Abstract
The nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) is an important regulator of lipid and glucose homeostasis and cellular differentiation. Studies of many cell types in vitro and in vivo have demonstrated that activation of PPARγ can reduce cellular proliferation. We show here that activation of PPARγ is sufficient to reduce the proliferation of cultured insulinoma cell lines. We created a model with mice in which the expression of the PPARG gene in β cells was eliminated (βγKO mice), and these mice were found to have significant islet hyperplasia on a chow diet. Interestingly, the normal expansion of β-cell mass that occurs in control mice in response to high-fat feeding is markedly blunted in these animals. Despite this alteration in β-cell mass, no effect on glucose homeostasis in βγKO mice was noted. Additionally, while thiazolidinediones enhanced insulin secretion from cultured wild-type islets, administration of rosiglitazone to insulin-resistant control and βγKO mice revealed that PPARγ in β cells is not required for the antidiabetic actions of these compounds. These data demonstrate a critical physiological role for PPARγ function in β-cell proliferation and also indicate that the mechanisms controlling β-cell hyperplasia in obesity are different from those that regulate baseline cell mass in the islet.
Peroxisome proliferator-activated receptor γ (PPARγ [NR1C3], encoded by PPARG) is a member of the nuclear hormone receptor superfamily of ligand-gated transcription factors. PPARγ has been shown to play a role in diverse biological processes, including adipogenesis and glucose and lipid homeostasis (33). Although the endogenous ligand of PPARγ is not known, there are several synthetic compounds that bind PPARγ with high affinity and activate the receptor. These include the thiazolidinedione (TZD) class of drugs, which are insulin sensitizers currently in use for the treatment of type 2 diabetes.
In addition to insulin sensitization, other actions of PPARγ have recently come to light, including the control of cellular proliferation. Activation of PPARγ has been shown to reduce growth in a variety of cell lines cultured from cancers of adipose tissue (40), colon (35), breast (29), prostate (30), liver (34), lung (41), and pancreatic acinar tissue (13). These findings have been extended to some human tumors, such as liposarcoma (11) and prostate cancer (30), and clinical trials with other forms of cancer are in progress. Interestingly, loss-of-function mutations in PPARG have been demonstrated for some human colon cancers (36) and loss of heterozygosity at 3p25, a broad region that includes PPARG, has been seen in many human prostate (30) and pancreatic endocrine (9, 10, 31, 38, 46) tumors.
The latter observation led us to wonder whether the activation of PPARγ in pancreatic β cells would also regulate cellular proliferation. PPARγ is expressed in the β cells of both rodents and humans (12, 47), and treatment of diabetic or prediabetic humans and rodents with PPARγ agonists leads to improvements in islet architecture, insulin content, and glucose-stimulated insulin secretion (GSIS) (6, 8, 17). Although these effects could be explained as the beneficial sequelae of reducing peripheral insulin resistance, troglitazone added directly to the culture medium of islets isolated from Zucker fatty rats also improves GSIS (37). Additionally, activation of PPARγ directly induces the expression of the glucose transporter Glut2 and glucokinase, critical participants in GSIS, in cultured primary rat islets and insulinoma cell lines (20, 21).
These studies suggested to us that PPARγ may exert a significant effect on β-cell function and that at least part of the therapeutic effect of TZD administration may be mediated through the pancreatic islet. We therefore sought to determine whether PPARγ could regulate the growth and function of β cells by using both cultured insulinoma cell lines and targeted gene elimination in mice.
MATERIALS AND METHODS
Animal protocols.
Study populations of mice were generated by crossing animals with exon 2 of PPARG flanked by loxP sites (PPARγfl/fl) to mice expressing Cre recombinase driven by the rat insulin promoter (PPARγcre). F1 PPARγfl/+ mice were then crossed with F1 PPARγfl/+ cre mice, thus generating the four study groups (PPARγfl/fl, PPARγ+/+, PPARγ+/+ cre, and PPARγfl/fl cre [heretofore and herein called βγKO]). Because mice were maintained on a mixed FVB-129-C57 background, littermates served as controls for all experiments. For studies involving a high-fat diet, mice were fed Harlan Teklad TD93075 special diet (55% fat by caloric content). Mice were genotyped by PCR using the following primers (see Fig. 2): F, CTCCAATGTTCTCAAACTTAC; R1, GATGAGTCATGTAAGTTGACC; and R2, GTATTCTATGGCTTCCAGTGC. All animal work was performed with the approval of the Animal Use Committees of Harvard and Brandeis Universities.
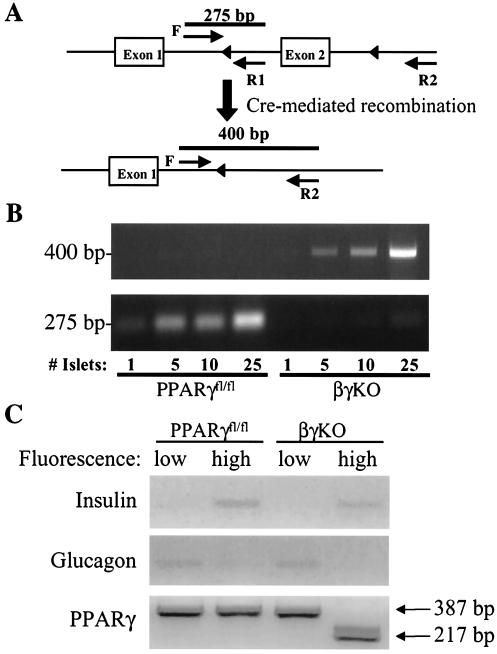
FIG. 2.
Deletion of PPARG exon 2 in βγKO mice. (A) Scheme showing exon 2 of PPARG targeted with loxP sites (triangles) before and after Cre-mediated recombination. Heavy lines indicate the expected PCR products from primers F, R1, and R2. The 400-bp band is a specific recombination product seen only when exon 2 is appropriately excised, while the 275-bp band represents the nonrecombined floxed allele. (B) Results of PCR from whole islets isolated from PPARγfl/fl and βγKO mice. (C) Dispersed islet cells were loaded with a calcium-sensitive fluorophore and treated with glucose, followed by flow sorting. RT-PCR for insulin and glucagon shows that cells with high fluorescence are primarily β cells but that those with low fluorescence are primarily non-β cells. RT-PCR of RNA from these cells yields a band of 387 bp for the wild-type transcript and 217 bp for the recombined transcript missing exon 2.
Blood glucose, plasma insulin, glucose tolerance tests, and in vivo insulin secretion.
Blood glucose values were determined from whole venous blood by using an automated glucose monitor (Glucometer Elite; Bayer). Insulin levels in serum were measured by enzyme-linked immunosorbent assay using mouse insulin as a standard (Crystal Chem, Chicago, Ill.). Glucose tolerance tests and acute insulin secretion tests were performed on animals that had been fasted overnight for 16 h. The peritoneal cavities of the animals were injected with 2 g of glucose/kg of body weight. Glucose levels were measured from blood collected from the animals' tails immediately before and 15, 30, 60, and 120 min after the injections (24).
Islet morphology and immunohistochemistry.
Tissues were fixed in Bouin's solution and 10% buffered formalin and embedded in paraffin. Sections of pancreas were stained for non-β-cell hormones with a cocktail of antibodies to glucagon, somatostatin, and pancreatic polypeptide (24, 27). Immunofluorescent staining for insulin was achieved with anti-insulin antibodies and detected by fluorescein antibodies (Jackson Immunoresearch). β-Cell growth was evaluated by bromodeoxyuridine (BrdU) labeling. Mice were injected with BrdU (Sigma) ∼6 h before sacrifice, followed by dissection of the pancreas for staining and sectioning as described above. BrdU-positive cells were identified with an anti-BrdU antibody (cell proliferation kit; Amersham, Piscataway, N.J.).
β-cell mass determination.
β-Cell mass was evaluated by point counting morphometry on immunoperoxidase-stained sections of pancreas (27, 28). Multiple sections (separated by 80 μm each) were obtained from each pancreas and analyzed systematically by using a grid system covering at least 175 fields per mouse. Separate images were acquired with a BX60 microscope (Olympus, New Hyde Park, N.Y.) as described previously (28). Relative volumes were calculated for β cells, non-β cells, and exocrine tissue. The extent of contaminating tissue (pancreatic ducts, lymph nodes, adipose, and intestine) was recorded to correct for the pancreatic weight. β-Cell mass was calculated by the following formula: islet β-cell mass = relative β-cell volume × corrected pancreatic weight.
Fluorescence sorting of islet cells.
We used the observation that glucose stimulation promotes greater intracellular calcium concentration in β cells than in non-β cells to sort islet cells (4, 18). Briefly, islets were dispersed after isolation to obtain single cells as described previously (19). Dispersed cells were suspended in Krebs-Ringer buffer (KRB) containing 0.1 mM glucose, 2 μM Fluo-4, and 0.02% Pluronic F-127 (Molecular Probes, Eugene, Oreg.) and incubated for 30 min at 37°C. Cells were then washed with 0.1 mM glucose KRB for 30 min, followed by stimulation with 20 mM glucose for 10 min. Samples were washed and kept at 37°C until analysis. Flow cytometry was used for sorting (excitation, 488 nm; emission, 502 to 542 nm), and islet cells were separated based on low (non-β cells) versus high (β cells) fluorescence. We extracted RNA from cell pellets and analyzed gene expression by reverse transcription-PCR (RT-PCR). We used nested PCR for amplifying PPARγ cDNA. Primer details are available on request.
Insulin release and content of islets.
Islets were isolated by using the intraductal collagenase method as described previously (27). The insulin released in vitro from isolated islets was measured by static incubation of islets cultured overnight (27). Insulin content in whole pancreas in acid-ethanol extracts was measured by enzyme-linked immunosorbent assay (Crystal Chem).
Cell culture and proliferation experiments.
INS-1 and HIT-T15 cells were grown in RPMI 1640 with 10% fetal bovine serum, 0.5 M HEPES, 102 mM l-glutamine, 50 mM sodium pyruvate, and 2.5 mM β-mercaptoethanol. RINmF and βTC3 cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum. Cells were plated on day 0 and counted with a hemocytometer on the days indicated in Fig 1. Troglitazone (10 μM) or vehicle (0.1% dimethyl sulfoxide) was added as appropriate. Retroviral supernatants expressing PPARγ1 or vector (pMSCVpuro) only were generated, and INS-1 cells were infected and selected in puromycin as described previously (32).
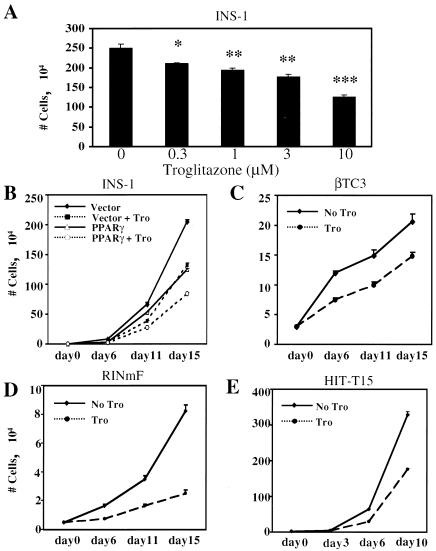
FIG. 1.
Effect of TZDs on cultured insulinoma cell growth. (A) INS-1 cells were treated with various doses of troglitazone for 15 days before being counted. Results are given as mean numbers of cells (104) ± standard errors of the means from at least three independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.001. (B) INS-1 cells were infected with a retrovirus expressing PPARγ1 or vector only and then treated with vehicle (solid lines) or 10 μM troglitazone (Tro) (dashed lines) before being counted as described for panel A. (C to E) βTC3, RINmF, and HIT-T15 cells were cultured in the presence of 10 μM troglitazone or vehicle for the indicated numbers of days before being counted as described for panel A.
RESULTS
PPARγ regulates cellular proliferation in cultured insulinoma cells.
We first assessed the effect of PPARγ activation on the proliferation of insulinoma cells in vitro. Rat INS-1 cells were treated with various doses of troglitazone or vehicle for 15 days, and cell numbers were counted; a dose-dependent reduction in cellular proliferation was noted (Fig. 1A). Inhibition of cellular proliferation could be seen with as little as 0.03 μM troglitazone, significantly below the Kd of the receptor for this ligand, making a nonspecific effect of troglitazone very unlikely. To modulate PPARγ activity in an independent way, we also expressed the receptor by retrovirally mediated gene transfer into INS-1 cells. This step had an effect identical to that of ligand administration, and the combination of PPARγ and ligand caused an additive repression of cell growth (Fig. 1B). This phenomenon is not restricted to INS-1 cells, as other β-cell-derived lines (βTC3, HIT-T15, and RINmF) responded similarly to the addition of 10 μM troglitazone (Fig. 1C to E).
PPARγ regulates cellular proliferation in islets in vivo.
The data above show that PPARγ exerts strong antiproliferative effects on β cells when it is expressed via viral vectors or activated by ligand. If this effect of PPARγ is biologically important, then the absence of the receptor in β cells may lead to islet hyperplasia. Unfortunately, this hypothesis could not be tested with a global PPARγ knockout model, as these mice die early in embryogenesis (5, 22). To test our hypothesis, we crossed mice bearing a version of PPARG with loxP sites flanking exon 2 (PPARγfl/fl) (Fig. 2A) with mice expressing Cre recombinase from a β-cell-specific promoter (rat insulin promoter [RIP]-Cre). Both of these lines have been described previously (2, 15). F1 PPARγfl/+ mice with and without Cre were mated to one another to generate PPARγfl/fl, PPARγ+/+, PPARγcre, and βγKO animals. βγKO mice were born in the expected Mendelian ratio and were not found to have a survival advantage or disadvantage during more than 2 years of study. Figure 2B shows the results of whole-islet PCR demonstrating that the recombined allele (represented by the 400-bp band) appears only in βγKO mice, as expected. The 275-bp band represents the nonrecombined floxed allele, present as expected in the PPARγfl/fl mice but virtually absent from βγKO mice. As the number of islets increases, a small amount of nonrecombined product in the βγKO mice becomes apparent, most likely reflecting the presence of non-β cells, which do not express the RIP-Cre transgene. In order to ascertain whether PPARγ mRNA reflected the recombination event in β cells, we took advantage of the observation that intracellular calcium levels are higher in β cells than in non-β cells after a glucose challenge (4, 18). This difference allows one to separate β cells from non-β cells using flow sorting on the basis of fluorescence in the presence of a calcium-activated fluorophore and glucose. Figure 2C shows that dispersed islet cells characterized by high and low fluorescence were characterized by the presence of insulin and glucagon mRNA, respectively. Using RT-PCR primers for PPARγ complementary to exons 1 and 3, we show that there is virtually no recombination of PPARG in non-β cells of βγKO mice. Conversely, β cells from βγKO mice yield no detectable nonrecombined PPARγ transcripts.
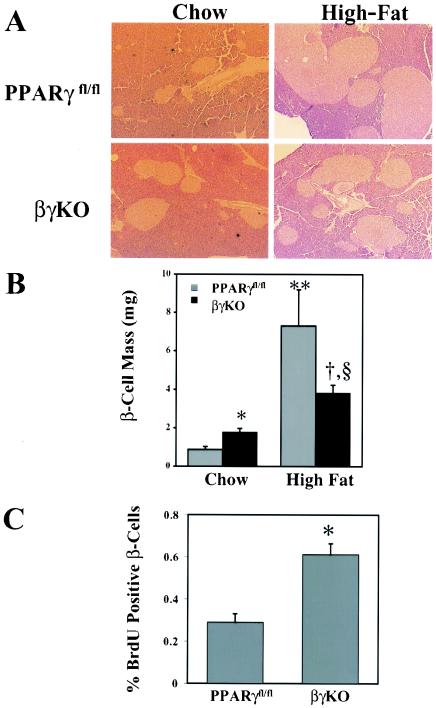
Islets from βγKO mice were approximately twice as large as those from PPARγfl/fl mice on a chow diet (Fig. 3A and B). Morphometric analysis and immunostaining for insulin revealed that the differences in islet sizes were largely accounted for by β-cell hyperplasia. Consistent with these results, BrdU incorporation was proportionately greater in βγKO islets than in PPARγfl/fl islets; this finding is also consistent with the notion that an increase in cell number rather than cell size contributes to the discrepancy in islet size (Fig. 3C). Insulin content was significantly greater in βγKO islets (3.45 ± 0.28 ng of insulin/ng of DNA) than in PPARγfl/fl islets (1.75 ± 0.13 ng of insulin/ng of DNA) (P = 0.001).
FIG. 3.
Islet morphology and histomorphometry in control and βγKO mice. (A) Hematoxylin and eosin staining of pancreata from PPARγfl/fl and βγKO mice maintained on either a chow or high-fat diet. (B) Quantification of β-cell mass from PPARγfl/fl and βγKO mice maintained on either a chow or high-fat diet. Four mice were tested for each determination. *, P < 0.05 for PPARγfl/fl versus βγKO mice on the chow diet; **, P < 0.001 for PPARγfl/fl mice on the chow versus the high-fat diet; §, P < 0.001 for βγKO mice on the chow versus the high-fat diet; †, P < 0.001 for PPARγfl/fl versus βγKO mice on the high-fat diet. (C) BrdU labeling of islets from chow-fed control and βγKO mice (*, P < 0.01; n = 4).
Surprisingly, when the mice were placed on a high-fat diet, quite a different effect was seen. Islets from PPARγfl/fl mice underwent the expected hyperplastic response that typifies the obese, insulin-resistant state, increasing in mass 8.3-fold. Islets from obese βγKO mice, on the other hand, showed only a 2.1-fold increase in β-cell mass from baseline. Thus, PPARγ is required for the normal expansion of islet mass seen in obesity, demonstrating an unexpected and exciting new role for this receptor in β-cell biology.
Metabolic function in βγKO mice.
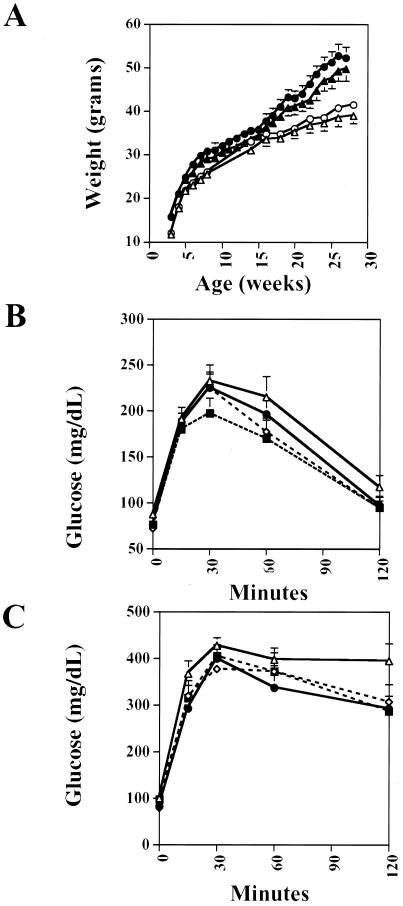
The islet hyperplasia in chow-fed βγKO mice led us to examine whether there were corresponding metabolic alterations in these animals. Male βγKO mice, both fasting and fed, had normal glucose and insulin levels in their sera compared to levels in the three control genotypes, PPARγfl/fl, PPARγ+/+, and PPARγcre (Table 1), as well as normal body weight (Fig. 4A). Switching mice to a high-fat diet for up to 55 weeks resulted in impaired glucose tolerance in all genotypes, with no differences in glucose or insulin levels noted between βγKO mice and controls. We then performed glucose and insulin tolerance testing to attempt to unmask a diabetic diathesis. In both chow-fed animals and mice fed a high-fat diet for 55 weeks, no significant differences were seen in glucose excursions after intraperitoneal glucose (Fig. 4B and C) or insulin administration or in glucose-stimulated acute-phase insulin secretion (data not shown). For all of these parameters, trends among female mice did not differ from those seen among males (data not shown).
TABLE 1.
Glucose and insulin levels in the sera of βγKO and control micea
| Phenotype | Glucose (mg/dl)
|
Insulin (ng/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Fastingb
|
Fed
|
Fasting
|
Fed
|
|||||
| Chow diet (n) | High-fat diet (n) | Chow diet | High-fat diet | Chow diet | High-fat diet | Chow diet | High-fat diet | |
| PPARγ+/+ | 76 ± 5.1 (13) | 120 ± 11.8 (6) | 138 ± 7.2 | 142 ± 2.2 | 448 ± 180 | 6,191 ± 1,356 | 2,518 ± 896 | ND |
| PPARγfl/fl | 87 ± 6.9 (6) | 111 ± 7.5 (14) | 147 ± 7.6 | 138 ± 9.9 | 256 ± 50 | 6,202 ± 878 | 1,682 ± 243 | ND |
| PPARγcre | 72 ± 5.3 (12) | 107 ± 9.9 (10) | 152 ± 8.1 | 126 ± 10.2 | 456 ± 83 | 6,456 ± 576 | 2,864 ± 777 | ND |
| βγKO | 76 ± 6.0 (8) | 101 ± 10.9 (10) | 137 ± 6.3 | 131 ± 9.3 | 455 ± 85 | 5,583 ± 421 | 2,555 ± 689 | ND |
Values given are means ± standard errors of the means. ND, not done.
n, number of mice.
FIG. 4.
Metabolic characteristics of male PPARγfl/fl and βγKO mice. (A) Body weights of PPARγfl/fl (triangles) and βγKO (circles) mice on the chow (open symbols) and high-fat (filled symbols) diets. (B) Glucose tolerance tests of male mice on chow (16 weeks of age). Phenotypes (numbers of mice) are as follows: PPARγ+/+ (n = 14), PPARγcre (n = 13), PPARγfl/fl (n = 11), and βγKO (n = 16). (C) Glucose tolerance tests of male mice on the high-fat diet (44 weeks of age). Phenotypes (numbers of mice) are as follows: PPARγ+/+ (n = 6), PPARγcre (n = 10), PPARγfl/fl (n = 15), and βγKO (n = 10).
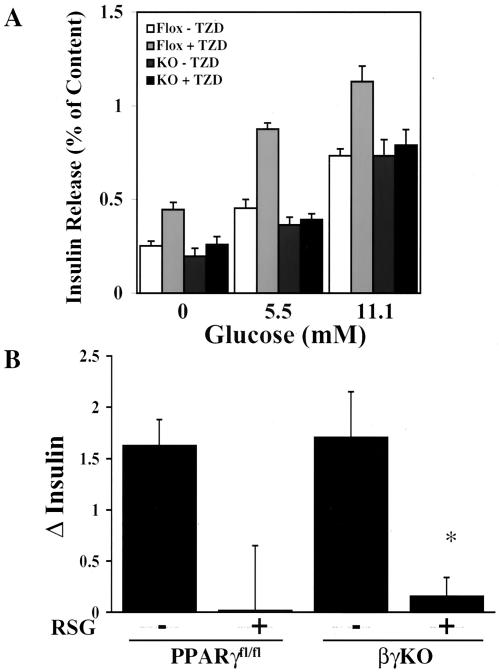
We next assessed the effect of a PPARγ agonist on insulin secretion from isolated islets. Troglitazone (10 μM) significantly improved insulin secretion from PPARγfl/fl islets in the presence and absence of glucose, consistent with previous reports (45). In contrast, troglitazone had no effect on βγKO islets (Fig. 5A). This result demonstrates that PPARγ is the sole target of troglitazone in β cells (at least with respect to effects on insulin secretion), and the knockout is likely to be virtually complete in those cells that can synthesize and secrete insulin. More importantly, these results suggested that TZDs might exert their antidiabetic actions, in part, through PPARγ in β cells. In order to definitively address that possibility, we took male PPARγfl/fl and βγKO mice and placed them on a high-fat diet for 10 weeks. The mice were then treated with either rosiglitazone (3 mg/kg of body weight/day) or vehicle for 3 weeks. For fasting mice, insulin levels in sera obtained after the drug treatment period were higher than those found immediately preceding drug administration in vehicle-treated mice, demonstrating that insulin resistance was still developing (Fig. 5B). Rosiglitazone, however, eliminated this rise in serum insulin levels equally in PPARγfl/fl and βγKO mice, thus demonstrating that TZD action within β cells does not significantly contribute to the antidiabetic effects of these drugs.
FIG. 5.
Effect of TZD drugs on insulin secretion and glucose homeostasis in vivo and in vitro. (A) Isolated islets from PPARγfl/fl (Flox) and βγKO (KO) mice were cultured overnight in 10 μM troglitazone (a TZD drug) or vehicle prior to exposure to glucose at the indicated concentrations. Insulin release is expressed as a percentage of the total content. *, P < 0.05; number of mice for all conditions, 4. (B) Male PPARγfl/fl and βγKO mice were fed a high-fat diet for 10 weeks, followed by 3 weeks of rosiglitazone (3 mg/kg/day) or vehicle. Serum insulin levels of fasting mice were measured before and after drug administration, and the net changes (Δ) over the treatment period are depicted. Results are means ± standard errors of the means. For each group, the number of mice was 8. *, P < 0.05; **, P < 0.01; RSG, rosiglitazone.
DISCUSSION
Despite intense investigation, we have a very incomplete understanding of the specific roles played by PPARγ in many of the tissues where it is expressed. The identification of the pancreatic β cell as a site of PPARγ expression, combined with data demonstrating the effects of TZD administration on islet architecture and function, suggested to us that PPARγ could be important in the biology of the β-cell. We have critically analyzed this issue by using Cre-lox technology to target PPARγ in a β-cell-specific manner. Counter to expectations, we could detect no significant metabolic abnormality in βγKO mice relative to the metabolism of PPARγfl/fl, PPARγ+/+, or PPARγcre controls. Our analysis included measurements of insulin and glucose in fasting and fed mice on both the lean (chow) and insulin-resistant (high-fat) diets. Glucose and insulin tolerance testing and levels of acute-phase insulin secretion similarly revealed no differences between βγKO and control mice. Although it is not possible to confirm that we have deleted PPARγ expression in every β cell, several lines of evidence indicate that we have eliminated its expression in the vast majority of cells. First, RT-PCR of β cells from the dispersed islets of βγKO mice reveals the absence of wild-type PPARγ mRNA. Second, PCR of whole islets (which contain numerous non-β cells, such as α and δ cells, endothelial cells, etc.) reveals a significant reduction in the expression of the floxed allele, while the 400-bp PCR product indicating the presence of the deleted allele is present only in βγKO islets. Third, the effect of troglitazone on insulin secretion is totally eliminated in the βγKO islets. Fourth, in other studies, the expression of the RIP-Cre transgene has been demonstrated to be virtually completely penetrant (24, 25).
The absence of metabolic consequences in βγKO mice does not exclude the β cell as an antidiabetic target of TZD drugs. To assess this possibility, we measured insulin secretion after TZD administration from isolated whole islets of control or βγKO mice. In control islets, troglitazone caused a roughly twofold induction of insulin secretion, even in the absence of glucose. This effect was totally eliminated in βγKO islets. When these studies were extended to the intact mouse, however, we found that the improved glucose homeostasis associated with TZD administration was preserved in βγKO animals. This finding demonstrates that PPARγ in β cells is unlikely to play a major role in the therapeutic response to TZDs. Even if there is a slight improvement in insulin secretion after TZD administration in vivo, this effect is likely masked by the peripheral insulin sensitization that dominates the clinical response to TZDs.
The most striking difference between control mice and βγKO mice was islet mass. Loss of PPARγ is associated with a hyperplastic response in the targeted β cells. PPARγ is known to regulate growth in a variety of cell types, including preadipocytes, myeloid leukemia cells, and epithelial tumor lines derived from breast, colon, and prostate. The fact that βγKO islets do not proliferate indefinitely suggests that other growth-regulating pathways eventually come into play, which may explain why tumor formation in the islet tissue of βγKO mice was not observed.
β-Cell hyperplasia is most commonly seen as a response to obesity and insulin resistance; it may be caused by a circulating growth factor or factors (14). Despite several theories, no specific factor has been definitively identified. Recent evidence has suggested that insulin itself might be important as a mediator of β-cell hyperplasia (1, 26). All the known components of the insulin signaling pathway are present in β cells (16) (reviewed in reference 23). Loss of insulin receptor substrate 1 (IRS-1) results in hyperplastic, but dysfunctional, islets (3, 27, 39), while loss of IRS-2 results in diabetes without compensatory β-cell hyperplasia (44). The overexpression of a constitutively active form of Akt increases β-cell mass in transgenic mice (7, 42). Furthermore, loss of β-cell insulin receptors (βIRKO) is associated with reduced β-cell hyperplasia during normal aging (24). Crossing βIRKO mice to animals with hepatic insulin resistance (due to the inactivation of insulin receptors in their livers) results in progeny that are insulin resistant but which do not appropriately expand their β-cell mass (R. N. Kulkarni, unpublished data). Given the obvious defect in β-cell hyperplasia after high-fat feeding in βγKO mice, it is tempting to speculate that the lack of PPARγ may cause local insulin resistance within β cells.
Other mechanisms may also contribute to our findings, of course. For example, excess lipid accumulation in the islet has been associated with the lipoapoptosis of β cells (43). TZD treatment of islets from Zucker fatty rats causes reductions in cellular triglyceride levels as well as reduced lipoapoptosis (17, 37). It is possible, therefore, that for βγKO animals on the high-fat diet, the smaller islet mass than that of controls results from enhanced apoptosis in the setting of reduced PPARγ activity.
One of the more intriguing aspects of our study is the discordance between islet mass and function, especially for mice in the obese, insulin-resistant state. Specifically, the fact that glucose and insulin levels are identical in βγKO and PPARγfl/fl mice on a high-fat diet implies that there must be a compensatory gain in β-cell function in the βγKO mice. This observation is supported by the elevated insulin content on a per cell basis that was seen in βγKO islets (at least in the chow-fed mice). PPARγ can thus be identified as a factor that couples peripheral insulin resistance to changes in β-cell proliferation, perhaps by promoting lipid deposition in the islet. In this scenario, removal of PPARγ makes the β cell work more efficiently. However, obese βγKO mice were still glucose intolerant, indicating that the absence of PPARγ cannot compensate fully for the β-cell dysfunction seen in states of peripheral insulin resistance.
Acknowledgments
We thank K. C. Hayes and the staff of the animal facility of the Foster Biomedical Laboratories at Brandeis University.
This work was supported by NIH grants KO8 DK02535 (to E.D.R.), RO3 DK58850 (to E.D.R.), R37DK31405 (to B.M.S.), RO1 DK33201 (to C.R.K.), and KO8 DK02885 (to R.N.K.).
REFERENCES
- 1.Accili, D. 2001. A kinase in the life of the beta cell. J. Clin. Investig. 108:1575-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama, T. E., S. Sakai, G. Lambert, C. J. Nicol, K. Matsusue, S. Pimprale, Y.-H. Lee, M. Ricote, C. K. Glass, H. B. Brewer, Jr., and F. J. Gonzalez. 2002. Conditional disruption of the peroxisome proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 22:2607-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki, E., M. A. Lipes, M. E. Patti, J. C. Bruning, B. Haag III, R. S. Johnson, and C. R. Kahn. 1994. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186-190. [DOI] [PubMed] [Google Scholar]
- 4.Aspinwall, C. A., W.-J. Qian, M. G. Roper, R. N. Kulkarni, C. R. Kahn, and R. T. Kennedy. 2000. Roles of insulin receptor substrate-1, phosphatidylinositol 3-kinase, and release of intracellular Ca2+ stores in insulin-stimulated insulin secretion in β-cells. J. Biol. Chem. 275:22331-22338. [DOI] [PubMed] [Google Scholar]
- 5.Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz, K., R. Peters, S. L. Kjos, J. Goico, A. Marroquin, M. E. Dunn, A. Xiang, S. Azen, and T. A. Buchanan. 1996. Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM. Diabetes 45:1572-1579. [DOI] [PubMed] [Google Scholar]
- 7.Bernal-Mizrachi, E., W. Wen, S. Stahlhut, C. M. Welling, and M. A. Permutt. 2001. Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Investig. 108:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, K. K., B. R. Henke, S. G. Blanchard, J. E. Cobb, R. Mook, I. Kaldor, S. A. Kliewer, J. M. Lehmann, J. M. Lenhard, W. W. Harrington, P. J. Novak, W. Faison, J. G. Binz, M. A. Hashim, W. O. Oliver, H. R. Brown, D. J. Parks, K. D. Plunket, W. Q. Tong, J. A. Menius, K. Adkison, S. A. Noble, and T. M. Willson. 1999. A novel N-aryl tyrosine activator of peroxisome proliferator-activated receptor-gamma reverses the diabetic phenotype of the Zucker diabetic fatty rat. Diabetes 48:1415-1424. [DOI] [PubMed] [Google Scholar]
- 9.Chung, D. C., S. B. Brown, F. Graeme-Cook, L. G. Tillotson, A. L. Warshaw, R. T. Jensen, and A. Arnold. 1998. Localization of putative tumor suppressor loci by genome-wide allelotyping in human pancreatic endocrine tumors. Cancer Res. 58:3706-3711. [PubMed] [Google Scholar]
- 10.Chung, D. C., A. P. Smith, D. N. Louis, F. Graeme-Cook, A. L. Warshaw, and A. Arnold. 1997. A novel pancreatic endocrine tumor suppressor gene locus on chromosome 3p with clinical prognostic implications. J. Clin. Investig. 100:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetri, G. D., C. D. Fletcher, E. Mueller, P. Sarraf, R. Naujoks, N. Campbell, B. M. Spiegelman, and S. Singer. 1999. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc. Natl. Acad. Sci. USA 96:3951-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois, M., F. Pattou, J. Kerr-Conte, V. Gmyr, B. Vandewalle, P. Desreumaux, J. Auwerx, K. Schoonjans, and J. Lefebvre. 2000. Expression of peroxisome proliferator-activated receptor γ (PPARγ) in normal human pancreatic islet cells. Diabetologia 43:1165-1169. [DOI] [PubMed] [Google Scholar]
- 13.Elnemr, A., T. Ohta, K. Iwata, I. Ninomia, S. Fushida, G. Nishimura, H. Kitagawa, M. Kayahara, M. Yamamoto, T. Terada, and K. Miwa. 2000. PPARgamma ligand (thiazolidinedione) induces growth arrest and differentiation markers of human pancreatic cancer cells. Int. J. Oncol. 17:1157-1164. [DOI] [PubMed] [Google Scholar]
- 14.Flier, S. N., R. N. Kulkarni, and C. R. Kahn. 2001. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc. Natl. Acad. Sci. USA 98:7475-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gannon, M., C. Shiota, C. Postic, C. V. Wright, and M. Magnuson. 2000. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26:139-142. [DOI] [PubMed] [Google Scholar]
- 16.Harbeck, M. C., D. C. Louie, J. Howland, B. A. Wolf, and P. L. Rothenberg. 1996. Expression of insulin receptor mRNA and insulin receptor substrate 1 in pancreatic islet beta-cells. Diabetes 45:711-717. [DOI] [PubMed] [Google Scholar]
- 17.Higa, M., Y.-T. Zhou, M. Ravazzola, D. Baetens, L. Orci, and R. H. Unger. 1999. Troglitazone prevents mitochondrial alterations, β cell destruction, and diabetes in obese prediabetic rats. Proc. Natl. Acad. Sci. USA 96:11513-11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara, H., P. Maechler, A. Gjinovci, P. L. Herrera, and C. B. Wollheim. 2003. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat. Cell Biol. 5:330-335. [DOI] [PubMed] [Google Scholar]
- 19.Josefsen, K., J. P. Stenvang, H. Kindmark, P. O. Berggren, T. Horn, T. Kjaer, and K. Buschard. 1996. Fluorescence-activated cell sorted rat islet cells and studies of the insulin secretory process. J. Endocrinol. 149:145-154. [DOI] [PubMed] [Google Scholar]
- 20.Kim, H. I., J. Y. Cha, S. Y. Kim, J. W. Kim, K. J. Roh, J. K. Seong, N. T. Lee, K. Y. Choi, K. S. Kim, and Y. H. Ahn. 2002. Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells. Diabetes 51:676-685. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H. I., J. W. Kim, S. H. Kim, J. Y. Cha, K. S. Kim, and Y. H. Ahn. 2000. Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Diabetes 49:1517-1524. [DOI] [PubMed] [Google Scholar]
- 22.Kubota, N., Y. Terauchi, H. Miki, H. Tamemoto, T. Yamauchi, K. Komeda, S. Satoh, R. Nakano, C. Ishii, T. Sugiyama, K. Eto, Y. Tsubamoto, A. Okuno, K. Murakami, H. Sekihara, G. Hasegawa, M. Naito, Y. Toyoshima, S. Tanaka, K. Shiota, T. Kitamura, T. Fujita, O. Ezaki, S. Aizawa, T. Kadowaki, et al. 1999. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4:597-609. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni, R. N. 2002. Receptors for insulin and insulin-like growth factor-1 and insulin receptor substrate-1 mediate pathways that regulate islet function. Biochem. Soc. Trans. 30:317-322. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni, R. N., J. C. Bruning, J. N. Winnay, C. Postic, M. A. Magnuson, and C. R. Kahn. 1999. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329-339. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni, R. N., M. Holzenberger, D. Q. Shih, U. Ozcan, M. Stoffel, M. A. Magnuson, and C. R. Kahn. 2002. β-Cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat. Genet. 31:111-115. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni, R. N., and C. R. Kahn. 2000. Genetic models of insulin resistance: alterations in beta-cell biology, p. 299-323. In J. F. Habener and M. Hussain (ed.), Molecular basis of pancreas development and function. Kluwer Academic Publishers, New York, N.Y.
- 27.Kulkarni, R. N., J. N. Winnay, M. Daniels, J. C. Brüning, S. N. Flier, D. Hanahan, and C. R. Kahn. 1999. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured β-cell lines. J. Clin. Investig. 104:R69-R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael, M. D., R. N. Kulkarni, C. Postic, S. F. Previs, G. I. Shulman, M. A. Magnuson, and C. R. Kahn. 2000. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6:87-97. [PubMed] [Google Scholar]
- 29.Mueller, E., P. Sarraf, P. Tontonoz, R. M. Evans, K. J. Martin, M. Zhang, C. Fletcher, S. Singer, and B. M. Spiegelman. 1998. Terminal differentiation of human breast cancer through PPAR gamma. Mol. Cell 1:465-470. [DOI] [PubMed] [Google Scholar]
- 30.Mueller, E., M. Smith, P. Sarraf, T. Kroll, A. Aiyer, D. S. Kaufman, W. Oh, G. Demetri, W. D. Figg, X. P. Zhou, C. Eng, B. M. Spiegelman, and P. W. Kantoff. 2000. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc. Natl. Acad. Sci. USA 97:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikiforova, M. N., Y. E. Nikiforov, P. Biddinger, D. R. Gnepp, L. A. Grosembacher, B. L. Wajchenberg, J. A. Fagin, and R. M. Cohen. 1999. Frequent loss of heterozygosity at chromosome 3p14.2-3p21 in human pancreatic islet cell tumours. Clin. Endocrinol. 51:27-33. [DOI] [PubMed] [Google Scholar]
- 32.Rosen, E. D., C.-H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 16:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen, E. D., and B. M. Spiegelman. 2001. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 276:37731-37734. [DOI] [PubMed] [Google Scholar]
- 34.Rumi, M. A., H. Sato, S. Ishihara, K. Kawashima, S. Hamamoto, H. Kazumori, T. Okuyama, R. Fukuda, N. Nagasue, and Y. Kinoshita. 2001. Peroxisome proliferator-activated receptor gamma ligand-induced growth inhibition of human hepatocellular carcinoma. Br. J. Cancer 84:1640-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarraf, P., E. Mueller, D. Jones, F. J. King, D. J. DeAngelo, J. B. Partridge, S. A. Holden, L. B. Chen, S. Singer, C. Fletcher, and B. M. Spiegelman. 1998. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat. Med. 4:1046-1052. [DOI] [PubMed] [Google Scholar]
- 36.Sarraf, P., E. Mueller, W. M. Smith, H. M. Wright, J. B. Kum, L. A. Aaltonen, A. de la Chapelle, B. M. Spiegelman, and C. Eng. 1999. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol. Cell 3:799-804. [DOI] [PubMed] [Google Scholar]
- 37.Shimabukuro, M., Y. T. Zhou, Y. Lee, and R. H. Unger. 1998. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J. Biol. Chem. 273:3547-3550. [DOI] [PubMed] [Google Scholar]
- 38.Speel, E. J., J. Richter, H. Moch, C. Egenter, P. Saremaslani, K. Rutimann, J. Zhao, A. Barghorn, J. Roth, P. U. Heitz, and P. Komminoth. 1999. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am. J. Pathol. 155:1787-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamemoto, H., T. Kadowaki, K. Tobe, T. Yagi, H. Sakura, T. Hayakawa, Y. Terauchi, K. Ueki, Y. Kaburagi, S. Satoh, et al. 1994. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372:182-186. [DOI] [PubMed] [Google Scholar]
- 40.Tontonoz, P., S. Singer, B. M. Forman, P. Sarraf, J. A. Fletcher, C. D. Fletcher, R. P. Brun, E. Mueller, S. Altiok, H. Oppenheim, R. M. Evans, and B. M. Spiegelman. 1997. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proc. Natl. Acad. Sci. USA 94:237-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsubouchi, Y., H. Sano, Y. Kawahito, S. Mukai, R. Yamada, M. Kohno, K. Inoue, T. Hla, and M. Kondo. 2000. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem. Biophys. Res. Commun. 270:400-405. [DOI] [PubMed] [Google Scholar]
- 42.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat. Med. 7:1133-1137. [DOI] [PubMed] [Google Scholar]
- 43.Unger, R. H., and L. Orci. 2001. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 15:312-321. [DOI] [PubMed] [Google Scholar]
- 44.Withers, D. J., J. S. Gutierrez, H. Towery, D. J. Burks, J. M. Ren, S. Previs, Y. Zhang, D. Bernal, S. Pons, G. I. Shulman, S. Bonner-Weir, and M. F. White. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900-904. [DOI] [PubMed] [Google Scholar]
- 45.Yang, C., T. J. Chang, J. C. Chang, M. W. Liu, T. Y. Tai, W. H. Hsu, and L. M. Chuang. 2001. Rosiglitazone (BRL 49653) enhances insulin secretory response via phosphatidylinositol 3-kinase pathway. Diabetes 50:2598-2602. [DOI] [PubMed] [Google Scholar]
- 46.Zhao, J., H. Moch, A. F. Scheidweiler, A. Baer, A. A. Schaffer, E. J. Speel, J. Roth, P. U. Heitz, and P. Komminoth. 2001. Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes Cancer 32:364-372. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, Y. T., M. Shimabukuro, M. Y. Wang, Y. Lee, M. Higa, J. L. Milburn, C. B. Newgard, and R. H. Unger. 1998. Role of peroxisome proliferator-activated receptor alpha in disease of pancreatic beta cells. Proc. Natl. Acad. Sci. USA 95:8898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]