Abstract
The serine/threonine kinase PAK4 is a target for the Rho GTPase Cdc42 and has been shown to regulate cell morphology and cytoskeletal organization in mammalian cells. To examine the physiological and developmental functions of PAK4, we have disrupted the PAK4 gene in mice. The absence of PAK4 led to lethality by embryonic day 11.5, a result most likely due to a defect in the fetal heart. Striking abnormalities were also evident in the nervous systems of PAK4-deficient embryos. These embryos had dramatic defects in neuronal development and axonal outgrowth. In particular, spinal cord motor neurons and interneurons failed to differentiate and migrate to their proper positions. This is probably related to the role for PAK4 in the regulation of cytoskeletal organization and cell and/or extracellular matrix adhesion. PAK4-null embryos also had defects in proper folding of the caudal portion of the neural tube, suggesting an important role for PAK4 in neural tube development.
The Rho GTPases, including Cdc42, Rac, and Rho, regulate the organization of the actin cytoskeleton in mammalian cells by promoting the formation of filopodia, lamellipodia, and stress fibers, respectively (63). These cytoskeletal changes play important roles in the regulation of cell morphology and migration. The Rho GTPases also regulate cell adhesion and proliferation, as well as activation of intracellular signaling pathways (2, 8, 13, 21, 44, 74). Although they were initially characterized in fibroblasts, the Rho GTPases also have important functions in other cell types. For example, the Rho GTPases have been shown to be important for the regulation of neurite outgrowth in Caenorhabditis elegans, Drosophila melanogaster, and chickens and in mammalian primary neurons and cell lines (9, 18, 30, 33, 34, 39, 54, 75). This is probably due in large part to the fact that filopodia and lamellipodia play key roles in the elongation of neurites (37).
In their activated GTP-bound states, the Rho GTPases bind to target proteins that mediate their various biological functions (63). Among the targets that have been identified for the Rho GTPases, the p21 activated kinase (PAK) family of serine/threonine kinases are thought to have important roles in regulating cell growth and morphology (4, 16, 17, 31, 51). The PAKs fall into two categories based on their amino acid sequences. The group A PAKs, consisting of mammalian PAK1, PAK2, and PAK3, are similar to each other throughout their entire sequences. They contain an amino-terminal GTPase-binding domain (GBD), which binds to activated Rac or Cdc42, and a carboxyl-terminal kinase domain. Although expression of the group A PAKs leads to changes in cell morphology, several studies have shown that they may not be directly required for all of the cytoskeletal rearrangements triggered by Cdc42 and Rac (40, 52). Their exact roles in Rho GTPase signaling pathways, therefore, remain to be fully clarified.
The second category of PAKs, group B, consists of human PAK4, PAK5, and PAK6. PAK4 is ubiquitously expressed (1), PAK5 is expressed primarily in the brain and pancreas (15), and PAK6 is expressed in the testes, prostate, and brain (35, 69). PAK4, -5, and -6 are highly similar to each other throughout their GBD and kinase domains but share only approximately 50% identity with the group A PAKs in these domains. Outside of the GBD and kinase domains, the group B PAKs are completely different from each other and from the group A PAKs. PAK4 was the first member of the group B PAKs to be identified (1). It binds preferentially to activated Cdc42, although it also binds to activated Rac (1, 48). In contrast to the other PAKs, PAK4 can promote filopodia formation in response to Cdc42 in several cell types, including fibroblasts, and this is dependent on its kinase activity and on its binding to activated Cdc42 (1). In neuroblastoma cells, activated PAK4 can also promote neurite outgrowth, a process which requires Cdc42 activation and filopodia formation (15). In fibroblasts, activated PAK4 decreases adhesion to the extracellular matrix and promotes proliferation, leading to anchorage-independent growth (48), which may explain why its overexpression is associated with tumorigenesis (10). PAK4 is also thought to be directly involved in cell migration, and this may be mediated specificaly through the integrin β5 subunit (73). The extracellular stimuli that activate PAK4 and substrates that are phosphorylated by PAK4 are only beginning to be elucidated. PAK4, for example, was shown to be activated by the cytokine HGF (68), and its substrates include the cytoskeletal regulatory protein LIMK1 and the proapoptotic protein BAD (14, 25).
Since the regulation of cell shape, adhesion, and motility are critical for all aspects of development, there has been growing interest in the physiological and developmental functions of the Rho GTPases and their target proteins. In Drosophila, the Rho GTPases have been implicated in a wide range of early developmental processes (cellularization, cytokinesis, gastrulation, and dorsal closure) (36), as well as later developmental processes (neuronal development, muscle differentiation, and the establishment of planar cell polarity) (36). The two best-characterized Drosophila PAKs are Drosophila PAK (DPAK), a group A PAK (26), and “mushroom body tiny” (MBT), a group B PAK similar to PAK4 (41). DPAK is important for dorsal closure and photoreceptor axon guidance (26, 27), and MBT is thought to be important for the development of neurons, specifically the Kenyon cells in the mushroom body (41).
In mammals and birds, most studies of the biological roles of PAKs and Rho GTPases have relied on the use of dominant-negative or activated mutants. For example, studies in which activated or dominant-negative mutants of the Rho GTPases are transfected into neurons from chicken or rat suggest an important role for the GTPases in neuronal development (9, 58). Likewise, transgenic mice expressing activated Rac in neurons have an increased number of dendritic spines (38). Whereas some important information can be obtained from such studies, the generation of loss-of-function mutants is crucial to understanding the physiological and developmental functions of these proteins in mammals. Mouse knockouts of Cdc42 and Rac1 have been analyzed, but result in early embryonic lethality (before embryonic day 6.5 [E6.5] and E9.5, respectively) (11, 55), so that information about their roles in mammalian development is still limited (11, 55). Thus far, no mouse knockouts of any of the PAK family members have been reported, so the physiological and developmental roles of these kinases in mammals are still completely unknown. Here we report on the effects of targeted disruption of PAK4 in mice. PAK4 is absolutely required for development, and PAK4-null embryos die prior to E11.5. Since PAK4-null embryos survive considerably longer than Cdc42- or Rac1-null embryos, however, we were able to examine organogenesis. The most likely cause of death in the PAK4-null embryos is a defect in the fetal heart. In addition, improper folding occurred in the caudal neural tubes of the PAK4-null embryos, resulting in the formation of two neural lumens. Most strikingly, PAK4-null embryos had severe abnormalities in the development and migration of neurons. Whereas neuronal progenitors appeared to form normally, neuronal differentiation was largely inhibited, axonal outgrowth was impaired, and neurons failed to migrate to their proper locations.
MATERIALS AND METHODS
Plasmids and antibodies.
Expression plasmids encoding hemagglutinin (HA)-tagged PAK4 wt, HA-tagged PAK4(N445,E474), HA-tagged PAK4ΔGBD, and empty vector SRα were described previously (48). The empty targeting vector pPNT was described in (62). The anti-Myc antibody, anti-PAK2 (γ-PAK) antibody, anti-PAK1 antibody, and horseradish peroxidase-conjugated donkey anti-goat immunoglobulin G (IgG) were obtained from Santa Cruz Biotechnology; anti-vinculin antibody and horseradish peroxidase-conjugated goat anti-mouse IgG were from Sigma. The monoclonal antibody to PAK4 was described previously (48). The anti-PAK5 antibody was generated by Covance, Inc. Fluorescein isothiocyanate (FITC)-conjugated phalloidin was obtained from Molecular Probes, and rhodamine-conjugated goat anti-mouse IgG antibody was from Pierce.
Guinea pig anti-Nkx6.1, mouse anti-Pax7, guinea pig anti-olig2, mouse anti-Isl1/2, rabbit anti-Lim1/2, rabbit anti-Dbx2, mouse anti-Shh, and mouse anti-neurofilament antibodies were gifts from T. Jessell. FITC-labeled donkey anti-guinea pig, Cy3-labeled donkey anti-mouse, Cy3-labeled donkey anti-guinea pig, FITC-labeled donkey anti-mouse, and Cy5-labeled donkey anti-rabbit secondary antibodies were obtained from Jackson Immunoresearch Laboratories.
Cloning of murine PAK4 cDNA.
To isolate mouse PAK4 cDNA clones, a pair of degenerate oligonucleotide primers were synthesized based on the amino acid sequences conserved among the kinase domains of human PAK4, MBT (PAK4 Drosophila homologue), and C45B11.1 (PAK4 C. elegans homologue). The two primers corresponding to the amino acid sequences KQQRRELL and HRDIKSD were used to generate a PCR product with cDNA from NIH 3T3 cells as a template. PCR products were gel purified and subcloned. Inserts were sequenced by automatic sequencer (ABI Prism 377). A 253-bp PCR product exhibiting 90% homology to the kinase domain of human PAK4 was labeled with [α-32P]dCTP (Amersham Life Sciences) by the random prime labeling method (Prime-It II kit; Stratagene) and used as a probe to screen a mouse adult brain cDNA lambda library (in the Uni-ZAP XR vector; Stratagene) according to the standard protocol. Seven independent positive clones were excised as plasmids. The longest one contained the complete coding frame of mouse PAK4.
Construction of target vector and generation of PAK4−/− mice.
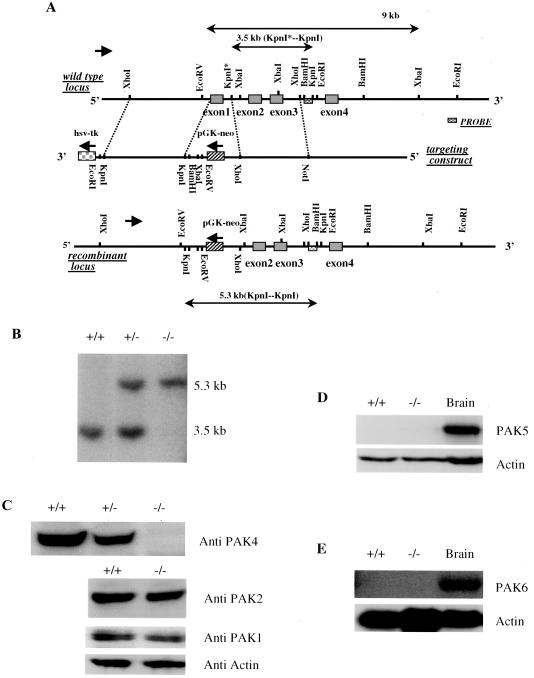
To isolate PAK4 genomic sequences, a murine 129 genomic library (Genome Systems) was screened with the 253-bp degenerate PCR products. Several genomic clones were isolated, subcloned into the pBluescript vector, and mapped by standard techniques. A 3.5-kb fragment 3′ to exon 1 and a 4.2-kb fragment 5′ to exon 1 were sequentially subcloned into the pPNT vector in the opposite transcriptional orientation from the PGK-neo gene to generate the targeting construct pPNT-PAK4KO. The final construct pPNT-PAK4KO was verified by restriction analysis and partial sequencing. The pPNT-PAK4KO was linearized at a unique NotI site and electroporated into D3 embryonic stem (ES) cells. G418- and GANC-resistant colonies were isolated and genotyped by Southern analysis with an external DNA probe. Digestion of genomic DNA with KpnI results in a 3.5-kb fragment in the wild-type allele and a 5.3-kb fragment in the recombinant allele. Among 240 G418-resistant clones, 29 had a recombinant PAK4 allele. D3 ES cells were obtained from Richard Hynes (20). Later, the electroporations were repeated in E14 ES cells. PAK4 knockouts from both types of ES cells gave identical results.
PAK4+/− cells were injected into C57BL/6 blastocysts. Chimeric male mice displaying >50% coat color chimerism were bred to C57BL/6 females to generate F1 offspring. Germ line transmission of the targeted PAK4 allele was verified by PCR and Southern blot analysis of tail DNA from F1 offspring with agouti coat color. Heterozygotes were crossed with C57BL/6 mice, and the heterozygote progeny were interbred to obtain homozygotes. Genotypes were verified by PCR and Southern blot analysis. Embryos were generated by timed pregnancies of heterozygotes, and the genomic DNA derived from yolk sacs were genotyped.
Two lines of mice carrying the targeted PAK4 allele in the germ line were established from two independent ES cell clones. The phenotypic consequences of PAK4 deficiency appeared to be equivalent in offspring of both of these separately derived lines.
Genotyping by PCR.
A competitive PCR method was used to assess the PAK4-null (PAK4−) and wild-type (PAK4+) alleles simultaneously. A reverse primer (5′-TGG TCA CTG GGA GAG GAT G-3′) common to the PAK4− and PAK4+ alleles and two forward primers specific to PAK4+ (5′-CAG CAG CCA TTC AGT CTT-3′) or PAK4−(5′-GGT GGA TGT GGA ATG TGT-3′) were combined in the same PCR. The PCRs were heated at 94°C for 5 min and then went through 30 cycles at 94°C for 45 s, 60°C for 1 min, and 72°C for 1 min. The PCR products were analyzed on 2% agarose gel. The expected sizes of the wild-type and knockout PCR products are ca. 300 and 200 bp, respectively.
Histological analysis.
Embryos generated by timed matings were isolated, fixed overnight in Bouin's solution, dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Yolk sacs were isolated for genotyping. Freshly dissected whole embryos were also observed and photographed by using a dissecting microscope.
Immunohistochemistry.
For immunohistochemical localization of proteins, embryos were fixed with ice-cold 4% paraformaldehyde for 2 h, washed with cold phosphate-buffered saline (PBS), equilibrated with 30% sucrose in PBS at 4°C overnight, and then mounted in Tissue-Tek OCT compound and frozen by using dry ice. The embryo blocks were cut at about 12 μm per section. Proteins were stained by incubating slides with the indicated antibodies in 1% horse serum in PBS with 0.1% Triton X-100 (PBST) overnight at 4°C. After a wash with PBST, the slides were incubated with the indicated immunofluorescence-tagged secondary antibodies for 1 h at room temperature, washed again with PBST, and then mounted with Vectashield mounting medium (Vector). Images were collected on a Zeiss LSM510 confocal microscope.
ES cell culture and generation of PAK4−/− ES cells.
ES cells were cultured on a uniform layer of mouse embryonic fibroblasts (MEFs) rendered quiescent by gamma irradiation. ES cells were grown in Dulbecco modified Eagle medium (Gibco-BRL) containing 15% fetal bovine serum (Gibco-BRL), 0.1 mM nonessential amino acids (Gibco-BRL), 2 mM l-glutamine (Gibco-BRL), 0.1 mM β-mercaptoethanol (Sigma), and 100 U of penicillin-streptomycin/ml (Gibco-BRL) at 37°C and 5% CO2. To generate PAK4−/− ES cells, the PAK4+/− ES cells were grown in a high concentration of G418 (2 mg/ml). The medium was supplemented with lymphocyte inhibitory factor (1,000 U/ml; Gibco-BRL). Genomic DNA from 50 resistant clones were genotyped by Southern blotting. Six percent of the resistant cells that were analyzed had a PAK4−/− genotype.
Generation of PAK4−/− MEFs.
E9.5 embryos were dissected from decidua, and all maternal tissues were removed under the dissection microscope. Yolk sacs were isolated and genotyped by PCR as described above, and each embryo was minced, treated with trypsin, and then cultured in individual wells on 24-well plates in Dulbecco modified Eagle medium containing 15% fetal bovine serum, glutamine, penicillin, and streptomycin. After confluence was reached, cells were trypsinized and replated, and nonadherent cells were removed 40 min later.
Western blots.
Western blot analysis was carried out as described earlier (48). Lysates from human testes, brain, and heart were from Clontech.
Northern blots.
To determine the relative expression levels of mouse PAK4 mRNA in different tissues and developmental stages, Northern analysis was performed with a mouse RNA master blot (Clontech) according to the manufacturer's protocol. A 400-bp cDNA fragment in the mouse PAK4 regulatory domain was prepared by random prime labeling method (Stratagene) to analyze the master blot.
To examine PAK6 levels, total RNA from different tissues of wild-type and PAK4-null mice was prepared by using RNAzol B (Tel-Test, Inc.). The isolated total RNA was separated and transferred to a positively charged nylon membrane (Amersham Bioscience) as described elsewhere (23). A 500 PAK6 cDNA fragment from the regulatory domain was radioactive labeled with a random primer labeling kit (Stratagene) and used to probe the membrane. The hybridization was performed in ExpressHyb solution from Clontech according to the manufacturer's protocol, and the membrane was exposed to a Kodak film at −80°C for 5 days. The same membrane was probed with radiolabeled α-actin cDNA probe as loading control.
Indirect immunofluorescence microscopy.
PAK4+/+ and PAK4−/− MEFs (E9.5) grown on fibronectin-coated coverslips were starved in serum-free medium for 24 h and then fixed in 4% paraformaldehyde for 10 min at room temperature. The cells were stained with anti-vinculin monoclonal antibody (hVIN-1; Sigma) and rhodamine-conjugated anti-mouse secondary antibody and then visualized by fluorescence microscopy as described previously (48).
BrdU assays.
Bromodeoxyuridine (BrdU) was injected intraperitoneally at 100 μg/g (body weight) into pregnant female mice at E9.5. The females were sacrificed 30 min after injection, and the embryos were fixed in 10% formalin, dehydrated, paraffin embedded, and sectioned for immunohistochemistry. The sections were labeled with the in situ cell proliferation Zymed BrdU staining kit according to the manufacturer's manual.
TUNEL assays.
Paraffin-embedded sections were performed with TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining by using an ApopTag peroxidase in situ apoptosis detection kit (Integrin) according to the manufacturer's manual.
RESULTS
Generation of PAK4-null mice.
A targeting vector was generated in which the first exon of murine PAK4, containing the initiating methionine and the critical GBD, was replaced by a neomycin resistance gene (Fig. 1A). The linearized targeting vector was electroporated into ES cells. Southern blot analysis of 240 G418-resistant clones revealed 29 clones containing the desired homologous recombination event (Fig. 1B). Several of these PAK4+/− ES cell clones were then grown in a high concentration of G418 in order to select G418-resistant clones that contained a homozygous (PAK4−/−) deletion. Western blot analysis of these cells indicated that the targeting strategy led to elimination of PAK4 protein, whereas the expression of PAK1 and PAK2 was unaffected (Fig. 1C). Western blot analysis of PAK5 and Northern blot analysis of PAK6 indicated no detectable expression in neither wild-type nor PAK4-null ES cells (Fig. 1D). Chimeric mice were generated by injecting several of the PAK4+/− ES cell clones into C57BL/6 blastocysts. Five clones resulted in germ line transmission of the disrupted PAK4 allele. PAK4 heterozygous mice appeared normal and were fertile. Heterozygotes from one of the ES clones were crossed to generate PAK4-null (PAK4−/−) mice.
FIG. 1.
Targeting of PAK4. (A) Construction of the PAK4 targeting vector. The genomic locus of the PAK4 gene is shown at the top. The first four exons are indicated. Exon 1 encodes the first 68 amino acids of PAK4, including the GBD. Exon 3 includes the critical lysine 348 in subdomain II of the kinase domain. The knockout vector was generated to replace exon 1 with a PGK-neo gene cassette flanked with a 4.2-kb 5′ homology region and a 3.5-kb 3′ homology region. The 5′ XhoI-EcoRV fragment was inserted into the targeting vector by ligation of a PCR product with KpnI adaptors on both ends. The 3′ KpnI-XhoI fragment was inserted into the targeting vector by using a 5′ XhoI-KpnI adaptor and a 3′ XhoI-NotI adaptor. In the recombinant locus, restriction sites derived from the targeting vector are indicated below those from the wild-type locus. The KpnI* site is replaced by XhoI site upon gene targeting, and this change is used to distinguish the knockout allele from the wild-type allele. KpnI digestion of genomic DNA results in a 3.5-kb fragment in the wild-type allele and a 5.3-kb fragment in the recombinant allele. (B) Southern analysis of genomic DNA from ES clones. Genomic DNA was isolated from PAK4+/+, PAK4+/−, and PAK4−/− ES cells, digested with KpnI, and analyzed by Southern blotting with a 3′ external probe. The probe is schematically shown in panel A. (C) Western analysis of cell lysates from PAK4+/+, PAK4+/−, and PAK4−/− ES cells. Cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride filter, and probed with monoclonal antibodies against PAK4; blots with the same lysates were also probed with PAK1, PAK2, and actin antibody. (D) Western analysis of ES cells. A Western blot of PAK4+/+ and PAK4−/− ES cells and wild-type brain lysates was probed with PAK5 polyclonal antibody and actin antibody. There is no detectable expression of PAK5 protein in either the wild-type cells or the PAK4-null ES cells. (E) Northern blot analysis of total RNA from PAK4+/+ and PAK4−/− ES cells and wild-type brain tissue. The blot was probed with a portion of the PAK6 cDNA that is specific to PAK6. There is no detectable expression of PAK6 mRNA in neither the wild-type nor PAK4-null ES cells. The same blot was also probed with a portion of actin cDNA as control.
Absence of PAK4 results in embryonic lethality.
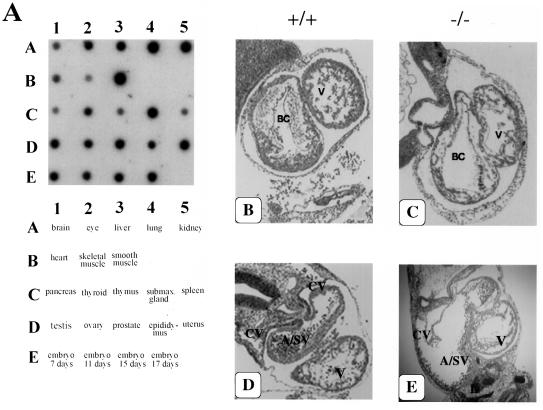
Genotyping of more than 100 newborn pups revealed that the absence of PAK4 results in embryonic lethality. PAK4-null embryos were found at Mendelian ratios up to embryonic day 10.5 (E10.5). No live PAK4-null embryos were found after that stage (Table 1). The early lethality of the PAK4-null embryos is consistent with the wide expression pattern of PAK4 in adults and early in embryogenesis (Fig. 2A). The most likely cause of death of the PAK4-null embryos is a defect in the heart. PAK4-null embryos had a thinning of the myocardial walls of the bulbus cordis and ventricle (Fig. 2B and C). This most likely led to poor ventricular function and pooling of blood in the atrium and/or sinus venosus region of the heart, causing it to appear distended and dilated (Fig. 2D and E).
TABLE 1.
Genotypes of offspring from PAK4+/− intercrossesa
| Stage | No. of animals with genotype:
|
|||
|---|---|---|---|---|
| +/+ | +/− | −/− | ND | |
| Postnatal | 118 | 251 | 0 | |
| E15.5 | 5 | 4 | 0 | |
| E13.5 | 1 | 7 | 0 | |
| E11.5 | 5 | 20 | 8* | 2 |
| E10.5 | 18 | 43 | 27 | |
| E9.5 | 90 | 161 | 82 | 8 |
| E9 | 10 | 12 | 3 | 2 |
PAK4 deficiency results in embryonic lethality. Genotypes of postnatal mice or mice at the indicated embryonic day were determined. +/+, Wild-type mice; +/−, heterozygous mice; −/−, homozygous (knockout) mice. *, Partly resorbed embryos. ND, not determined.
FIG. 2.
PAK4 expression pattern and analysis of the heart defects in PAK4 knockout mice. (A) Northern blot analysis of PAK4 expression. A mouse RNA master blot (Clontech) was probed with a 0.4-kb cDNA fragment in the regulatory domain of mouse PAK4. PAK4 is expressed in all of the tissues tested and can be detected as early as E7. (B and C) H&E-stained cross section of the heart showing the bulbus cordis (BC) and primitive ventricle (V) from E10.5 wild-type (+/+) (B) and PAK4-deficient (−/−) (C) embryos. (The dorsal aorta and first branchial arch artery can also be seen in the PAK4−/− embryo, although they cannot be seen in this section of the wild-type embryo.) This region of the heart appears normal in the knockouts, except for the thin myocardial wall. (D and E) Cross sections deeper into the hearts are shown. The region where the common atrium merges with the sinus venosus (A/SV) can be seen above the ventricle. In the knockout, the A/SV region (which can be seen communicating with one of the anterior cardinal veins [CV]) is dilated and distorted.
PAK4 is required for normal neuronal development.
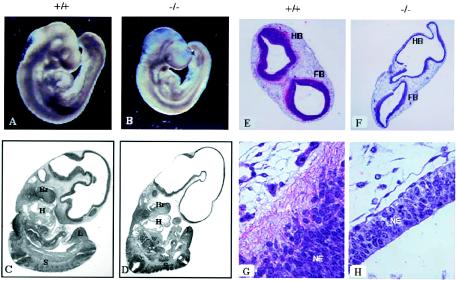
The most striking abnormalities in the PAK4-null embryos were defects in the brain and neural tube. Gross examination of the PAK4-null embryos (E9.5) revealed that the regions around the head and parts of the neural tube appeared translucent in the mutant embryos (Fig. 3A and B). This was also evident on H&E-stained sagittal sections of PAK4 knockouts (Fig. 3C and D), although many other aspects of embryogenesis appeared to occur surprisingly normally in the PAK4-null embryos (Fig. 3A to D). Histological analysis of H&E-stained cross sections revealed that the neuroepithelium around the hindbrain and forebrain was unusually thin in the PAK4-null embryos compared to wild-type embryos (Fig. 3E and F), and loss of eosinophilic staining suggests a lack of neurite outgrowth in these embryos (Fig. 3G and H). To confirm that neurite outgrowth was impaired in the knockouts, cross sections of the hindbrain were stained with antibodies against the neuronal marker neurofilament, which stains axons of differentiated neurons (19). Staining with anti-neurofilament antibody was greatly reduced in the knockouts (Fig. 3I and J), indicating a defect in axonal outgrowth. In the wild-type embryos, neurofilament positive cells were located at the lateral edge of the hindbrain, which reflects the normal lateral migration of neurons. The small number of neurofilament positive cells that could be seen in the knockouts, however, could be seen both medially and laterally (Fig. 3I and J), suggesting a defect in neuronal migration. In E9.5 embryos, the defects in the neuroepithelium were not associated with a severe defect in proliferation (Fig. 3K and L) or an increase in apoptosis (Fig. 3M and N), although in other parts of the Pak4-null embryos there was a more pronounced defect in proliferation and increase in apoptosis (data not shown). Our results suggest, therefore, that lack of neuronal differentiation and a corresponding decrease in cell size due to the defect in axonal outgrowth contributed to the abnormal appearance of the brain rather than a severe defect in cell proliferation. Consistent with this apparent role for PAK4 in neuronal development, we found that in contrast to mRNA levels, PAK4 protein levels were especially high in the brain (Fig. 3O).
FIG. 3.
Neuronal defects in PAK4-null embryos. (A and B) Images of PAK4 wild-type (+/+) (A) and PAK4-deficient (−/−) (B) E9.5 embryos. PAK4-null embryos are generally smaller than wild-type embryos and are translucent around the head and neural tube regions. (C and D) H&E-stained sagittal sections of E10.5 PAK4+/+ (C) and PAK4−/− (D) littermates. The large empty area in the head region is apparent in the knockouts, although many other aspects of development appear normal. For example, branchial arches (Br), two heart chambers (H), somites (S), and limb buds (L) can be seen in both wild-type and knockout embryos, and turning occurs normally in the knockout and wild-type embryos. (E and F) H&E-stained cross sections of the brains of E9.5 PAK4 wild-type (+/+) (E) and PAK4-deficient (−/−) (F) embryos (4× lens). The neuroepithelium, which stains dark blue, is thinner in the knockouts than in wild-type embryos. The wavy appearance of the brains in the PAK4-null mice is most likely due to postmortem collapse of these extremely thin neuroepithelial walls. Hindbrain (HB) and forebrain (FB) are labeled. (G and H) High-magnification images (40× lens) of the neuroepithelium (NE) of the hindbrains of wild-type (G) and PAK4-deficient (H) embryos. Pink (eosinophilic) stained cells are most likely axons (white A) and can only be seen in the wild-type embryos. (I and J) Lack of axon extension in PAK4 knockout embryos. Hindbrains of wild-type (I) and PAK4-deficient (J) E9.5 embryos were stained with antibodies to neurofilament (red) and Nkx6.1 (green), which stains part of the neuroepithelium. The region where they overlap appears yellow. The results indicate that neurofilament staining is dramatically reduced in the knockouts. (K and L) BrdU-labeled cross sections of PAK4+/+ (K) and PAK4−/− (L) littermates at E9.5. Sections focusing on the neuroepithelium are shown. The relative amounts of BrdU-positive (brown) cells are comparable in the neuroepithelia of the control and mutant embryos. (M and N) TUNEL staining of sagittal sections of PAK4+/+ (M) and PAK4−/− (N) littermates at E9.5. Sections focusing on the neuroepithelia are shown. TUNEL-positive cells (brown), which are apoptotic, were rarely observed in neuroepithelia of either PAK4 wild-type or PAK4-null embryos (although more sporadic positive staining was seen in the tissues outside the neuroepithelium in the knockouts). (O) Western blot analysis of PAK4 protein levels. Equal amounts of lysates generated from different tissues (testis, brain, and heart) were blotted and probed with a monoclonal antibody to PAK4. For comparison, lysates from 293 cells transfected with HA-tagged PAK4 were also analyzed.
PAK4 is required for differentiation and migration of spinal cord motor neurons and interneurons.
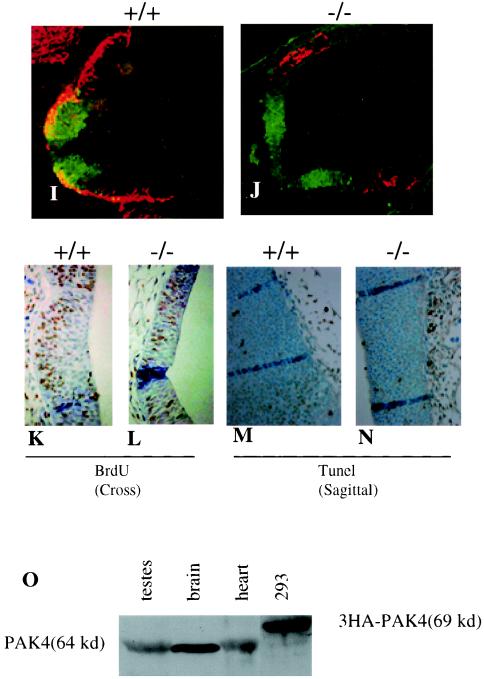
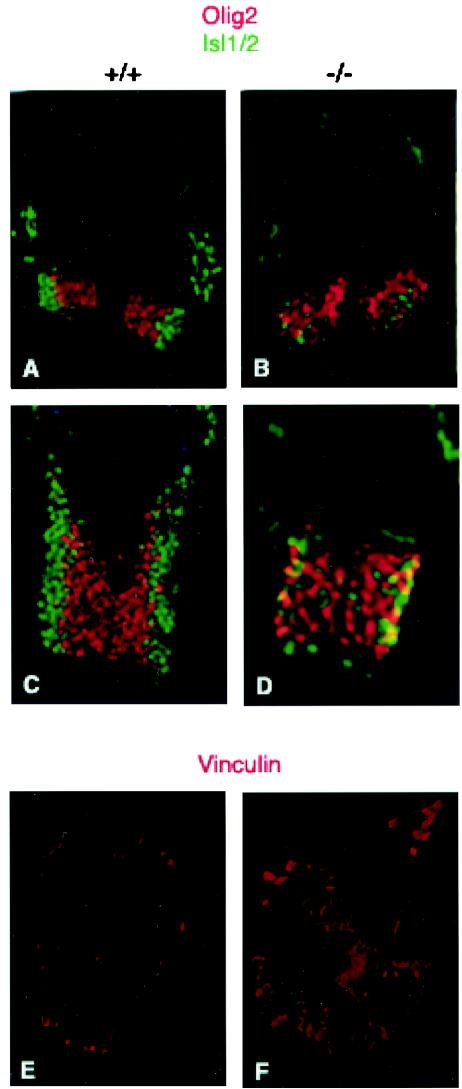
To study neuronal differentiation in the knockouts in more detail, we examined the developing spinal cord neurons, focusing on ventrally expressed motor neurons and interneurons. To determine whether motor neuron progenitors formed normally and whether neuronal differentiation occurred in the knockouts, cross sections of the neural tubes were stained with anti-olig2 antibody, which stains motor neuron progenitors (46), and anti Isl1/2, which stains differentiated motor neurons that derive from these progenitors (47, 61). In a normal developing neural tube, olig2 is expressed medially, whereas Isl1/2 is found laterally, due to the lateral migration of the differentiated neurons (Fig. 4A and C). Interestingly, we found that although motor neuron progenitors (olig2 positive) formed normally, there was a defect in motor neuron differentiation (Isl1/2 positive) in the PAK4-null embryos (Fig. 4B and D). Similar results were found in all parts of the neural tube that we have analyzed (data not shown). Interestingly, although some Isl1/2-positive cells were present in the mutant embryos, they were not in their proper lateral positions (Fig. 4B and D), indicating a corresponding defect in motor neuron migration. This did not appear to be due simply to a developmental delay in motor neuron differentiation, since most of the Isl1/2-positive cells did not stain with Math3 (data not shown), which is found in newly differentiated neurons in the neural tube (B. Novitch and T. Jessell, unpublished data). These results further support the idea that neuronal differentiation and migration are impaired in the PAK4 mutant embryos. Development of ventral interneurons, as assessed by staining with anti-LIM1/2 antibody (22), was also severely impaired in the mutant embryos (data not shown).
FIG. 4.
Defects in motor neuron and interneuron development in PAK4 knockout embryos. (A to D) Cross sections of different parts of the neural tubes from E9.5 embryos were stained with anti-olig2 (red) and anti-Isl1/2 (green) antibodies. Neural tubes from PAK4 wild-type (+/+) (A and C) and PAK4-null (−/−) (B and D) embryos were analyzed. There were fewer Is1/2-positive cells in the knockouts. Isl1/2-positive cells that were present in the knockouts were not in the correct lateral positions. (E and F) PAK4-deficient cells had increased levels of focal adhesions. Cells isolated from E9.5 PAK4+/+ (E) and PAK4−/− (F) embryos grown on glass coverslips were starved in serum-free medium for 24 h and then subjected to indirect immunofluorescence microscopy using antivinculin antibody. Whereas PAK4+/+ cells only contained low levels of vinculin when grown in serum-free medium, PAK4-null cells had large clusters of vinculin.
The regulation of axonal outgrowth and neuronal migration is controlled by a variety of factors. Interactions with the extracellular matrix via integrins, for example, play important roles in both of these processes (12, 43). Interestingly, we have found that cells lacking PAK4 have greatly elevated levels of focal adhesions (Fig. 4E and F), suggesting a misregulation of adhesion molecules in the absence of PAK4. Improper regulation of adhesion molecules in neuronal cells could provide one explanation for the migration defect and the lack of proper neuronal differentiation observed in the PAK4-null embryos.
PAK4 knockouts have caudal neural tube defects.
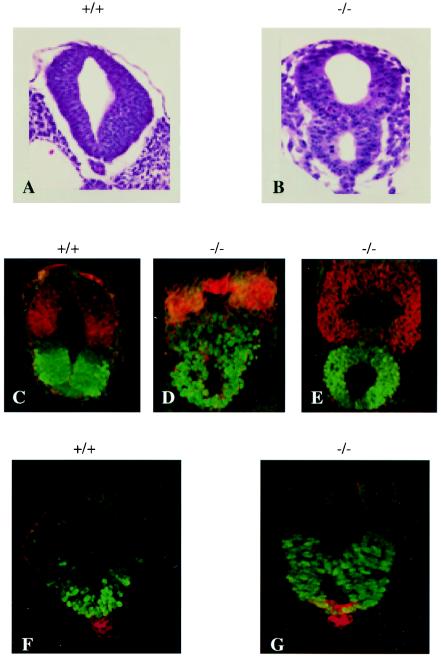
In addition to defects in neurite outgrowth, PAK4-null embryos had defects in neural tube development. Although the neural tube appeared normal throughout most of its length in the mutant embryos, the caudal end of the tube had an abnormal appearance. In this region, what at first appeared to be a duplication of the neural tube could be seen in PAK4-null embryos (Fig. 5A and B). In some embryos this apparent duplication could be seen even more rostrally along the neural tube. To see whether the apparent ectopic structure was indeed neural tube tissue and to verify that correct dorsal ventral patterning was established, cross sections of the neural tubes were stained with antibodies against dorsal and ventral neural tube markers. These included anti-Nkx6.1, which stains ventral neural progenitors, and anti-Pax7, which stains dorsal progenitors (6). Interestingly, correct dorsal ventral patterning occurred even in the mutant neural tubes (Fig. 5C to E), and the absence of duplicated gene expression suggests that there was single improperly folded tube rather than two separate tubes. Furthermore, staining with sonic hedgehog antibody, which stains the notochord (22), indicates that only a single notochord is present (Fig. 5F and G). Thus, rather than a true duplication, an improper folding or “pinching” appeared to occur between the ventral and dorsal parts of the caudal neural tube.
FIG. 5.
Caudal neural tube defects in PAK4-null embryos. (A and B) Cross sections of the caudal portions of neural tubes from wild-type (A) and PAK4-deficient (B) E10.5 embryos. In the PAK4-deficient embryo, what appears to be two neural tubes can be seen adjacent to a single notochord. A layer of neuroepithelium can be seen between the two lumens. (C to E) The expression of Pax7 (red) and Nkx6.1(green) was analyzed in cross sections of the caudal neural tubes of E9.5 wild-type (C) and PAK4-deficient (D and E) embryos. The dorsal-ventral patterning appears normal in the PAK4 mutant neural tubes (compare panel C with panels D and E). (F and G) Analysis of Shh (red) and Nkx6.1 (green) expression in cross sections of caudal neural tubes from E9.5 wild-type (F) and PAK4-deficient (G) embryos. Shh is expressed normally in a single notochord and floor plate of the mutant neural tube (see panel G). The results from panels C to G suggest the presence of a single improperly folded neural tube rather than two separate neural tubes.
DISCUSSION
We have described the targeted disruption of PAK4 in mice, and this is the first report of a knockout of any of the PAK family members. Remarkably, although there are six mammalian PAK family members, and all of them have been shown to promote morphological changes in cell lines (17, 29), we found that PAK4 is absolutely required for development. This indicates a lack of redundancy between the functions of PAK4 and those of other PAK family members. In fact, we have found that unlike PAK4, PAK5 knockout mice are both viable and fertile (35a). The absence of PAK4 led to lethality before E11.5. The most likely cause of death was a defect in the heart associated with a thinning of the myocardium and a dilation of the atrium and sinus venosus. Interestingly, transgenic mice expressing the Rho GTPase inhibitor Rho GDIα in the heart also have severe cardiac defects leading to embryonic lethality by E11.5. The GDI transgenic hearts are dilated and surrounded by a distended pericardium. Since Rho GDIα inhibits all three major members of the Rho GTPase family (66), these results suggest an important role for Rho GTPases in heart development. However, the defects in the GDI transgenic hearts are more severe than those of the PAK4 knockouts and include abnormalities in cardiac looping and septation. This is associated with a decrease in proliferation of cardiomyocytes and improper regulation of cyclin A and the cyclin-dependent kinase inhibitor p21 (66). The basis for the cardiac defect in PAK4-null embryos is not yet known. However, our results reflect the fact that properly regulated cell growth and morphogenesis are essential for normal development and suggest that changes in cell growth and morphology caused by the absence of PAK4 may contribute to improper development of the heart.
PAK4-deficient embryos also had severe defects in the nervous system, although dorsal ventral patterning of the nervous system occurred normally. Development and migration of neuronal cells was seriously impaired in the PAK4 knockouts. PAK4 mutant embryos had unusually thin neuroepithelia in the hindbrain and forebrain and a corresponding defect in neuronal differentiation and axonal outgrowth. This could be detected even in intact embryos by the appearance of a nearly translucent head and neural tube. The abnormalities in the nervous system were most likely not secondary effects due to the defect in fetal circulation because other mouse knockouts that die from cardiovascular defects at similar developmental stages do not have translucent or abnormally shaped heads (65, 70). Even the Rho GDIα transgenic embryos with the severe cardiac abnormalities described above do not have transparent heads as seen in the PAK4 knockouts (66).
To examine neuronal differentiation in detail, we focused on developing spinal cord neurons. In the developing embryo, sensory neurons and their attendant interneurons develop at the dorsal part of the neural tube, whereas motor neurons and interneurons develop in the ventral part (5). By E9.5, ventral neurons, but not dorsal neurons, are well developed in the mouse. We therefore focused on ventral motor neuron and interneuron development in the knockouts. We found that whereas neuronal progenitors formed normally, there was a defect in the differentiation and lateral migration of both motor neurons and ventral interneurons. This did not appear to be associated with the folding defect present in PAK4-null embryos because the lack of neuronal differentiation was apparent all along the neural tube and was not limited to the caudal end where the improper folding occurred.
The defect in neuronal differentiation most likely reflects the fact that PAK4 is associated with cytoskeletal changes including the formation of filopodia (1, 48), which have essential roles in the developing nervous system (3, 45, 56). Both filopodia and lamellipodia play key roles in the guidance of neuronal growth cones toward attractive cues and away from repulsive cues, and neurite extension occurs when these filopodia and lamellipodia are stabilized. Stabilization of these structures is followed by extension of new filopodia and lamellipodia so that the cycle of axon guidance and growth can continue (37, 45). Both neurite outgrowth and migration are thus largely controlled by these cytoskeletal structures. Consistent with this, Cdc42 and Rac were shown to play important roles in neurite outgrowth in cultured mammalian cells (24, 33, 49, 59, 60, 64), and Drosophila and C. elegans Cdc42 and Rac homologues have important roles in axonal extension and guidance (30, 75). Likewise, our results suggest that PAK4 has an important role in the differentiation of neurons. Interestingly, the Drosophila PAK4 homologue, MBT, is also thought to have an important role in neuronal development (41). In contrast to PAK4, however, MBT most likely has a role in regulating the growth or survival neuronal cells rather than in neuronal differentiation (41).
Association of integrins with the extracellular matrix plays a critical role in the regulation of axonal outgrowth and neuronal migration, and integrins are thought to have both positive and negative roles in neuronal migration (12, 43). Interestingly, cells overexpressing activated PAK4 have a decrease in focal adhesions (48), which are made up of integrins and accessory proteins (72). Conversely, cells isolated from PAK4 deficient embryos had a greatly increased level of focal adhesions (Fig. 4). Elevated levels of focal adhesions are likely to lead to an increase in adhesion to the extracellular matrix and could thus potentially prevent neurons from extending axonal processes and from migrating normally. It will be interesting to determine whether improper regulation of adhesion molecules in the neurons of PAK4-null embryos may be directly related to the defects in differentiation and migration.
It will also be interesting to determine which PAK4 substrates are important mediators in its role in neurite outgrowth. One likely candidate is LIM kinase. PAK4 strongly phosphorylates LIM kinase 1 (LIMK1) (14). Knockouts of LIMK1, however, have a much milder defect than the PAK4 knockouts. LIMK1 knockouts are viable and appear normal, although they have defects in spatial learning, alterations in long-term potentiation, and abnormalities in dendritic spine formation (42). Furthermore, we have found that the basal level of LIMK1 phosphorylation is not abrogated in PAK4 knockouts, and similar results were observed in the PAK5 knockouts (data not shown). These results suggest that PAK4 and PAK5 are not absolutely required for basal LIMK1 phosphorylation, although in the future it will also be important to determine whether LIMK1 phosphorylation in response to Cdc42 or Rac is altered in Pak4-null cells.
Another striking abnormality in the PAK4-null mice was what appeared to be a duplication or misfolding of the caudal portion of the neural tube. The absence of duplicated gene expression patterns and the absence of a second notochord, however, indicate that the caudal neural tubes in the PAK4 knockouts were most likely not actually duplicated. Instead, there appeared to be an alteration in neural tube constriction. One possible explanation is that abnormal proliferation and/or migration of neural epithelial cells led to the formation of what appeared to be two lumens instead of one. Proper neural tube development depends on actin reorganization (50), and therefore improper cytoskeletal organization in Pak4-null embryos could contribute to the defects in neural tube development.
It is interesting that mouse knockouts of other cytoskeletal regulatory proteins also have neural tube defects, although they are not identical to the abnormalities seen in the PAK4 knockouts. For example, approximately 30% of p190 Rho GAP-null mice have severe cranial neural tube closure defects (7). p190 Rho GAP is directly involved in regulating the activity of Rho, and it is thought to have an important role in cytoskeletal organization (57). The neural tube defects in the p190 Rho GAP-null mice appear to be associated with an abnormal accumulation of F-actin but not with an increase in apoptosis (7). Other cytoskeletal regulatory proteins have also been found to contribute to neural tube development. N-Wasp, for example, is thought to control cytoskeletal organization by inducing Arp2/3-mediated actin assembly (67). Neural tubes of N-Wasp-null embryos do close, but they appear undulated, and the neuroepithelium has a wavy appearance (53). Other examples are the Abl and Arg tyrosine kinases, which are both thought to have important roles in cytoskeletal organization. In double knockouts of Abl and Arg, neural tube closure is delayed and can be incomplete (32). In addition, the neural tubes “buckle” and part of the neuroepithelium collapses into the neural tube. This does not appear to be due to an increase in proliferation of the neuroepithelial cells but appears instead to be associated with disruption of actin filaments (32). All of these results suggest that abnormalities in cytoskeletal organization and cell shape may contribute to the abnormal morphology of the neural tube. It is interesting that the neural tubes of Pak4-null embryos did appear to close but were clearly abnormal in appearance. Since Pak4 is thought to have an important role in cytoskeletal organization, one possibility is that defects in cell morphology could cause the neuroepithelium to collapse into the lumen of the neural tube, similar to what was found with Arg and Abl double knockouts (32), and leading to the appearance of a duplicated neural tube.
Although several cytoskeletal regulatory proteins have been shown to have important roles in neural tube development, as described above, it is interesting that other types of signaling proteins have also been implicated in neural tube abnormalities that are similar to those of the Pak4 knockouts. One of the most interesting is the Wnt family of secreted proteins. In particular, caudal neural tubes in Wnt3A knockout embryos appeared quite similar to the PAK4 knockout caudal neural tubes, although in the Wnt3A knockouts the neural tubes may have a true duplication (71). In addition to an apparent caudal neural tube duplication, the Wnt3A knockouts lack caudal somites. The absence of Wnt3A is thus thought to cause cells normally destined to become mesoderm (from which somites develop) to adopt a neural fate instead (71). The PAK4 mutants did have caudal somites, but they were underdeveloped compared to the somites in wild-type embryos. Furthermore, the defects in the PAK4-null neural tubes appeared to be associated with an increased number of cells between the two lumens. PAK4 could potentially also have a role, therefore, in specifying mesodermal or neuronal fate in the caudal part of the embryo. Interestingly, we have found that activated PAK4 can stimulate expression of a TCF/LEF reporter (J. Qu and A. Minden, unpublished results), which is a target for the Wnt signaling pathway (28). Taken together, these results suggest the possibility of cross talk between PAK4 and the Wnt signaling pathway, and future experiments will be required to determine whether both pathways play similar roles in neural tube development.
In summary, these findings demonstrate that PAK4 has an essential role in animal development. Despite the presence of five other PAK family members and other Rho GTPase targets, none of these could compensate for the lack of PAK4 during development. We found that PAK4 has an essential role in the morphological development of the heart and neural tube, as well as in differentiation and migration of neurons.
.
Acknowledgments
We thank members of A. A. Beg's lab and L. Yamasaki's lab and L. Yamasaki for advice and assistance with the generation of the PAK4 knockout mice. We thank D. Kelley, J. Kitajewski, and T. Jessell for helpful discussions. We thank C. Yang and R. Peraza at the Rockefeller animal facility for help in generation of the knockout mice and Radma Mahmood and Robert Russell for help in analyzing PAK4-null embryos.
This work was supported by a grant from the NIH (CA76342) and an American Scientist Development Grant Award from American Heart Association to A.M. and a grant from the NIH (CA074892) to A.A.B.
REFERENCES
- 1.Abo, A., J. Qu, M. S. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17:6527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995-27998. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, D., and T. P. O'Connor. 1994. Cytoskeletal events in growth cone steering. Curr. Opin. Neurobiol. 4:43-48. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, A. L., and A. Hall. 2000. Rho GTPase and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 5.Briscoe, J., and J. Ericson. 2001. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 11:43-49. [DOI] [PubMed] [Google Scholar]
- 6.Briscoe, J., A. Pierani, T. M. Jessell, and J. Ericson. 2000. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101:435-445. [DOI] [PubMed] [Google Scholar]
- 7.Brouns, M. R., S. F. Matheson, K. Q. Hu, I. Delalle, V. S. Caviness, J. Silver, R. T. Bronson, and J. Settleman. 2000. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development 127:4891-4903. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. L., L. Stowers, M. Baer, J. Trejo, S. Coughlin, and J. Chant. 1996. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr. Biol. 6:598-605. [DOI] [PubMed] [Google Scholar]
- 9.Brown, M. D., B. J. Cornejo, T. B. Kuhn, and J. R. Bamburg. 2000. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J. Neurobiol. 43:352-364. [DOI] [PubMed] [Google Scholar]
- 10.Callow, M. G., F. Clairvoyant, S. Zhu, B. Schryver, D. B. Whyte, J. R. Bischoff, B. Jallal, and T. Smeal. 2002. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277:550-558. [DOI] [PubMed] [Google Scholar]
- 11.Chen, F., L. Ma, M. C. Parrini, X. Mao, M. Lopez, C. Wu, P. W. Marks, L. Davidson, D. J. Kwiatkowski, T. Kirchhausen, S. H. Orkin, F. S. Rosen, B. J. Mayer, M. W. Kirschner, and F. W. Alt. 2000. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr. Biol. 10:758-765. [DOI] [PubMed] [Google Scholar]
- 12.Clegg, D. O. 2000. Novel roles for integrins in the nervous system. Mol. Cell. Biol. Res. Commun. 3:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 14.Dan, C., A. Kelly, O. Bernard, and A. Minden. 2001. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J. Biol. Chem. 276:32115-32121. [DOI] [PubMed] [Google Scholar]
- 15.Dan, C., N. Nath, M. Liberto, and A. Minden. 2002. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol. Cell. Biol. 22:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11:220-230. [DOI] [PubMed] [Google Scholar]
- 17.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350-355. [DOI] [PubMed] [Google Scholar]
- 18.Daniels, R. H., P. S. Hall, and G. M. Bokoch. 1998. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 17:754-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd, J., S. B. Morton, D. Karagogeos, M. Yamamoto, and T. M. Jessell. 1988. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron 1:105-116. [DOI] [PubMed] [Google Scholar]
- 20.Doetschman, T. C., H. Eistetter, M. Katz, W. Schmidt, and R. Kemler. 1985. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 87:27-45. [PubMed] [Google Scholar]
- 21.Dutartre, H., J. Davoust, J. P. Gorvel, and P. Chavrier. 1996. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J. Cell Sci. 109:367-377. [DOI] [PubMed] [Google Scholar]
- 22.Ericson, J., P. Rashbass, A. Schedl, S. Brenner-Morton, A. Kawakami, V. van Heyningen, T. M. Jessell, and J. Briscoe. 1997. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90:169-180. [DOI] [PubMed] [Google Scholar]
- 23.Fourney, R. M., Miyakoshi J., R. S. Day III, and P. M. C. 1988. Northern blotting: efficient RNA staining and transfer. Focus 10:5-7. [Google Scholar]
- 24.Gebbink, M. F., O. Kranenburg, M. Poland, F. P. van Horck, B. Houssa, and W. H. Moolenaar. 1997. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J. Cell Biol. 137:1603-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gnesutta, N., J. Qu, and A. G. Minden. 2001. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J. Biol. Chem. 276:14414-14419. [DOI] [PubMed] [Google Scholar]
- 26.Harden, N., J. Lee, H. Y. Loh, Y. M. Ong, I. Tan, T. Leung, E. Manser, and L. Lim. 1996. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell. Biol. 16:1896-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hing, H., J. Xiao, N. Harden, L. Lim, and S. L. Zipursky. 1999. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell 97:853-863. [DOI] [PubMed] [Google Scholar]
- 28.Huelsken, J., and W. Birchmeier. 2001. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11:547-553. [DOI] [PubMed] [Google Scholar]
- 29.Jaffer, Z. M., and J. Chernoff. 2002. p21-Activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann, N., Z. P. Wills, and D. Van Vactor. 1998. Drosophila Rac1 controls motor axon guidance. Development 125:453-461. [DOI] [PubMed] [Google Scholar]
- 31.Knaus, U. G., and G. M. Bokoch. 1998. The p21Rac/Cdc42-activated kinases (PAKs). Int. J. Biochem. Cell Biol. 30:857-862. [DOI] [PubMed] [Google Scholar]
- 32.Koleske, A. J., A. M. Gifford, M. L. Scott, M. Nee, R. T. Bronson, K. A. Miczek, and D. Baltimore. 1998. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21:1259-12572. [DOI] [PubMed] [Google Scholar]
- 33.Kozma, R., S. Sarner, S. Ahmed, and L. Lim. 1997. Rho family GTPases and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol. 17:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamoureux, P., Z. F. Altun-Gultekin, C. Lin, J. A. Wagner, and S. R. Heidemann. 1997. Rac is required for growth cone function but not neurite assembly. J. Cell Sci. 110:635-641. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S. R., S. M. Ramos, A. Ko, D. Masiello, K. D. Swanson, M. L. Lu, and S. P. Balk. 2002. AR and ER interaction with a p21-activated kinase (PAK6). Mol. Endocrinol. 16:85-99. [DOI] [PubMed] [Google Scholar]
- 35a.Li, X., and A. Minden. 2003. Targeted disruption of the gene for the PAK5 kinase in mice. Mol. Cell. Biol. 23:7134-7142. [DOI] [PMC free article] [PubMed]
- 36.Lu, Y., and J. Settleman. 1999. The role of rho family GTPases in development: lessons from Drosophila melanogaster. Mol. Cell. Biol. Res. Commun. 1:87-94. [DOI] [PubMed] [Google Scholar]
- 37.Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1:173-180. [DOI] [PubMed] [Google Scholar]
- 38.Luo, L., T. K. Hensch, L. Ackerman, S. Barbel, L. Y. Jan, and Y. N. Jan. 1996. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379:837-840. [DOI] [PubMed] [Google Scholar]
- 39.Luo, L., Y. J. Liao, L. Y. Jan, and Y. N. Jan. 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8:1787-1802. [DOI] [PubMed] [Google Scholar]
- 40.Manser, E., H. Y. Huang, T. H. Loo, X. Q. Chen, J. M. Dong, T. Leung, and L. Lim. 1997. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17:1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melzig, J., K. H. Rein, U. Schafer, H. Pfister, H. Jackle, M. Heisenberg, and T. Raabe. 1998. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr. Biol. 8:1223-1226. [DOI] [PubMed] [Google Scholar]
- 42.Meng, Y., Y. Zhang, V. Tregoubov, C. Janus, L. Cruz, M. Jackson, W. Y. Lu, J. F. MacDonald, J. Y. Wang, D. L. Falls, and Z. Jia. 2002. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 35:121-133. [DOI] [PubMed] [Google Scholar]
- 43.Milner, R., and I. L. Campbell. 2002. The integrin family of cell adhesion molecules has multiple functions within the CNS. J. Neurosci. Res. 69:286-291. [DOI] [PubMed] [Google Scholar]
- 44.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 45.Mueller, B. K. 1999. Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci. 22:351-388. [DOI] [PubMed] [Google Scholar]
- 46.Novitch, B. G., A. I. Chen, and T. M. Jessell. 2001. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 31:773-789. [DOI] [PubMed] [Google Scholar]
- 47.Pfaff, S. L., M. Mendelsohn, C. L. Stewart, T. Edlund, and T. M. Jessell. 1996. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84:309-320. [DOI] [PubMed] [Google Scholar]
- 48.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. de Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 21:3523-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarner, S., R. Kozma, S. Ahmed, and L. Lim. 2000. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol. Cell. Biol. 20:158-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoenwolf, G. C., and J. L. Smith. 1990. Mechanisms of neurulation: traditional viewpoint and recent advances. Development 109:243-270. [DOI] [PubMed] [Google Scholar]
- 51.Sells, M. A., and J. Chernoff. 1997. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 7:162-167. [DOI] [PubMed] [Google Scholar]
- 52.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 53.Snapper, S. B., F. Takeshima, I. Anton, C. H. Liu, S. M. Thomas, D. Nguyen, D. Dudley, H. Fraser, D. Purich, M. Lopez-Ilasaca, C. Klein, L. Davidson, R. Bronson, R. C. Mulligan, F. Southwick, R. Geha, M. B. Goldberg, F. S. Rosen, J. H. Hartwig, and F. W. Alt. 2001. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat. Cell Biol. 3:897-904. [DOI] [PubMed] [Google Scholar]
- 54.Steven, R., T. J. Kubiseski, H. Zheng, S. Kulkarni, J. Mancillas, A. Ruiz Morales, C. W. Hogue, T. Pawson, and J. Culotti. 1998. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in Caenorhabditis elegans. Cell 92:785-795. [DOI] [PubMed] [Google Scholar]
- 55.Sugihara, K., N. Nakatsuji, K. Nakamura, K. Nakao, R. Hashimoto, H. Otani, H. Sakagami, H. Kondo, S. Nozawa, A. Aiba, and M. Katsuki. 1998. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 17:3427-3433. [DOI] [PubMed] [Google Scholar]
- 56.Suter, D. M., and P. Forscher. 1998. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr. Opin. Neurobiol. 8:106-116. [DOI] [PubMed] [Google Scholar]
- 57.Tatsis, N., D. A. Lannigan, and I. G. Macara. 1998. The function of the p190 Rho GTPase-activating protein is controlled by its N-terminal GTP binding domain. J. Biol. Chem. 273:34631-34638. [DOI] [PubMed] [Google Scholar]
- 58.Threadgill, R., K. Bobb, and A. Ghosh. 1997. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19:625-634. [DOI] [PubMed] [Google Scholar]
- 59.Tigyi, G., D. J. Fischer, A. Sebok, F. Marshall, D. L. Dyer, and R. Miledi. 1996. Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite-protective effects of cyclic AMP signaling. J. Neurochem. 66:549-558. [DOI] [PubMed] [Google Scholar]
- 60.Tigyi, G., D. J. Fischer, A. Sebok, C. Yang, D. L. Dyer, and R. Miledi. 1996. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 66:537-548. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchida, T., M. Ensini, S. B. Morton, M. Baldassare, T. Edlund, T. M. Jessell, and S. L. Pfaff. 1994. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79:957-970. [DOI] [PubMed] [Google Scholar]
- 62.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 63.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 64.van Leeuwen, F. N., H. E. Kain, R. A. van der Kammen, F. Michiels, O. W. Kranenburg, and J. G. Collard. 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267-276. [DOI] [PubMed] [Google Scholar]
- 66.Wei, L., K. Imanaka-Yoshida, L. Wang, S. Zhan, M. D. Schneider, F. J. DeMayo, and R. J. Schwartz. 2002. Inhibition of Rho family GTPases by Rho GDP dissociation inhibitor disrupts cardiac morphogenesis and inhibits cardiomyocyte proliferation. Development 129:1705-1714. [DOI] [PubMed] [Google Scholar]
- 67.Welch, M. D. 1999. The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 9:423-427. [DOI] [PubMed] [Google Scholar]
- 68.Wells, C. M., A. Abo, and A. J. Ridley. 2002. PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J. Cell Sci. 115:3947-3956. [DOI] [PubMed] [Google Scholar]
- 69.Yang, F., X. Li, M. Sharma, M. Zarnegar, B. Lim, and Z. Sun. 2001. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 276:15345-15353. [DOI] [PubMed] [Google Scholar]
- 70.Yang, J., M. Boerm, M. McCarty, C. Bucana, I. J. Fidler, Y. Zhuang, and B. Su. 2000. Mekk3 is essential for early embryonic cardiovascular development. Nat. Genet. 24:309-313. [DOI] [PubMed] [Google Scholar]
- 71.Yoshikawa, Y., T. Fujimori, A. P. McMahon, and S. Takada. 1997. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev. Biol. 183:234-242. [DOI] [PubMed] [Google Scholar]
- 72.Zamir, E., and B. Geiger. 2001. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114:3583-3590. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, H., Z. Li, E. K. Viklund, and S. Stromblad. 2002. P21-activated kinase 4 interacts with integrin αvβ5 and regulates αvβ5-mediated cell migration. J. Cell Biol. 158:1287-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934-23936. [DOI] [PubMed] [Google Scholar]
- 75.Zipkin, I. D., R. M. Kindt, and C. J. Kenyon. 1997. Role of a new Rho family member in cell migration and axon guidance in Caenorhabditis elegans. Cell 90:883-894. [DOI] [PubMed] [Google Scholar]