Abstract
Our studies address questions pertaining to the regulation of D cyclin-cdk4 activity, and the following results were obtained. Conditions that increased the abundance of the D cyclins also increased the abundance of enzymatically active D cyclin-cdk4 complexes in mouse embryo fibroblasts (MEFs) lacking both p27Kip1 and p21Cip1 (p27/p21−/−). Such conditions included ectopic expression of cyclin D1 and inhibition of D cyclin degradation by the proteasome inhibitor MG132. However, as determined by treatment of wild-type MEFs with MG132, maximal accumulation of D cyclin-cdk4 complexes required p27Kip1 and p21Cip1 and coincided with the formation of inactive D cyclin-cdk4-p27Kip1 or -p21Cip1 complexes. p27Kip1 or p21Cip1 also increased the abundance of D cyclin-cdk4 complexes and reduced amounts of cdk4 activity when ectopically expressed in p27/p21−/− MEFs. Lastly, increases in the stability of the D cyclins accounted for their greater abundance in wild-type MEFs than in p27/p21−/− MEFs. We conclude that (i) D cyclin-cdk4 complexes are formed and become active in the absence of p27Kip1 and p21Cip1 and (ii) p27Kip1 and p21Cip1 maximize the accumulation but inhibit the activity of D cyclin-cdk4 complexes. We suggest that D cyclin-cdk4 complexes are more stable when bound to p27Kip1 or p21Cip1 and that formation of ternary complexes also stabilizes the D cyclins.
The cyclin-dependent kinases (CDKs) mediate the passage of cells through the cell cycle (13). Two families of proteins regulate CDK activity: the cyclins, which are required for activity, and the CDK inhibitors (CKIs). Two CKIs, p27Kip1 and p21Cip1, associate with complexes containing cyclins D, E, and A and their CDK partners. The D cyclins (D1, D2, and D3) increase in abundance and combine with cdk4 or cdk6 in mid- to late G1, and the resultant complexes phosphorylate the retinoblastoma protein (Rb) and sequester p27Kip1 and p21Cip1. Sequestration of p27Kip1 and p21Cip1 prevents their interaction with, and the consequent inactivation of, cyclin E-cdk2 and cyclin A-cdk2. When active, these complexes further phosphorylate Rb and elicit additional events required for the initiation and execution of S phase.
Although p27Kip1 and p21Cip1 clearly inhibit cdk2 activity, their effects on cdk4 activity are unresolved. Some studies suggest that p27Kip1 and p21Cip1 function as obligate assembly factors for D cyclin-cdk4 complexes (4) and that D cyclin-cdk4 complexes containing a single p27Kip1 or p21Cip1 are active (2, 9, 19). Other studies argue that D cyclin-cdk4 complexes containing p27Kip1 or p21Cip1 are always inactive. For example, we found that antibody to p27Kip1 or p21Cip1 coprecipitated D cyclin-cdk4 complexes from extracts of growing fibroblasts but did not remove cdk4 activity (1). Moreover, as reported by us and others, p27Kip1 inhibited cdk4 activity in vitro over a wide range of concentrations (1, 9). We suggest that D cyclin-cdk4 complexes fulfill their enzymatic and sequestration obligations by devoting different portions of the D cyclin-cdk4 pool to each function (1).
Inhibitory effects of p27Kip1 and p21Cip1 on cdk4 activity indicate that these CKIs cannot be required for D cyclin-cdk4 complex formation. Consistent with this premise, we found that D cyclin-cdk4 complexes were present in mouse embryo fibroblasts (MEFs) lacking both p27Kip1 and p21Cip1 (p27/p21−/−), although in smaller amounts than in wild-type MEFs (1). We suggest that the low abundance of D cyclin-cdk4 complexes in p27/p21−/− MEFs reflects the inherent instability of binary D cyclin-cdk4 complexes (9) rather than the need for p27Kip1 or p21Cip1 for complex assembly.
As described in a previous report (1), most of the D cyclin-cdk4 complexes in asynchronously cycling wild-type MEFs contain p27Kip1 or p21Cip1, whereas the remaining complexes, although almost undetectable, account for all of the D cyclin-associated activity. Thus, in both wild-type and p27/p21−/− MEFs, mechanisms limit the extent and duration of D cyclin-cdk4 activation. In the presence of p27Kip1 and p21Cip1, inactive ternary complexes predominate, whereas in the absence of these CKIs, active binary complexes are short-lived. Data presented here further support the two main premises of our model: p27Kip1 and p21Cip1 stabilize but are not required for the assembly of D cyclin-cdk4 complexes, and p27Kip1 and p21Cip1 inhibit the activity of D cyclin-cdk4 complexes.
MATERIALS AND METHODS
Cell culture.
Breeding pairs for wild-type and p27/p21−/− mice were obtained from J. Roberts. After removal of the head and internal organs, 15- to 16-day-old embryos were minced and plated individually in 100-mm-diameter culture dishes containing Dulbecco's modified Eagle's medium with 4 mM l-glutamine, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 10% fetal calf serum.
Immunoprecipitation and Western blotting.
Cells were harvested by scraping and were incubated in lysis buffer (50 mM HEPES [pH 7.5], 100 mM NaCl, 2 mM EDTA, 0.5% NP-40, 10% glycerol, 0.1 mM sodium orthovanadate, 0.5 mM NaF, 0.1 mM phenylmethylsulfonyl fluoride, 2.5 μg of leupeptin/ml, and 1 mM dithiothreitol) for 30 min on ice. In some experiments, 50 mM Tris (pH 7.5) and 0.1% Tween were used instead of HEPES and NP-40; similar results were obtained with both buffers. For immunoprecipitations, cell extracts were incubated with antibody for 1 to 2 h at 4°C. Immune complexes were recovered with protein A-agarose beads (1 to 2 h, 4°C) and were washed twice with lysis buffer. For Western blots, cell extracts or immune complexes were resolved on sodium dodecyl sulfate (SDS) gels and were electrophoretically transferred to nitrocellulose membranes. Membranes were blocked in phosphate-buffered saline containing 0.1% Tween 20 and 5% instant milk and were incubated with antibody in phosphate-buffered saline containing 0.1% Tween 20 for 2 h at room temperature. Proteins recognized by the antibody were detected by enhanced chemiluminescence using a horseradish peroxidase-coupled secondary antibody (Pierce).
In vitro kinase assays.
Immune complexes were washed twice with lysis buffer and once with a buffer containing 100 mM HEPES (pH 7.5), 20 mM MgCl2, 10 mM MnCl2, and 20 mM dithiothreitol. Washed complexes were resuspended in kinase reaction buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 5 mM MnCl2, and 10 mM dithiothreitol) containing 10 μCi of [γ-32P]ATP, 10 μM ATP, and 1 μg of glutathione S-transferase (GST)-Rb. Reaction mixtures were incubated for 30 min at 30°C and were applied to SDS gels. Phosphoproteins were visualized by autoradiography.
Northern blotting.
Cells were solubilized in Trizol reagent (Gibco BRL/Life Technologies), and RNA (15 μg/lane) was resolved on 1% agarose-formaldehyde gels, transferred to Biobond nylon membranes (Sigma), and cross-linked to membranes in a Stratalinker. The coding region of the mouse cyclin D1 gene was 32P labeled by using a DECAprime II kit (Ambion, Inc.) and was incubated with the membrane overnight in PerfectHyb Plus (Sigma) at 65°C. The blots were washed twice at room temperature with 2× SSC-0.1% SDS (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and radiolabeled bands were visualized by autoradiography.
Constructs.
To produce flag-cyclin D1, the coding sequence of the cyclin D1 gene was subcloned in frame into p3×Flag-CMV-10 (Sigma). Transient transfections were done on exponentially growing cells by using Lipofectamine. Ad-p27Kip1 and Ad-p21Cip1 were obtained from J. Nevins. Viral stocks were prepared and purified as described previously (12, 16).
Antibodies.
Antibodies were obtained from the following sources: polyclonal cyclin D3, Santa Cruz; monoclonal cyclin D3 and monoclonal p27Kip1, Transduction Labs; monoclonal cdk4, Pharmingen; and monoclonal p21Cip1, Neomarker. Polyclonal antibodies to cyclin D1, cdk4, and p27Kip1 were prepared in our laboratory. Monoclonal antibodies were used for Western blots of immunoprecipitates.
RESULTS
Effects of D cyclin overexpression and proteasome inhibitors on the abundance and activity of D cyclin-cdk4 complexes.
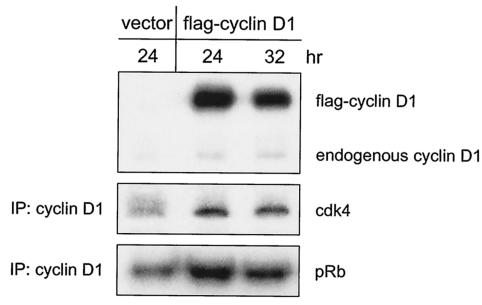
We have found that MEFs express cyclin D1 and cyclin D3 but little or no cyclin D2 and that cdk4 is the predominant D cyclin partner in these cells (1; unpublished observations). We and others have also shown that D cyclin-cdk4 complexes are present in p27/p21−/− MEFs, although at lower levels than in wild-type MEFs (1, 4). As a potential means of increasing D cyclin-cdk4 abundance in p27/p21−/− MEFs, we overexpressed cyclin D1 in these cells by transient transfection. cdk4 is more abundant in mouse fibroblasts than is cyclin D1 (11, 15) and thus should not be rate limiting for complex formation in transfected cells. At 24 and 32 h after transfection, amounts of ectopically expressed cyclin D1 (flag-cyclin D1) exceeded those of endogenous cyclin D1 (Fig. 1). There were more cyclin D1-cdk4 complexes in cells transfected with flag-cyclin D1 than in cells transfected with vector alone, and increases in the amounts of complex were accompanied by increases in the amounts of cyclin D1-cdk4 activity. These findings show that overexpression of cyclin D1 enhances the formation of catalytically active D1-cdk4 complexes in the absence of p27Kip1 and p21Cip1.
FIG. 1.
Effect of cyclin D1 overexpression on cyclin D1-cdk4 abundance and activity in p27/p21−/− MEFs. Exponentially growing p27/p21−/− MEFs were transfected with 15 μg of plasmid alone (vector) or with plasmid encoding flag-tagged cyclin D1. Cells transfected with plasmid alone were harvested 24 h after transfection. Cells transfected with plasmid encoding cyclin D1 were harvested 24 or 32 h after transfection. Amounts of cyclin D1 (both ectopic and endogenous) were determined by Western blotting of cell extracts with antibody to cyclin D1. The association of cdk4 with cyclin D1 was determined by Western blotting of cyclin D1 immune complexes with antibody to cdk4. Amounts of cdk4 activity were determined by in vitro kinase assays of cyclin D1 immunoprecipitates with GST-Rb as substrate. IP, immunoprecipitation; pRb, phosphorylated Rb.
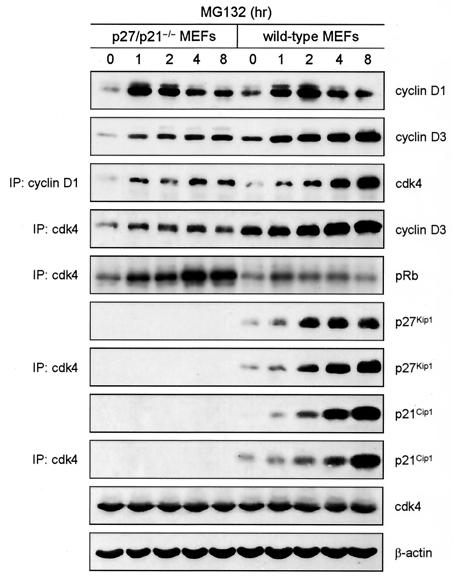
To increase the abundance of endogenous cyclin D1 and cyclin D3, we treated p27/p21−/− MEFs with MG132, an inhibitor of proteasome activity and thus of D cyclin degradation (7, 8). For comparative purposes, wild-type MEFs were also exposed to MG132. Cyclin D1 and cyclin D3 were more abundant in untreated wild-type MEFs than in untreated p27/p21−/− MEFs, and amounts of both cyclins increased in both populations within 1 h of MG132 addition (Fig. 2). This finding implies that wild-type MEFs and p27/p21−/− MEFs contain a pool of D cyclins that are rapidly synthesized and, to achieve a steady state, are rapidly degraded. In wild-type MEFs, this pool presumably consists of D cyclins not bound to cdk4 (i.e., free D cyclins) and, to a much lesser extent, D cyclins associated with cdk4 (but not p27Kip1 or p21Cip1). We found that free cyclin D3 accounts for ∼20% of total cyclin D3 in wild-type MEFs and almost all of the cyclin D3 in p27/p21−/− MEFs (1). Previous studies showed that cyclin D1, whether free or complexed to cdk4, has a half-life of 30 to 60 min and thus is relatively unstable (7, 8). Two hours after exposure to MG132, amounts of cyclin D1 declined in wild-type and p27/p21−/− MEFs, presumably via a proteasome-independent pathway (5). Amounts of cyclin D3 increased slightly after 2 h and were greater in wild-type MEFs than in p27/p21−/− MEFs. MG132 had no effect on the abundance of cdk4, which has a half-life of ∼4 h (10).
FIG. 2.
Effect of MG132 on the abundance and activity of D cyclin-cdk4 complexes in wild-type and p27/p21−/− MEFs. Logarithmically growing MEFs received 20 μM MG132 for the indicated times. Amounts of cyclin D1, cyclin D3, cdk4, p27Kip1, p21Cip1, and β-actin (loading control) were determined by Western blotting of cell extracts. Amounts of cyclin D1-associated cdk4 were determined by Western blotting of cyclin D1 immunoprecipitates with antibody to cdk4. Amounts of cdk4-associated cyclin D3, p27Kip1, and p21Cip1 were determined by Western blotting of cdk4 immunoprecipitates with antibody to cyclin D3. Amounts of cdk4 activity were determined by in vitro kinase assays of cdk4 immunoprecipitates (IP). pRb, phosphorylated Rb.
In p27/p21−/− MEFs exposed to MG132, increases in the amounts of the D cyclins were accompanied by increases in the amounts of cdk4-associated cyclin D1 and cyclin D3 and cdk4 activity. Thus, increases in D cyclin abundance resulting from inhibition of proteasome activity result in the accumulation of active D cyclin-cdk4 complexes in cells lacking p27Kip1 and p21Cip1. Although MG132 also increased D cyclin-cdk4 abundance in wild-type MEFs, two important differences between MG132-treated wild-type and p27/p21−/− MEFs are noted. First, amounts of cdk4-associated D cyclin were greater in wild-type MEFs than in p27/p21−/− MEFs. Second, unlike p27/p21−/− MEFs, MG132 had a biphasic effect on cdk4 activity in wild-type MEFs. Amounts of cdk4 activity increased slightly in wild-type MEFs exposed to MG132 for 1 h and then decreased. The decrease in activity corresponded with increases in the abundance of total and cdk4-associated p27Kip1 and p21Cip1.
We suggest that elevated amounts of D cyclin-cdk4 complex account for the small increase in cdk4 activity in wild-type MEFs exposed to MG132 for 1 h. However, once p27Kip1 and p21Cip1 begin to accumulate, inactive ternary complexes are formed. The formation of ternary complexes also correlates with increases in D cyclin-cdk4 abundance, presumably resulting from the stabilization of these complexes. Collectively, the data in Fig. 2 show that p27Kip1 and p21Cip1 promote the accumulation but are not required for the assembly of D cyclin-cdk4 complexes and that D cyclin-cdk4 complexes containing p27Kip1 or p21Cip1 are not active.
Effects of p27Kip1 and p21Cip1 on the abundance and associated activities of cyclins D1 and D3.
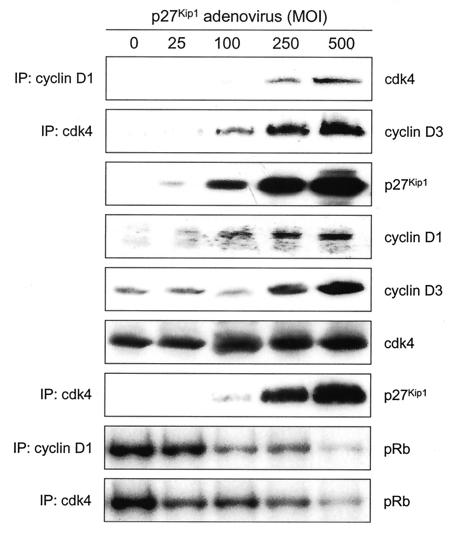
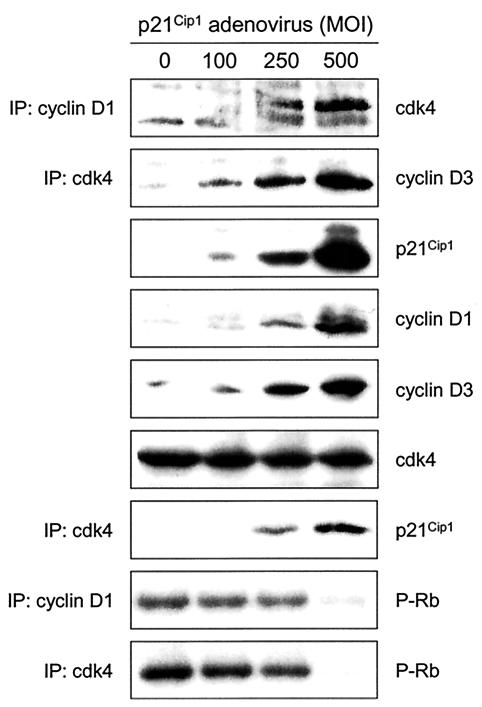
As an alternative means of assessing the effects of p27Kip1 and p21Cip1 on the abundance and activity of D cyclin-cdk4 complexes, we infected exponentially growing p27/p21−/− MEFs with adenovirus encoding p27Kip1 (Ad-p27Kip1) or p21Cip1 (Ad-p21Cip1) at different multiplicities of infection (MOIs). Cells were harvested 25 h after infection, and amounts and activities of cyclin D1-cdk4 and cyclin D3-cdk4 were determined. p27/p21−/− MEFs infected with Ad-p27Kip1 (Fig. 3) or Ad-p21Cip1 (Fig. 4) contained more cyclin D1- and cyclin D3-cdk4 complexes than did uninfected p27/p21−/− MEFs. Increases in the amounts of these complexes paralleled increases in the amounts of p27Kip1 or p21Cip1. Infection of p27/p21−/− MEFs with Ad-p27Kip1 or Ad-p21Cip1 also increased the abundance of cyclins D1 and D3 but had no effect on the abundance of cdk4. Ectopically expressed p27Kip1 or p21Cip1 associated with cdk4 in a dose-dependent manner but did not result in cdk4 activation. In fact, amounts of cdk4 activity were greatest in uninfected cells and decreased as amounts of p27Kip1 or p21Cip1 increased. These findings show that ectopic expression of p27Kip1 or p21Cip1 in p27/p21−/− MEFs increases the abundance of D cyclin-cdk4 complexes, inhibits the activity of preexisting D cyclin-cdk4 complexes, and prevents the activation of newly formed D cyclin-cdk4 complexes. Previous studies (17) also showed inhibition of cyclin D1-cdk4 activity by ectopic expression of Ad-p27Kip1 (at a single MOI) in p27/p21−/− MEFs.
FIG. 3.
Amounts and associated activities of cyclins D1 and D3 in p27/p21−/− MEFs infected with Ad-p27Kip1. Asynchronously growing p21/p27−/− MEFs were infected with Ad-p27Kip1 at the indicated MOIs. Cells were harvested 25 h after infection. Amounts of p27Kip1, cyclin D1, cyclin D3, and cdk4 were determined by Western blotting of cell extracts. Amounts of cyclin D1-associated cdk4, cdk4-associated cyclin D3, and cdk4-associated p27Kip1 were determined by immunoprecipitation (IP) and immunoblot analysis. Kinase activity was assayed in cyclin D1 and cdk4 immunoprecipitates with GST-Rb as substrate. pRb, phosphorylated Rb.
FIG. 4.
Amounts and associated activities of cyclins D1 and D3 in p27/p21−/− MEFs infected with Ad-p21Cip1. Experiments were performed as described in the legend to Fig. 3, except that p27/p21−/− MEFs were infected with Ad-p21Cip1 rather than Ad-p27Kip1.
Effects of p27Kip1 and p21Cip1 on D cyclin expression and stability.
The data in Fig. 3 and 4 show that ectopic expression of p27Kip1 or p21Cip1 in p27/p21−/− MEFs increases the abundance of cyclins D1 and D3. This could result from an increase in the expression of these proteins, the stability of these proteins, or both. To determine whether p27Kip1 increases the expression of cyclin D1 at the mRNA level, exponentially proliferating p27/p21−/− MEFs were infected with Ad-p27Kip1 at an MOI of 500. Cells were harvested 30 or 40 h after infection, and amounts of cyclin D1 protein and mRNA were determined by Western blotting and Northern blotting, respectively. Cyclin D1 protein was more abundant in cells infected with Ad-p27Kip1 than in uninfected cells or cells infected with adenovirus alone (Fig. 5). In contrast, amounts of cyclin D1 mRNA were similar in control cells, adenovirus-infected cells, and Ad-p27Kip1-infected cells. Ectopic expression of p27Kip1 in p27/p21−/− MEFs also had no effect on cyclin D3 mRNA abundance (data not shown). Thus, p27Kip1 does not affect the expression of the D cyclins at the mRNA level. Previous studies have shown that ectopic expression of p27Kip1 in p27/p21−/− MEFs does not appreciably increase the rate of translation of the cyclin D1 transcript (4).
FIG. 5.
Lack of effect of p27Kip1 on cyclin D1 mRNA expression. Exponentially growing p27/p21−/− MEFs were infected with adenovirus without insert (Ad) or adenovirus encoding p27Kip1 (Ad-p27Kip1) at an MOI of 500. Cells were harvested 30 or 40 h after infection. Amounts of cyclin D1 protein and mRNA were determined by Western blotting and Northern blotting, respectively. Amounts of 28S and 18S rRNA are shown as loading controls. The asterisk denotes a nonspecific band.
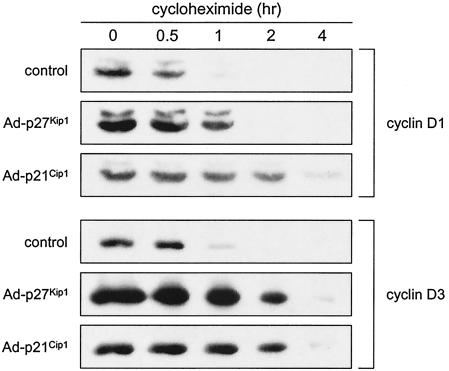
To determine the effects of p27Kip1 and p21Cip1 on the stability of cyclins D1 and D3, growing p27/p21−/− MEFs were infected with Ad-p27Kip1 or Ad-p21Cip1, and 25 h later infected and uninfected cells received cycloheximide. Cells were harvested at various times after addition of cycloheximide, and amounts of cyclins D1 and D3 were determined by Western blotting. For ease of comparison, we have normalized (as much as possible) the amounts of cyclin D1 or D3 at time zero by using different exposures of the Western blots. Both proteins degraded more rapidly in uninfected cells than in cells infected with Ad-p27Kip1 or Ad-p21Cip1 (Fig. 6). Thus, cyclins D1 and D3 are more stable in the presence than in the absence of p27Kip1 and p21Cip1. Stabilization of cyclin D1 by ectopic expression of p27Kip1 in p27/p21−/− MEFs has been described previously (4). Because p27Kip1 and p21Cip1 associate with D cyclin-cdk4 complexes rather than with D cyclins alone (3, 14), they presumably stabilize the D cyclins via the formation of ternary complexes.
FIG. 6.
Effect of p27Kip1 and p21Cip1 on the stability of cyclin D1 and cyclin D3. Growing p27/p21−/− MEFs were infected with Ad-p27Kip1 or Ad-p21Cip1 at an MOI of 500. Twenty-five hours later infected and uninfected cultures received 10 μg of cycloheximide/ml. Cells were harvested at the indicated times, and amounts of cyclin D1 and cyclin D3 were determined by Western blotting. Different exposures of the Western blots were chosen to obtain roughly equivalent amounts of cyclin D1 or cyclin D3 at time zero.
DISCUSSION
It was previously shown that cdk4 is active in p27/p21−/− MEFs and that D cyclin-cdk4 complexes containing p27Kip1 or p21Cip1 are inactive (1). Thus, p27Kip1 and p21Cip1 are not and cannot be required for the assembly of active D cyclin-cdk4 complexes. p27Kip1 and p21Cip1 do, however, promote the accumulation of these complexes. We and others showed that D cyclin-cdk4 complexes were much more abundant in wild-type MEFs than in p27/p21−/− MEFs and that p27Kip1 and p21Cip1 increased D cyclin-cdk4 abundance when ectopically expressed in p27/p21−/− MEFs (Fig. 3 and 4) (1, 4, 17). However, as described here, ectopic expression of p27Kip1 or p21Cip1 in p27/p21−/− MEFs reduced cdk4 activity in a dose-dependent manner. Thus, p27Kip1 and p21Cip1 have opposite effects on the abundance versus the activity of D cyclin-cdk4 complexes.
Total amounts of cyclins D1 and D3 are lower in p27/p21−/− MEFs than in wild-type MEFs (1, 4, 17). This finding suggests that loss of p27Kip1 and p21Cip1 reduces the abundance of these cyclins, which perhaps limits the formation of D cyclin-cdk4 complexes. Consistent with this premise, we found that D cyclin-cdk4 complexes increased in abundance when amounts of the D cyclins were artificially elevated in p27/p21−/− MEFs. Two methods were used to increase D cyclin abundance in these cells: ectopic expression of cyclin D1 and inhibition of D cyclin degradation by the proteasome inhibitor MG132. Amounts of cyclin D1- and cyclin D3-cdk4 complexes increased rapidly and maximally after addition of MG132 to p27/p21−/− MEFs, as did amounts of both cyclins. However, MG132 had a greater effect on D cyclin-cdk4 abundance in wild-type MEFs than in p27/p21−/− MEFs. Thus, overexpression of the D cyclins is insufficient for the maximal accumulation of D cyclin-cdk4 complexes; i.e., p27Kip1 and p21Cip1 are also required. Rather than the low abundance of the D cyclins, we suggest that the instability of binary D cyclin-cdk4 complexes accounts for the low abundance of D cyclin-cdk4 complexes in p27/p21−/− MEFs.
Additional experiments showed that amounts of cyclin D1 mRNA were similar in p27/p21−/− MEFs infected with adenovirus alone or with adenovirus encoding p27Kip1. On the other hand, cyclin D1 and cyclin D3 were more rapidly degraded in uninfected p27/p21−/− MEFs than in p27/p21−/− MEFs infected with Ad-p27Kip1 or Ad-p21Cip1. This finding implies that D cyclins in ternary complexes are more stable than are free D cyclins or D cyclins in binary complexes. Thus, the abundance of the D cyclins reflects the abundance of ternary complexes, which in turn is dependent upon amounts of p27Kip1 and p21Cip1. Cyclin D1 has a half-life of only 30 to 60 min regardless of whether or not it is associated with cdk4 (7, 8). Proteasome-mediated degradation of cdk4-associated cyclin D1 (but not of free cyclin D1) requires its phosphorylation at threonine 286 by glycogen synthase 3β (6, 8). Whether this kinase phosphorylates cyclin D1 in ternary complexes remains to be determined.
As described above, ectopic expression of cyclin D1 in p27/p21−/− MEFs increased D cyclin-cdk4 abundance (albeit submaximally), as did inhibition of proteasome activity. Importantly, in these experiments increases in complex formation were accompanied by increases in cdk4 activity. These findings clearly show that D cyclin-cdk4 complexes are formed and become active in the absence of p27Kip1 and p21Cip1. On the other hand, conditions that increased the abundance of p27Kip1 or p21Cip1 reduced cdk4 activity; such conditions included ectopic expression of these CKIs in p27/p21−/− MEFs and treatment of wild-type MEFs with MG132. It is possible that D cyclin-cdk4 assembly requires proteins that do not interfere with cdk4 activation. Although previous studies implicate p34SEI-1 in this process (18), we found that overexpression of p34SEI-1 in growing p27/p21−/− MEFs did not increase D cyclin-cdk4 association or activity (data not shown). However, a recent study suggests that p34SEI-1 plays a more prominent role in D cyclin-cdk4 complex formation in quiescent cells stimulated to reenter the cell cycle than in exponentially proliferating cells (17).
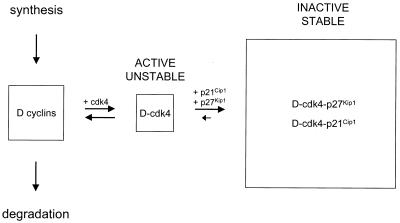
Based on our findings, we propose the following model (Fig. 7). In growing wild-type cells, newly synthesized D cyclins are either rapidly degraded or incorporated into binary complexes with cdk4. As noted above, binary complexes may contain additional proteins required for complex assembly. Binary complexes are active but unstable and do not accumulate, and D cyclins in binary complexes are degraded as rapidly as are free D cyclins. Association of D cyclin-cdk4 complexes with p27Kip1 or p21Cip1 results in the formation of stable ternary complexes. These complexes accumulate and thus account for the bulk of D cyclin-cdk4 complexes in wild-type cells, but are not active. The formation of ternary complexes also increases the half-life of the D cyclins. We suggest that the instability of binary complexes facilitates the rapid turn-off of cdk4 activity in response to growth inhibitory signals. Conversely, the low dissociation rates of ternary complexes allow the efficient sequestration of p27Kip1 and p21Cip1, thus preventing their redistribution to cdk2-containing complexes.
FIG. 7.
Model showing the effects of p27Kip1 and p21Cip1 on the abundance and activity of D cyclin-cdk4 complexes in wild-type cells. The D cyclins in three different pools are shown, and the activity and stability of binary and ternary complexes are indicated. See the text for details.
Acknowledgments
This work was supported by the Cortner-Couch Endowed Chair for Cancer Research and NIH Grant CA67360 to W.J.P.
We thank Nancy Olashaw for manuscript preparation and acknowledge the helpful service of the Molecular Imaging Core Laboratory at the Moffitt Cancer Center.
REFERENCES
- 1.Bagui, T. K., R. J. Jackson, D. Agrawal, and W. J. Pledger. 2000. Analysis of cyclin D3-cdk4 complexes in fibroblasts expressing and lacking p27Kip1 and p21Cip1. Mol. Cell. Biol. 20:8748-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blain, S. W., E. Montalvo, and J. Massague. 1997. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 272:25863-25872. [DOI] [PubMed] [Google Scholar]
- 3.Chen, J., P. Saha, S. Kornbluth, B. D. Dynlacht, and A. Dutta. 1996. Cyclin-binding motifs are essential for the function of p21CIP1. Mol. Cell. Biol. 16:4673-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21Cip1 and p27Kip1 CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, Y. H., S. J. Lee, P. Nguyen, J. S. Jang, J. Lee, M. L. Wu, E. Takano, M. Maki, P. A. Henkart, and J. B. Trepel. 1997. Regulation of cyclin D1 by calpain protease. J. Biol. Chem. 272:28479-28484. [DOI] [PubMed] [Google Scholar]
- 6.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehl, J. A., F. Zindy, and C. J. Sherr. 1997. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 11:957-972. [DOI] [PubMed] [Google Scholar]
- 8.Germain, D., A. Russell, A. Thompson, and J. Hendley. 2000. Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J. Biol. Chem. 275:12074-12079. [DOI] [PubMed] [Google Scholar]
- 9.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 10.Matsushime, H., M. E. Ewen, D. K. Strom, J. Y. Kato, S. K. Hanks, M. F. Roussel, and C. J. Sherr. 1992. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71:323-334. [DOI] [PubMed] [Google Scholar]
- 11.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J. Y. Kato. 1994. D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevins, J. R. 1980. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 65:768-785. [DOI] [PubMed] [Google Scholar]
- 13.Olashaw, N., and W. J. Pledger. 2002. Paradigms of growth control: relation to Cdk activation. Sci. STKE 2002:re7. [Online.] [DOI] [PubMed] [Google Scholar]
- 14.Polyak, K., J. Y. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 15.Quelle, D. E., R. A. Ashmun, S. A. Shurtleff, J. Y. Kato, D. Bar-Sagi, M. F. Roussel, and C. J. Sherr. 1993. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 7:1559-1571. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz, J. K., C. H. Bassing, I. Kovesdi, M. B. Datto, M. Blazing, S. George, X. F. Wang, and J. R. Nevins. 1995. Expression of the E2F1 transcription factor overcomes type β transforming growth factor-mediated growth suppression. Proc. Natl. Acad. Sci. USA 92:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto, M., N. Martin, D. P. Wilks, K. Tamai, T. J. Huot, C. Pantoja, K. Okumura, M. Serrano, and E. Hara. 2002. Activation of cyclin D1-kinase in murine fibroblasts lacking both p21Cip1 and p27Kip1. Oncogene 21:8067-8074. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto, M., T. Nakamura, N. Ohtani, L. Hampson, I. N. Hampson, A. Shimamoto, Y. Furuichi, K. Okumura, S. Niwa, Y. Taya, and E. Hara. 1999. Regulation of CDK4 activity by a novel CDK4-binding protein, p34SEI-1. Genes Dev. 13:3027-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8:1750-1758. [DOI] [PubMed] [Google Scholar]