystallin in their crystallographic model. ASL and chicken δ I crystallin share 64% amino acid sequence identity, whereas turkey and chicken are expected to share >90% sequence identity. Data between 8 and 4 Å resolution were used in all rotation and translational searches. Initial rotation searches and Patterson correlation refinement were carried out using coordinates of TDIC subunit A, which had lower average atomic temperature factors than the three other monomers in that structure. The search yielded two solutions with maximum rotation function values with unpruned TDIC side-chain coordinates. Subsequent translation function searches were inconclusive.
In addition to TDIC (30), the structure of E. coli fumarase (31) also has been determined. The three-dimensional fold of these proteins is identical, and both associate to form a tetramer in a similar manner with almost identical 222 symmetry. This tetramer association almost certainly represents a common feature of the superfamily, and therefore it is reasonable to expect that the arrangement of ASL monomers would be similar. As Vm calculations indicated the presence of two monomers in the asymmetric unit of the ASL cell, a series of searches was carried out using various TDIC dimer arrangements. Three unique monomer combinations are possible, denoted as AB, AC, or AD. Each was used as a dimeric probe in a series of rotation searches. All rotation searches gave the same pair of solutions as that found in the previous searches using TDIC subunit A alone and after PC refinement, no dimer combination gave a significantly better result than the others. All translation searches were carried out in space groups P3121 and P3221. The solution was apparent as the highest peak in all translation searches at 22 SD above the mean in space group P3121 for the AC dimer. This allowed the correct enantiomorphic space group (P3121) to be determined. The final rotation/translation solution had an R factor of 39% (Rfree, ref. 32; 41%).
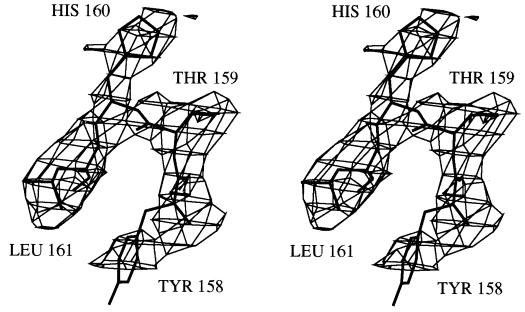
Rigid body refinement was carried out using xplor with the individual monomers refined as distinct bodies to give an Rfactor of 34.9% and Rfree of 36.3% for data [F > 2σ(F)] between 8 and 4 Å resolution. The initial model thus produced was examined with the graphics package o (33), and residues were mutated computationally to the correct ASL sequence. Rigorous testing of potential xplor simulated annealing refinement protocols was carried out by examination of the behavior of Rfree with the aim of making the best use of the relatively low-resolution data. Only those protocols where both the Rfree and Rfactor decreased were considered valid. As a result of these tests, the decision was made to truncate the data to 4.2 Å to diminish the possibility of overfitting the structure due to the incompleteness of the data in the highest resolution shell. It also proved valid to choose an appropriate weighting scheme comparable to that implemented in the program prolsq (34) and based on the magnitude of the difference between observed and calculated structure factors for ranges of sinQ/λ (35). This strategy resulted in lower weights for the generally stronger (yet more poorly modeled) lowest-resolution reflections, which otherwise would dominate a refinement run. Strong noncrystallographic restraints (ncs-weight = 1,500 kcal⋅mol−1⋅Å−2) were maintained during all stages of refinement. This resulted in a final rms difference between noncrystallographic symmetry-related monomers of 0.01 Å for all atoms. Several rounds of refinement were done with a simulated heat stage to 4,000 K followed by cooling to 300 K and several rounds of minimization. These rounds were alternated with sessions of rebuilding in o (33). With the individual B factors fixed at 15 Å2, the overall B factor was refined and is listed in Table 2. Both averaged (36, 37) and unaveraged maps with coefficients 2Fo − Fc and Fo − Fc were used during rebuilding. Extensive use of simulated annealing omit trials to remove model bias and frequent comparison with the refined coordinates of two recently solved high-resolution (2.5 and 2.0 Å) structures of two enzymatically active duck δ II crystallin mutants (M. Abu-Abed, M.A.T., C. Slingsby, and P.L.H., unpublished work; M.A.T., K. Dole, and P.L.H., unpublished work) aided in fitting difficult sections and confirming correctly built regions. Refinement was completed by three rounds of positional refinement in xplor. Fig. 2 is a simulated annealing 2Fo − Fc omit map of residues 158–161, a loop between the two regions of β-sheet and shows the location of His-160, one of the putative catalytic resides. The map shows the quality of the electron density in the loop regions. The electron density for the rest of the structure was, in general, of equal or better quality, with the exception of the electron density for residues 71–79 and the N-terminal 19 residues. These residues, therefore, have not been included in the final model. In addition, Glu-22 has been truncated to Cβ as have Arg-95 and Arg-246. The side chain of Arg-111 has been pruned to Cα. An assessment of structure quality was made using the program procheck (38) and showed that no residues resided in disallowed regions of a Ramachandran plot and that the geometry was generally excellent. Refinement results are listed in Table 2.
Table 2.
Refinement statistics
| Refinement | |
|---|---|
| Reflections, F > 2σF | 6,474 |
| Resolution | 8–4.2 Å |
| Rfactor* | 18.79% |
| Rfree† | 29.82% |
| Reflections, all data | 6,919 |
| R factor, all data | 21.16% |
| Overall B factor, Å2 | 20.5 |
| rms bonds, Å | 0.006 |
| rms angle, ° | 1.58 |
Rfactor = Σ (|Fo| − |Fc|)/Σ |Fo|.
Rfree = Σ (|Fos| − |Fcs|)/Σ |Fos| where s refers to a subset of data not used in refinement comprising 8% of the data and selected from thin-resolution shells dispersed at intervals through the data set.
Figure 2.
A simulated annealing 2Fo − Fo omit map for residues 158–161. All atoms displayed in the map were removed from the structure before a round of simulated annealing refinement. The map is contoured at 1 σ.
RESULTS AND DISCUSSION
Overall Topology.
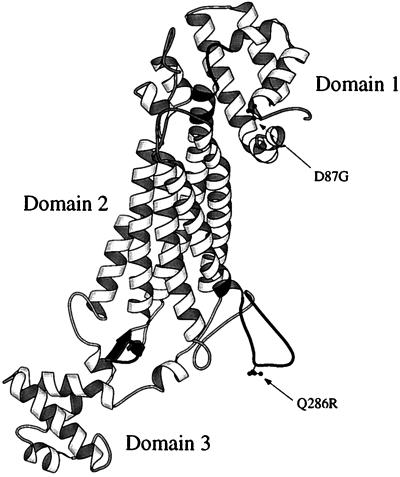
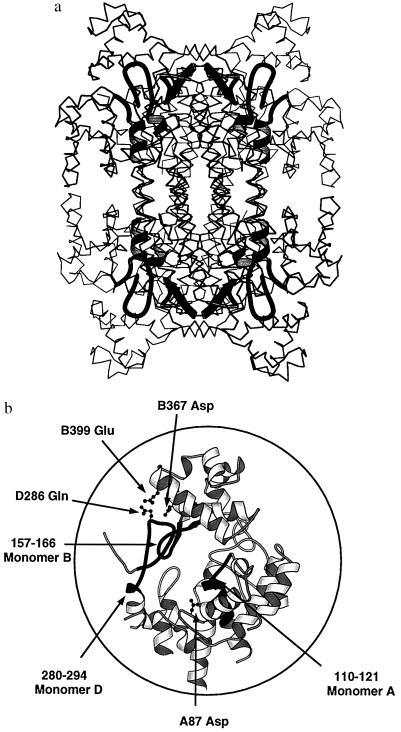
As expected from the close sequence similarities between ASL and the delta crystallins (64–72% identity) and the ASL activity demonstrated by some delta crystallins, the overall three-dimensional structures are very similar with rms deviations in dimer Cα positions of 0.96 Å as compared with TDIC (30) and 0.99 Å vs. duck δ II crystallin (M.A.T., K. Dole, and P.L.H., unpublished work). Duck δ II crystallin is a 2.0-Å resolution structure of an enzymatically active delta crystallin (M.A.T., K. Dole, and P.L.H., unpublished work). Although ASL is a low-resolution structure, the simulated annealing omit trials and the general good quality of the electron density maps (see Fig. 2) allowed some significant differences in the backbone atoms of the TDIC and ASL to be modeled. Each monomer is approximately 100 Å long and can be divided into three predominantly helical domains, comprising residues 1–112, 112–362, and 371–436. Fig. 3 is a schematic three-dimensional diagram of the overall topology of the monomer. ASL is catalytically active as a tetramer. Fig. 4a is a schematic diagram showing the 222 symmetric arrangement of the four monomers in the tetramer. It is drawn perpendicular to the long axis of the helix bundle in domain 2 and viewed down another of the 2-fold axes. The formation of the tetramer can be described as the arrangement of two dimeric pairs where each dimer is formed through mainly hydrophobic interactions between helices h8, h11, and h12. The numbering of the helices is consistent with that described previously for TDIC (30). Less extensive interactions between dimers result in the formation of the tetramer with helix h12 of each monomer forming the primary, innermost interface in the 20-helix bundle of the tetramer.
Figure 3.
Schematic diagram showing the three-dimensional topology of an ASL monomer drawn with the program molscript (40). The amino acid side chains of residues D87 and Q287 have been drawn in a ball-and-stick representation. The three regions of highly conserved sequence are shaded in black.
Figure 4.
Schematic diagrams showing the arrangement of the conserved regions (shaded in black) in (a) the tetramer and (b) the active site. The location of key residues are shown in a ball-and-stick representation. The residues are labeled according to the monomer on which they reside (A–D) and the residue number and type.
Location of Active Site.
The three regions of highly conserved sequence homology are believed, because of their intolerance to mutation, to participate in the formation of a general active site among superfamily members (30, 31). These regions are shown in Fig. 1 and are mapped onto the structure of the ASL monomer in Fig. 3. The spatial relationship between conserved regions becomes more obvious in the tetrameric arrangement of monomers in the active protein (Fig. 4a) where three different monomers contribute a section of conserved sequence to form a bowl-shaped indentation at each of the four “corners” of the ASL tetramer (Fig. 4 a and b). The ASL residues that contribute to the active are similar to those found in the TDIC and the E. coli fumarase structure. Further evidence that these regions comprise the active site was provided by the crystal structure of E. coli fumarase C complexed with the inhibitors tungstate and 1,2,4,5-benzenetetracarboxylic acid (31) as the inhibitor binding occurs in this putative active site pocket. Tungstate and benzenetetracarboxylate are thought to mimic fumarate in the enzyme complex and, by extension, may be analogous to the fumarate/succinate moiety in an argininosuccinate complex with ASL.
Earlier biochemical studies of ASL (9) implicated a histidine and a carboxylic acid as residues directly involved in catalysis. Indeed, His-160 is found at the putative ASL active site and corresponds to His-188 in fumarase C, which forms a hydrogen bond to the inhibitors through the oxygen atoms of the oxyanion. The role of His-160 in the catalytic mechanism has not yet been established. The catalytic mechanism requires a proton abstractor, a proton donor, and stabilizers of the carbanion intermediate either through interactions with the carbanion itself or through neutralization of the negatively charged carboxylates of the substrate. The side chain of His-160 could perform any of these functions depending on its protonation state. The structure of the fumarase C/tungstate inhibitor complex suggests that this histidine is involved in bonding to and neutralizing a negatively charged carboxylate group. However, there are few other residues in the vicinity that seem capable of proton abstraction. Presumably histidine acts as a proton abstractor to form the carbanion, and cleavage is achieved upon subsequent protonation by an acid. In the ASL structure His-160 ND1 is in a position to form potential hydrogen bonds with Glu-294 OE1, Asn-289 OD1, and Pro-290 O. The proximity of Glu-294 and its strong hydrogen bond through OE1 with the His-160 ND1 also is observed in the TDIC and fumarase structures and led Weaver et al. (31) to suggest a type of charge relay, which increases the nucleophilicity of His-160, making it a good candidate for the role of proton abstractor. The strong hydrogen bond to Glu-294 renders His-160 less likely to carry a positive charge and therefore less likely to offer stabilization of the negatively charged intermediate.
The basic residue Lys-287 is the only positively charged amino acid in this region that is strictly conserved among all superfamily members and may be responsible for neutralizing one of the three negative charges residing on the carbanion dicarboxylate. Indeed, mutation of the corresponding residue (Lys-326) to arginine in aspartase eliminates catalytic activity by elevating Km (39). The neutral residue Asn-289 is also strictly conserved and may be available for hydrogen bonding to substrate. The role of Met-284 also has been speculated on (5) in light of absolute conservation of this residue in sequences of superfamily members; however, there is no experimental data to suggest its possible role. The side chain of this residue is oriented into the active site at a distance of 11 Å from the His-160 side chain in the present structure. Other positively charged residues in the vicinity of the putative carboxylate binding site are Arg-113, Lys-323, and His-388, which are conserved among ASL and δ crystallin sequences but not across the superfamily. Simpson et al. (30) speculate that Glu-294, because of its proximity to His-160, is a good candidate for proton donation, whereas Weaver et al. (31) suggest for fumarase that the proton donor may be Ser-139 or Ser-140 (corresponding to Ser-112 or Arg-113 of ASL). It is possible that the candidate proton donor is found in this part of the structure; however, only two residues, Asp-115 and Thr-119, are strictly conserved across the superfamily. Whereas Thr-119 is buried and somewhat distant from the active site, Asp-115 could form a number of potential hydrogen bonds with neighboring main chain atoms of Ser-112 and Glu-116 and would appear to be important in stabilizing the turn containing residues Ser-112 and Arg-113.
Location of Q286 and D87.
The D87G and Q286R mutations associated with the most sucessful complementation event observed at the ASL locus yield tetrameric proteins with very low or no enzymatic activity, respectively. Gln-286, although located in the loop comprising the third conserved region between helices h11 and h12 (see Figs. 3 and 4b) is one of the least conserved residues across the superfamily (Fig. 1). The adjacent Lys-287 has been implicated in the reaction mechanism in light of its strict conservation throughout the superfamily and likely is involved in stabilizing the carbanionic intermediate by neutralizing a negative charge either on the carbanion or on one the carboxylate groups of the nascent fumarate. The effect of the glutamine → arginine mutation is difficult to explain in the absence of the structure of a complexed substrate or inhibitor molecule. The small size difference between glutamine and arginine is unlikely to affect enzymatic activity, because the glutamine side chain extends upward from the loop toward the solvent and away from the active site. It seems more likely that the additional positive charge perturbs the mechanism in some way. Replacement of the glutamine with a positively charged arginine may enable the formation of a salt bridge between Asp-367 or possibly Glu-399 of a different monomer (see Fig. 4b) resulting in a distortion and/or rigidification of the conserved loop, the flexibility of which may be required for activity.
The D87G point mutation results in a reduction of the specific enzymatic activity to approximately 5% that of wild type. Residue Asp-87 is located at the beginning of a helix in domain one (see Figs. 3 and 4b) and is in close proximity to His-89, a residue we postulate as being important for the binding of the substrate and in defining the substrate specificity of the enzyme (M. Abu-Abed, M.A.T., C. Slingsby, and P.L.H., unpublished work). Side-chain density is weak for residues in this region, implying some disorder due to flexibility; however, potential hydrogen bonds could be formed between Asp-87 and main chain of Thr-90 and between Asp-87 and Glu-86 (Å). This residue therefore could play a role in stabilizing the helix by hydrogen bonding to the nitrogen of the main chain of Thr-90 (i.e., N-capping this helix). This hydrogen bonding capability would be lost upon mutation to glycine as would the negative charge, both of which may play a role in enzyme structure stabilization or in formation of the enzyme/substrate complex.
Complementation in Hybrid Tetramer.
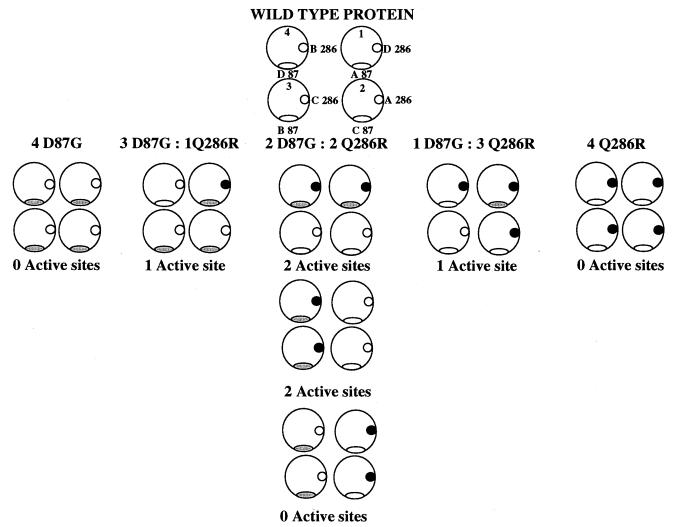
Whereas the structure of ASL presented here is a low-resolution structure and the side-chain conformations are less precisely defined than they would be in a high-resolution structure, it is, however, obvious that the individual mutations, D87G and Q286R, which participate in most successful complementation event observed at the ASL locus, are located at different loci in the active site cleft (Fig. 3b) and thus detrimentally affect different aspects of the catalytic mechanism. Any active site containing one or the other or both mutations would be compromised. Because a monomer will contain only one of the point mutations, there are five statistically distinguishable monomer combinations that can be formed. These unique combinations can give rise to the following tetramers in D87G:Q286R polypeptide ratios 4:0, 3:1, 2:2, 1:3, and 0:4. Examination of each of the four active sites in the protein reveals that for any one monomer (e.g., monomer A), residues 87 and 286 are found in two different active sites (active sites 1 and 2 in Fig. 5). The second half of the diagram illustrates the results for the four active sites for each of the various possible tetramer combinations. When the set of all possible hybrid tetramer combinations is considered there is a degeneracy corresponding to the binomial distribution 1:4:6:4:1 to give an overall expected enzymatic activity of ≈25% of that of the wild-type protein, assuming that zero activity is observed for each of the homotetrameric mutants and that the monomers combine in a randomly distributed manner. This expected level of activity is close to the 30% of wild-type activity seen in the reconstruction of the complementation event in COS cells (13), the difference between the two values being accounted for by the 5% activity exhibited by the D87G tetramer.
Figure 5.
A pictorial representation of the active sites of the statistically available combinations of mutants in D87G:Q286R intragenic complementation of the ASL tetramer. For clarity the diagram has been drawn to show the interaction of only the D87 (ovals) and Q286 (circles) and the shading of the symbols represents the presence of the point mutations D87G and Q286R, respectively. Each large circle represents one of the four active sites found in the protein. In the schematic diagram of the native protein, residues 286 and 87 also are labeled according to which monomer they are found on. The monomers are designated A-D. Due to the molecular symmetry of the tetramer, in the case of the 2D87G:2Q286R tetramer there are three distinctly different ways of combining the monomers that will give rise to either two or zero native active sites being formed. For each combination of monomers, one also must consider which monomer contains the mutation, in the case of 3D87G:1Q286R tetramer, the Q286R mutation could be present on monomer A, B, C, or D, and this gives rise to four different possible solutions. The set of all possible combinations has a degeneracy corresponding to a binomial distribution of 1:4:6:4:1.
Although the exact roles of Q286 and D87 are still in question, the structure of ASL has provided experimental proof that Crick and Orgel’s (17) initial intragenic complementation hypothesis regarding the statistical regeneration of wild-type active sites was correct. Furthermore, it appears likely that the phenomenon of intragenic complementation is as yet an unappreciated source of phenotypic variation in genetic diseases involving multimeric proteins.
Acknowledgments
We thank M. S. Hershfield for providing the purified protein for the initial crystallization trials and the ASL plasmid, C. Slingsby for providing the turkey δ I crystallin coordinates, and G. D. Smith for his help with the weighting schemes used in the crystallographic refinement and comments on the manuscript. This work is supported by a grant from the Natural Science and Engineering Research Council of Canada to P.L.H. R.R.M. is an International Research Scholar of the Howard Hughes Medical Institute.
Footnotes
Abbreviations: ASL, argininosuccinate lyase; TDIC, turkey δ I crystallin.
Data deposition: The coordinates and structure factors have been deposited in the Protein Data Bank, Brookhaven National Laboratory, Upton, NY, 11973 (accession nos. 1AOS and R1AOSSF, respectively).
References
- 1.Wistow G, Piatigorsky J. Science. 1987;236:1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- 2.Piatigorsky J, O’Brien W E, Norman B L, Kalumuck K, Wistow G J, Borras T, Nickerson J M, Wawrousek E F. Proc Natl Acad Sci USA. 1988;85:3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh L-S, Elzanowski L T, Hunt L T, Barker W C. Comp Biochem Physiol. 1988;2:433–437. doi: 10.1016/0305-0491(88)90247-7. [DOI] [PubMed] [Google Scholar]
- 4.Matsubasa T, Takiguchi M, Amaya Y, Matsuda I, Mori M. Proc Natl Acad Sci USA. 1989;86:592–596. doi: 10.1073/pnas.86.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods S A, Miles J S, Guest J R. FEMS Microbiol Lett. 1988;51:181–186. [Google Scholar]
- 6.Stone R L, Zalkin H, Dixon J E. J Biol Chem. 1993;268:19710–19716. [PubMed] [Google Scholar]
- 7.Williams S E, Woolridge E M, Ransom S C, Landro J A, Babbitt P C, Kozarick J W. Biochemistry. 1992;31:9768–9776. doi: 10.1021/bi00155a033. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa P, Wistow G J, Cialkowski M J P, O’Brien W E. J Biol Chem. 1991;266:22319–22322. [PubMed] [Google Scholar]
- 9.Lee H-J, Chiou S-H, Chang G-G. Biochem J. 1992;283:597–603. doi: 10.1042/bj2830597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiou S-H, Lo C-H, Chang C-Y, Itoh T, Kaji H, Samejma T. Biochem J. 1991;273:295–300. doi: 10.1042/bj2730295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondoh H, Araki I, Yasuda K, Matsubasa T, Mori M. Gene. 1991;99:267–271. doi: 10.1016/0378-1119(91)90137-z. [DOI] [PubMed] [Google Scholar]
- 12.Brusilow S W, Horwich A L. In: The Metabolic Basis of Inherited Disease. 6th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. Vol. 1. New York: McGraw–Hill; 1995. pp. 1187–1232. [Google Scholar]
- 13.Walker D C, Christodoulou J, Craig H J, Simard L R, Ploder L, Howell P L, McInnes R R. J Biol Chem. 1997;272:6777–6783. doi: 10.1074/jbc.272.10.6777. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa P, Cialkowski M, O’Brien W E. J Biol Chem. 1991;266:5286–5290. [PubMed] [Google Scholar]
- 15.Walker D C, McCloskey D A, Simard L R, McInnes R R. Proc Natl Acad Sci USA. 1990;87:9625–9629. doi: 10.1073/pnas.87.24.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McInnes R, Shih V, Chilton S. Proc Natl Acad Sci USA. 1984;81:4480–4484. doi: 10.1073/pnas.81.14.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crick F H C, Orgel L E. J Mol Biol. 1964;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- 18.Pookanjanatavip M, Yuthavong Y, Greene P J, Santi D V. Biochemistry. 1992;31:10303–10309. doi: 10.1021/bi00157a018. [DOI] [PubMed] [Google Scholar]
- 19.Larimer F W, Lee E H, Mural R J, Soper T S, Hartman F C. J Biol Chem. 1987;262:15327–15329. [PubMed] [Google Scholar]
- 20.Scrutton N S, Berry A, Deonarain M P, Perham R N. Proc R Soc London Ser B. 1990;242:217–224. doi: 10.1098/rspb.1990.0127. [DOI] [PubMed] [Google Scholar]
- 21.Distefano M D, Moore M J, Walsh C T. Biochemistry. 1990;29:2703–2713. doi: 10.1021/bi00463a013. [DOI] [PubMed] [Google Scholar]
- 22.Wente S R, Schachman H K. Proc Natl Acad Sci USA. 1987;84:31–35. doi: 10.1073/pnas.84.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravel R, Akerman B R, Lamhonwah A-M, Loyer M, Leon-del-Rio A, Italiano I. Am J Hum Genet. 1994;55:51–58. [PMC free article] [PubMed] [Google Scholar]
- 24.Gravel R A, Lam K F, Scully K J, Hsia Y. Am J Hum Genet. 1977;29:378–388. [PMC free article] [PubMed] [Google Scholar]
- 25.Qureshi A A, Crane A M, Matiaszuk N V, Rezvani I, Ledley F D, Rosenblatt D S. J Clin Invest. 1994;93:1812–1819. doi: 10.1172/JCI117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner M A, Achyuthan A M, McInnes R R, Hershfield M, Howell P L. J Mol Biol. 1994;239:336–338. doi: 10.1006/jmbi.1994.1372. [DOI] [PubMed] [Google Scholar]
- 27.Kabsch W. J Appl Cryst. 1988;21:67–71. [Google Scholar]
- 28.Kabsch W. J Appl Cryst. 1988;21:916–924. [Google Scholar]
- 29.Brünger, A. T. (1993) xplor Version 3.1 (Yale Univ. Press, New Haven).
- 30.Simpson A, Bateman O, Driessen H, Lindley P, Moss D, Mylvaganan S, Narebor E, Slingsby C. Nat. Struct Biol. 1994;1:724–733. doi: 10.1038/nsb1094-724. [DOI] [PubMed] [Google Scholar]
- 31.Weaver T M, Levitt D G, Donnelly M I, Wilkens Stevens P P, Banaszak L J. Nat Struct Biol. 1995;2:654–662. doi: 10.1038/nsb0895-654. [DOI] [PubMed] [Google Scholar]
- 32.Brunger A T. Nature (London) 1992;355:472–474. doi: 10.1038/355472a0. [DOI] [PubMed] [Google Scholar]
- 33.Jones T A, Zou J-Y, Cowan S W, Kjeldgaard M. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 34.Hendrickson W A, Konnert J H. In: Incorporation of Stereochemical Information into Crystallographic Refinement. Diamond R, Ramaseshan S, Venkatesan K, editors. Vol. 13. Bangalore, India: International Union of Crystallography; 1980. pp. 1–23. [Google Scholar]
- 35.Smith G D. Acta Crystallogr. 1997;D52:41–48. doi: 10.1107/S0907444996011535. [DOI] [PubMed] [Google Scholar]
- 36.Kleywegt G J, Jones T A. In: Halloween… Masks and Bones. Bailey S, Hubbard R, Waller D, editors. Daresbury, United Kingdom: Proceedings of the CCP4 Study Weekend; 1994. [Google Scholar]
- 37.Jones T A. In: a, yaap, asap, @#*?: A Set of Averaging Programs. Dodson E J, Gover S, Wolf W, editors. Daresbury, United Kingdom: Proceedings of the CCP4 Study Weekend; 1992. pp. 92–105. [Google Scholar]
- 38.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 39.Saribas A S, Schindler J F, Viola R E. J Biol Chem. 1994;269:6313–6319. [PubMed] [Google Scholar]
- 40.Kraulis P J. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]